Abstract
Twelve indigenous microalgae, comprising of three fresh water and nine marine strains, were evaluated for their potential as source of dietary polyunsaturated fatty acids (PUFAs) under autotrophic growth conditions. The microalgal lipids showed higher content of omega-3 PUFAs as reflected in lower omega-6 to omega-3 ratios between 0.1 and 0.75. Alpha linolenic acid (ALA), was the predominant omega-3 PUFA in fresh water green algal strains. Scenedesmus dimorphus and Chlorococcum sp. showed higher content of ALA with an average productivity of 3 mgL−1 day−1. Long chain omega-3 PUFAs, eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) were observed in marine strains. Nannochloropsis and Chlorella sp. had higher content of EPA with Chlorella sp. showing an average productivity of 2 mgL−1 day−1. Isochrysis sp. showed higher DHA content with average productivity of 0.37 mgL−1 day−1. Presence of EPA and DHA contributed to higher degree of unsaturation in lipids of marine strains. Among the marine strains, the growth and lipid profile of Nannochloropsis sp. and Chlorella sp. remained unaffected by growth medium, whereas strains like T. theli showed a differential response to media. Spirulina platensis SP6 from CFTRI algal culture repository was also included as a reference strain since its nutritional benefits have been well elucidated. Gamma Linolenic acid (GLA) was the predominant PUFA in Spirulina sp. with an average productivity of 0.73 mgL−1 day−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) such as alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) have been attributed with various health benefits like cardiovascular protection, prevention of age-associated cognition disorders, fetal neurodevelopment and anti-inflammatory properties in humans (Doughman et al. 2007). The minimum recommended dietary intake of n-3 PUFAs as prescribed by WHO and FAO is 250 mg day−1 while American Heart Association has recommended up to 500 mg day−1 for a healthy adult (Lichtenstein et al. 2006). Humans, like other members of animal kingdom, are incapable of de novo synthesis of these PUFAs and depend on dietary sources to meet the requirements. The common food sources of n-3 PUFAs are fish and shellfish, flaxseed (linseed), hemp oil, soya oil, canola (rapeseed) oil, chia seeds, pumpkin seeds, sunflower seeds, leafy vegetables and walnuts. However, fish oil obtained from marine fish such as salmon; mackerel and herring is the major commercial source of EPA and DHA (Guedes et al. 2011).
The marine organisms such as crustaceans and fish require PUFAs for their optimal nutrition and stress tolerance and acquire these fatty acids by feeding on PUFA containing microalgae/phytoplanktons. The microalgae as source of PUFAs are exploited in aquaculture as primary live feed as well as secondary feed by supporting the growth and reproduction of zooplanktons such as rotifers, cladocerans, copepods, brine shrimps that are in-turn fed to the crustacean and fishes (Brett and Muller-Navarra 1997).
The global demand for n-3 PUFAs is increasing with an estimated production of 24.87 kilotons in 2013 and expected to grow at 13.7 % from 2014 to 2020 (www.iffo.net, accessed on April 20, 2014). This increased demand of PUFAs will exert further pressure on world’s marine fishery resources which are depleting due to excessive fish capture and intense fishery practices (FAO 2010). In addition, many toxic chemicals such as methyl mercury, dioxins and poly chlorinated biphenyl (PCB) are found in fish oil due to the increasing pollution levels of oceanic ecosystem caused by anthropogenic activities. These toxic contaminants are hydrophobic in nature and bind to the lipid deposits in fish causing bioaccumulation down the food chain (Storelli et al. 2004). Further, fish oil has unpleasant odour; high cholesterol levels (Melanson et al. 2005) and the proportion of constituent fatty acids are difficult to control. Therefore, it is important to search alternative sources of PUFAs, such as microalgae.
