Abstract
We report the presence of ACC deaminase in Methylobacterium fujisawaense and its