Microalgae have been recognized as potent dietary supplement to enhance the nutritional content of foods owing to the presence of many nutraceuticals such as chlorophylls, carotenoids (lutein, beta-carotene, astaxanthin), essential amino acids, unsaturated fatty acids (EPA, DHA, ALA), β-glucan and dietary fibre (Becker 2004; Fogliano et al. 2010). Microalgae, as a source of PUFAs, have advantage over other plant-based sources as they have higher photosynthetic and surface area productivity (ten folds) than terrestrial crop plants (Rittmann 2008). They can be cultivated in non-arable land in open outdoor ponds throughout the year (Borowitzka 1999) with minimal nutritional input requirement. Thus, microalgal strains with high biomass and PUFA productivity could be important. Few microalgal species such as Schyzochytrium sp., Crypthecodinium cohnii, Phaeodactylum tricornutum have been reported earlier for PUFA production under heterotrophic growth conditions (Wen and Chen 2003). However large scale algal cultivation under autotrophic mode may be preferred as outdoor cultivation systems are well established for food grade algal biomass production such as Spirulina sp., Dunaliella sp., Scenedesmus sp., and Chlorella sp., (Borowitzka 1999). According to Jorquera et al. (2010) outdoor autotrophic based pond cultivation of microalgae have a net energy ratio greater than one over closed systems of biomass production indicating minimal energy requirements to operate the process.
Hence in the present study 12 indigenous microalgae from both freshwater and marine habitats, were evaluated for their fatty acid profiles with a special focus towards n-3 fatty acid productivity under autotrophic growth conditions. The main aim of the work was to identify potential PUFA accumulating strains which can find application as functional foods. The microalgae were cultivated under autotrophic conditions for understanding the effects of phototrophic effects on lipid accumulation and fatty acid composition of the indigenous strains. The biomass production under batch conditions, daily average biomass and lipid productivity, PUFA productivity of all the strains were evaluated for identifying the potential strain for further optimization. Nutritionally rich microalga Spirulina platensis (SP6 strain) maintained in the CFTRI algal culture repository was included in the study for comparison with indigenous freshwater strains in terms of biomass, lipid and PUFA productivity. Further, the marine microalgae were evaluated for their biomass, lipid and PUFA productivity under natural and artificial sea water growth medium for a better understanding on the effects of growth medium on microalgal production.
Materials and methods
Microalgal culture: isolation and identification
Freshwater microalgae, Scenedesmus dimorphus, Chlorella sp., and Chlorococcum sp., used in the study were isolated from natural water habitats and purified as per the method described by Vidyashankar et al. (2013). The geographical co-ordinates of the isolate’s natural habitats are presented in Table 1. The fresh water strains were identified by microscopy and ITS-2 gene sequencing (NCBI accession number – Scenedesmus dimorphus - KJ680137; Chlorococcum sp. - KJ680138; Chlorella sp. - KJ680144). The isolates are perpetually maintained by regular subculturing. The strains were cultivated in autotrophic bold basal medium (BBM) (Kanz and Bold 1969) for the study.
The Marine strains were obtained from culture collection of Regional Research Centre, Central Marine Fisheries Research Institute (CMFRI), Tuticorin, Tamilnadu, India and were maintained in autotrophic enriched natural sea water (ENSW) medium (Table 1). The ENSW and artificial sea water (ASW) medium were used for cultivation of marine microalgal strains in the present study (Table 1). Spirulina platensis- strain SP6, a nutritionally rich microalga maintained at microalgal culture collection of CSIR-CFTRI was included in the study for a comparison with other microalgae in terms of its fatty acid composition and Zarrouk medium (Zarrouk 1966) was used for its cultivation. The growth media were autoclaved at 121 °C for 20 min, vitamin solutions such as thiamine hydrochloride, cyanocobalamine and biotin, supplemented for marine media, were membrane sterilised (0.22 μm). The initial pH of BBM, ASW and ENSW was adjusted to 7.5 with sterile 1 M NaOH or HCl solutions. The initial pH of Zarrouk medium was 8.5.
Microalgae cultivation and growth measurements
All the cultures were incubated at 25 ± 1 °C under 30 μE m−2 s−1 light intensity with a photoperiod of 16:8 h light and dark cycles. The growth experiments were carried out in Erlenmeyer flasks (250 mL). The microalgal growth was monitored by measuring the optical density (OD) at 560 nm at regular intervals and estimating the total chlorophyll and total carotenoid content in biomass. The growth was expressed as biomass (gL−1) using an OD versus biomass correlation standard graph (data not shown) for each microalgal species individually. The growth profile for motile marine microalgae species such as Tetraselmis gracilis, Isochrysis sp. and Pavlova sp. were monitored by measuring the cell number using haemocytometer and expressed as number of cells per mL culture. Pigments were extracted with acetone and quantified by applying Lichtenthaler equations as described by Vidyashankar et al. (2013). The Specific growth rate (μ day−1) of the microalgal strains was calculated by the equation
where Xm and Xo are the concentrations of biomass at the end and beginning of a batch run, respectively, and t is the duration of the run.
The cultures were allowed to reach stationary phase for harvesting. In general the marine strains reached stationary phase between 14 and 18 days and fresh water strains by 21 days of incubation. The cells were harvested by centrifugation at the end of incubation period, washed with de-ionized water and lyophilized.
Lipid extraction and fatty acid composition analysis
The total lipids were extracted from a known quantity (100 mg) of freeze dried biomass with chloroform: methanol (2:1). The biomass was ground in a pestle and mortar with solvent until decolourized. The extracts were filtered (Whatman filter paper No. 1), dried under vacuum, weighed and expressed as percent on dry weight basis.
The fatty acid composition was determined by converting the crude lipids to fatty acid methyl esters (FAMEs) by trans-esterification with methanolic hydrogen chloride reagent (Christie 1982). The dried FAME extracts were diluted with n-hexane (1 mg ml−1) and 1 μl of the FAME solution was injected in a Gas Chromatograph equipped with flame ionization detector (FID). The FAMEs were separated using Rtx-1 (poly(dimethylsiloxane)) capillary column (30 m × 0.32 mm ID ×0.25 μm film thickness) and identified by comparing their retention times with standard FAME mixture (C-8 to C-24 FAME mix, SUPELCO) as described by Vidyashankar et al. (2013). The FAMEs were confirmed by GC-MS analysis (Turbomass Gold, Perkin Elmer, U.S.A) by comparing the fragmentation pattern with authentic standards.
Degree of unsaturation (DU) of the polyunsaturated fatty acids (PUFAs) was calculated for each PUFA and summarized over the entire PUFA profile of all microalgal samples using the Eq. (2) (Floreto and Teshima 1998).
where C x:n is the carbon number with number of double bonds, ω refers to omega (n-3) fatty acids and γ – gamma refers to GLA. DB is the number of double bonds
Statistical analysis
The cultivation experiments were performed twice in triplicates. Results were expressed as the mean ± SD of the replicates. Difference between the groups were statistically analyzed using one way ANOVA followed by Tukey Kramer multiple comparison test at significance level of p < 0.05. The statistical analysis was carried out using GraphPad InStat software, version 3.06, 2003.
Results and discussion
Growth characteristics of microalgae
The growth profile of freshwater microalgae are presented in Fig. 1. The freshwater strains reached stationary phase between 18 and 21 days of incubation, except in Scenedesmus dimorphus where the plateau was observed at 24th day of incubation under batch cultivation. Among the indigenous fresh water strains, S. dimorphus had highest specific growth rate (0.102 ± 0.001) followed by Chlorococcum sp. (0.069 ± 0.006) and least in Chlorella sp., (0.043 ± 0.003) (data not shown). The average biomass concentration at the end of a batch cultivation and daily biomass productivity of the fresh water microalgae is presented in Table 2. S. dimorphus was high biomass producer (0.98 g L−1) and the biomass productivity of the fresh water strains in batch cultivation ranged between 45 mgL−1 day−1 (Chlorella sp.) and 70 mgL−1 day−1 (S. dimorphus) (Table 2). The biomass concentration and productivity of the reference strain Spirulina platensis were 0.80 g L−1 and 57 mg L−1 day−1 respectively (Table 2).
The marine strains were evaluated for their growth and productivity in both the enriched natural sea water (ENSW) and artificial sea water (ASW) media. The ASW medium offers advantage as unlike the physicochemical properties of natural sea water, which vary between the seasons (Berges et al. 2001), its chemical composition can be controlled precisely. The total salinity levels in both the ASW and ENSW media was maintained uniformly at 30–33 parts per thousand (PPT). The biomass productivity of microalgal strains vary depending upon the cultivation conditions such as light illumination intensity, medium composition, temperature (Hempel et al. 2012). Therefore, to avoid any effect of varying culture condition, the microalgal strains in the present study were cultured in their respective medium under uniform conditions.
The average specific growth rate, average biomass concentration at the end of a batch cultivation, and biomass productivity of marine microalgae is presented in Table 3. The growth pattern of marine microalgal strains showed significant variations in ASW and ENSW media suggesting that response to the growth media is species specific. Nannochloropsissp., T. chuii and Chlorella sp., did not show any significant variation in their growth in ENSW or ASW medium and showed a high biomass concentration of >0.6 g L−1 irrespective of the growth medium (Fig. 2). The growth response of Synechocystis sp. also remained same in both the media, however, the biomass concentration was significantly lower at 0.3 g L−1 (Fig. 2). A reduction of 26–50 % in biomass concentration was observed for T. theli and Chromulina frieburgensis when cultivated in ASW compared to ENSW. A slower growth response was seen for these strains in ASW medium (Fig. 2). Motile microalgal species such as Pavlova sp., T. gracilis and Isochrysis sp. showed a reduced growth and biomass concentration when cultivated in ASW compared to ENSW medium (Fig. 3). The specific growth rate (μ day−1), which is the increase in cell density per unit time, was nearly twofold higher in ENSW medium compared to ASW medium for all the marine strains. The differential response of marine microalgal strains to growth media is manifested in their growth profile in ENSW and ASW media (Fig. 2). Except for Nannochloropsis sp. and Chlorella sp., growth of marine microalgal strains was slower in ASW with an extended lag phase of about 6 days compared to 3 days in ENSW medium. However, cells reached stationary phase earlier (12 days) in ENSW compared to ASW where the growth plateaued out at 15–18th day (Fig. 1). A similar increase in biomass concentration of marine strains when cultivated in ENSW was observed by Berges et al. (2001) who attributed the effect to the enrichment of natural sea water with macronutrients, trace metals like molybdenum, nickel, and natural organic compounds. Higher growth rates of certain strains like Nannochloropsis and Chlorella, in both natural and artificial media, indicate to their suitability for commercial cultivation.
Lipid content and productivity of microalgae
Eukaryotic algae predominantly contain saturated or mono-unsaturated fatty acids, with triglycerides as common storage lipids. The fatty acid composition show a wide variation in microalgal strains which have been reported to be taxonomic class specific and could be used as a chemotaxonomic marker (Sahu et al. 2013). The lipid content and lipid productivity of the fresh water microalgae under study is presented in Table 2. Among the freshwater chlorophyceae (green algae) strains, Chlorella sp. and Chlorococcum sp. showed significantly high lipid content of about 25 % w/w followed by Scenedesmus dimorphus with a content of 16.57 % w/w.Spirulina platensis showed a lower lipid content of about 5 %. The average lipid productivity of fresh water chlorophyceae strains was in the range of 11–13 mgL−1d−1 (Table 2).
The lipid content and productivity of the marine microalgae under study is presented in Table 4. Pavlova sp. (Haptophyceae); Nannochloropsis sp. (Eumastigophyceae) and Chlorella sp. (Chlorophyceae) showed the highest lipid content which varied from about 26 % w/w in ENSW to about 30 % w/w in ASW medium. The lipid productivity of Nannochloropsis was high at >15 mg L−1d−1 in both the media while Chlorella sp. showed a higher lipid content of 25 % w/w; and lipid productivity of 14 mg L−1d−1 in ASW medium.
In ASW medium, T. theli showed an enhanced lipid content of 22.85 % w/w and lipid productivity of 10.95 mg L−1d−1. The lipid productivity of all the other strains also showed an enhancement in ASW medium but remained <10 mg L−1d−1. The reduced lipid productivity in these strains when cultivated in ENSW medium may be attributed to enhanced growth (Table 3), as the natural sea water enriched with micro nutrients alleviate nutritional stress required for lipid accumulation. It has been reported widely that availability of nutrients plays an important role in lipid accumulation by microalgae (Harwood and Jones 1989); where nutrient sufficient conditions promote the growth and nutrient deficient conditions, specifically nitrogen limitation and other abiotic stress, induce lipid accumulation. Under these stressed or nutrient deficient conditions microalgae channelize their metabolism towards accumulation of lipid as preliminary storage material (Guschina and Harwood 2009). However growth and lipid accumulation under a cultivation condition is species specific. ASW is an effective medium for studying the effect of mineral nutrition towards lipid/PUFA accumulation in microalgae since media composition can be precisely controlled unlike in natural sea water medium.
Fatty acid composition of microalgae
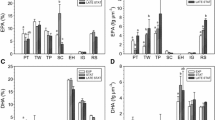
The fatty acid composition of fresh water and marine microalgae is represented in Table 5. PUFA productivities for fresh water and marine strains are represented in Tables 2 and 4 respectively. The fatty acid composition was unique with respect to PUFA distribution. Alpha linolenic acid (ALA, C-18:3, n-3, Δ9,12,15) was the major PUFA in freshwater chlorophyceae strains Scenedesmus sp., Chlorella sp. and Chlorococcum sp.; and gamma linolenic acid (GLA, C-18:3, n-6, Δ6,9,12) was the dominant PUFA in fresh water cyanophycean alga Spirulina platensis. Eicosapentaenoic acid (EPA, C-20:5, n-3, Δ5,8,11,14,17) was the major PUFA in marine microalgae except Isochrysis sp. where docosahexaenoic acid (DHA, C- 22:6, n-3, Δ4,7,10,13,16,19) was the predominant PUFA.
Among the fresh water chlorophyceae strains, the ALA content contributed about 30 % and 28 % w/w of the total FAME in Chlorococcum sp. and Scenedesmus dimorphus respectively with a productivity of 3.9 mgL−1 day−1 for the former and 3.35 mgL−1 day−1 for the later. The Chlorella sp. showed an ALA content and productivity of 11.39 % w/w of total FAME and 1.36 mgL−1 day−1 respectively. The results obtained in the present study were similar to Guedes et al. (2011) who reported high ALA contents in chlorophyceae species. Among the saturated fatty acids, palmitic acid (C-16:0) was predominant component of FAME of all the algal strains with Chlorella sp., showing over 50 % w/w. A lower content and productivity of ALA combined with very high levels of palmitic acid render Chlorella sp. unsuitable for nutritional applications. The other major n-6 PUFA observed in fresh water chlorophyceae strains and Spirulina was linoleic acid (LA, C-18:2) contributing between 8 and 20 % of total FAME. The gamma linolenic acid (GLA), an omega-6 fatty acid with wide nutritional and pharmaceutical applications (Deng and Chow 2010), was unique to Spirulina platensis which showed a high content (24 % w/w of total FAME) of this fatty acid with a productivity of 0.73 mgL−1 day−1.
The major n-3 PUFA of total FAME of marine strains was EPA with Pavlova sp. and Chlorella sp. showing a higher content at 13.45 % and 12.23 % respectively and Tetraselmis gracilis showing the least at 2.96 %. In addition to EPA, another important n-3 long chain PUFA DHA was detected only in three marine algae, Isochrysis sp. (8.2 % of FAME), Nannochloropsis (0.5 % of FAME) and Chlorella sp. (0.5 % of FAME). Linoleic acid (LA, C-18:2) was the only n-6 PUFA observed in marine algae. The EPA productivity of marine microalgae is presented in Table 4. As observed with lipid content and productivity, the strains showed an enhanced EPA productivity in ASW medium. The Nannochloropsis sp., Chlorella sp. and T.theli with higher lipid content and productivity, showed EPA productivity of 1.34, 2.12 and 1.41 mgL−1 day−1 respectively.
Therefore, based on the growth, lipid content, lipid and EPA productivity, the marine microalgal strains of Nannochloropsis sp. and Chlorella sp. could be considered as the candidate organisms for PUFA (EPA) production as they accumulate PUFA without compromising the growth. Also, a differential response to ENSW and ASW medium in strains like T. theli could be utilised for two stage cultivation system for PUFA (EPA) production. The first stage would involve biomass production in nutrient sufficient natural sea water (ENSW) medium followed by induction of lipid accumulation in nutrient deficient ASW medium in second stage. The results obtained in the present study are also encouraging for the potential application of selected fresh water microalgal strains e.g., Scenedesmus dimorphus and Chlorococcum sp. as source of PUFA (mainly ALA) compared to some earlier bioprospection studies such as Guedes et al. (2011) and Hempel et al. (2012).
The fatty acid composition indicated very high therapeutic value for these microalgal lipids in terms of n-6 to n-3 ratio. The n-6 to n-3 ratio for microalgae were very low in the range between 0.75 and 0.1 indicating that omega three fatty acids (EPA and DHA in marine strains and ALA in freshwater strains) are 2 to 10 fold higher than omega six fatty acid (LA) except in Spirulina where the n-6 to n-3 ratio is very high owing to the presence of GLA (n-6) as dominant PUFA (Table 5). The ratio of n-6 to n-3 fatty acids is very important in any food, since both of these fatty acids compete for the same enzyme to synthesize prostaglandins, a pro-inflammatory marker. It was observed that a lower ratio of n-6 to n-3 fatty acids is more desirable in reducing the risk of many of the chronic diseases such as cardiovascular disease, cancer, inflammatory and autoimmune diseases (Simopoulos 2002, 2008). The study also validates the earlier reports suggesting Spirulina, as a major source of GLA, a nutritionally important n-6 PUFA.
The presence of EPA and DHA in marine microalgae is also reflected in higher degree of un-saturation (DU) of their lipids (4.00 to 4.96), strongly supporting their candidature as source of dietary PUFAs (Table 5). The presence of long chain polyenoic PUFAs in marine microalgae could be an adaptive response to colder marine habitats which induces fatty acid desaturation required for membrane fluidity (Thompson et al. 1992). Several authors have observed accumulation of PUFA in psychro-tolerant strains and reported increase in PUFA production under cold environments (Teoh et al. 2004; Fogliano et al. 2010). Thus screening for psychro-tolerant strains would be beneficial for industrial production of PUFAs.
The fatty acid composition of few microalgal species such as Nannochloropsis sp., Porphyridium cruentum, Isochrysis galbana and Parietochloris incise under autotrophic growth conditions and Phaeodactylum tricornutum, Crypthecodinium cohnii, Schyzochytrium sp. under heterotrophic growth condition is well documented (Cohen and Ratledge 2005). However a detailed description on the fatty acid composition and productivity of indigenous strains mainly with respect to dietary PUFAs are rare except a few, such as Guedes et al. (2011). The present study is first of its kind in terms of description of fatty acid composition and evaluation of the PUFA productivity of certain indigenous marine and freshwater microalgal strains.
The n-3 PUFA rich microalgae evaluated in the present study could be used directly as n-3 PUFA fortifying agents in foods such as cookies, pasta, bread etc. Fortification of foods with n-3 PUFA rich microalgal biomass would enrich their nutritional properties by balancing/reducing the n-6 to n-3 PUFA ratio. The direct use of PUFA enriched microalgal biomass as functional foods would improve the economics of food processing industry as critical downstream processing can be avoided. Apart from the PUFA, the microalgal biomass would also contribute other nutritionally important compounds such as carotenoids, chlorophylls, dietary fibre to processed food products. Alternatively, the microalgal biomass can be either used as feedstock for PUFA rich oil production or as feed for poultry (Fraeye et al. 2012) to obtain n-3 PUFA fortified eggs. Thus the microalgae could be important alternative source of PUFA to marine fish oil and identification and evaluation of indigenous PUFA accumulating microalgae therefore assumes significance.
Conclusion
The present study was focussed on evaluation of a few of the indigenous marine and freshwater microalgae as potential source of PUFA under autotrophic mode. Freshwater chlorophycean microalgae Chlorococcum sp. and Scenedesmus dimorphus can be the possible candidates for ALA production with an average productivity of 3 mgL−1 day−1. Marine microalgae Nannochloropsis sp. and Chlorella sp. could be the source of EPA with productivity of 1 to 2 mgL−1 day−1. Growth medium composition significantly affected the biomass and PUFA productivity of some marine strains such as Tetraselmis theli. A two stage cultivation system for improving overall lipid or PUFA productivity could be designed for such marine strains, where the biomass obtained with growth promoting medium is incubated in lipid/PUFA enhancing medium. From the present study it can be concluded that microalgae could be a potential vegetarian source of dietary PUFAs.
References
Becker W (2004) Microalgae in human and animal nutrition. In: Richmond A (ed) Microalgae for aquaculture in handbook of microalgal culture: biotechnology and applied phycology. Blackwell Science, Iowa, pp 312–352
Berges JA, Franklin DJ, Harrison PJ (2001) Evolution of an artificial sea water medium: improvements in enriched sea water, artificial water, over the last two decades. J Phycol 37:1138–1145
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Brett M, Muller-Navarra D (1997) The role of highly unsaturated fatty acids in aquatic food web processes. Freshwater Biol 38:483–499
Christie WW (1982) Lipid analysis, 2nd edn. Pergamon press, New York, pp 93–96
Cohen Z, Ratledge C (2005) Single cell oils. AOCS Press, Illinois, Chapter 4
Deng R, Chow TJ (2010) Hypolipidemic, antioxidant, and anti-inflammatory activities of microalgae Spirulina. Cardiovasc Ther 28:33–45
Doughman SD, Krupanidhi S, Sanjeevi CB (2007) Omega-3 fatty acids for nutrition and medicine: considering microalgae oil as a vegetarian source of EPA and DHA. Curr Diabetes Rev 3:198–203
FAO Fisheries and Aquaculture Technical Paper No. 569 (2011) Review of the state of world marine fishery resources. FAO, Rome
Floreto EAT, Teshima S (1998) The fatty acid composition of seaweeds exposed to different levels of light intensity and salinity. Bot Mar 41:467–481
Fogliano V, Andreoli C, Martello A, Caiazzo M, Lobosco O, Formisano F, Carlino PA, Meca G, Graziani G, Rigano VM, Vona V, Carfagna S, Rigano C (2010) Functional ingredients produced by culture of Koliella antarctica. Aquacult 299:115–120
Fraeye I, Bruneel C, Lemahieu C, Buyse J, Muylaert K, Foubert I (2012) Dietary enrichment of eggs with omega-3 fatty acids: a review. Food Res Int 48:961–969
Guedes AC, Amaro HM, Barbosa CR, Pereira RD, Malcata FX (2011) Fatty acid composition of several wild microalgae and cyanobacteria, with a focus on eicosapentaenoic, docosahexaenoic and α-linolenic acids for eventual dietary uses. Food Res Int 44:2721–2729
Guschina IA, Harwood JL (2009) Algal lipids and effect of the environment on their biochemistry. In: Arts MT, Brett MT, Kainz M (eds) Lipids in aquatic ecosystems. Springer, New York, pp 1–24
Harwood JL, Jones AL (1989) Lipid metabolism in algae. Adv Bot Res 16:1–53
Hempel N, Petrick I, Behrendt F (2012) Biomass productivity and productivity of fatty acids and amino acids of microalgae strains as key characteristics of suitability for biodiesel production. J Appl Phycol 24:1407–1418
Jorquera O, Kiperstok A, Sales EA, Embirucu M, Ghirardi ML (2010) Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour Technol 101:1406–1413
Kanz T, Bold HC (1969) Morphological and taxonomical investigation of Nostoc and Anabaena in culture. In: physiological studies. University of Texas, Texas, Austin; publication No. 6924
Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA (2006) Diet and lifestyle recommendations revision 2006 — a scientific statement from the American heart association nutrition committee. Circulation 114:82–96
Melanson SF, Lewandrowski EL, Lewandrowski KB (2005) Measurement of organochlorines in commercial over-the-counter fish oil preparations. Arch Pathol Lab Med 129:74–77
Rittmann EB (2008) Opportunities for renewable bioenergy using microorganisms. Biotechnol Bioeng 100:203–212
Sahu A, Pancha I, Jain D, Paliwal C, Ghosh T, Shailesh P, Bhattacharya S, Mishra S (2013) Fatty acids as biomarkers of algae. Phytochem 89:53–58
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 233:674–688
Storelli MM, Storelli A, Marcotrigiano GO (2004) Polychlorinated biphenyls, hexa chloro benzene, hexachloro cyclohexane isomers, and pesticide organo chlorine residues in cod-liver oil dietary supplements. J Food Protect 67:1787–1791
Teoh M, Chu W, Merchant H, Phang S (2004) Influence of culture temperature on the growth, biochemical compositions, and fatty acid profiles of six Antarctic microalgae. J Appl Phycol 16:421–430
Thompson PAM, Guo M, Harrison PJ (1992) Effects of variation in temperature on the fatty acid composition of eight species of marine phytoplankton. J Phycol 28:488–497
UTEX - The Culture collection of Algae. Artificial Sea Water Medium. http://web.biosci.utexas.edu/utex/mediaDetail.aspx?mediaID=23
Vidyashankar S, Deviprasad K, Chauhan VS, Ravishankar GA, Sarada R (2013) Selection and evaluation of CO2 tolerant indigenous microalga Scenedesmus dimorphus for unsaturated fatty acid rich lipid production under different culture conditions. Bioresour Technol 144:28–37
Wen ZY, Chen F (2003) Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol Adv 21:273–294
Zarrouk C (1966) Contribution à l’étuded’unecyanophycée. Influence de divers’ facteurs physiques etchimiquessur la croissance et la photosynthèse de Spirulina maxima. Ph.D. Thesis, Université de Paris, Paris
Acknowledgments
Authors thank Dr. N. Bhaskar, Meat & Marine Sciences department, CSIR-CFTRI and Dr. Sugumar Gopalrajan, FCRI, Tuticorin for the help in procurement of marine microalgae strains. VS acknowledges the UGC, Govt. of India for the award of Senior Research Fellowship. Authors thank Director, CSIR-CFTRI for constant encouragement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Research highlights
• 12 microalgae strains were evaluated as source of dietary PUFAs in autotrophic growth.
• Microalgal lipids had higher ratio of n-3 PUFAs as reflected by lower n-6 to n-3 ratio (0.1 to 0.75).
• ALA was predominant n-3 PUFA in freshwater while EPA was major in marine strains.
• Degree of unsaturation of PUFAs was higher in marine strains.
Rights and permissions
About this article
Cite this article
Vidyashankar, S., Sireesha, E., Chauhan, V.S. et al. Evaluation of microalgae as vegetarian source of dietary polyunsaturated fatty acids under autotrophic growth conditions. J Food Sci Technol 52, 7070–7080 (2015). https://doi.org/10.1007/s13197-015-1781-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1781-8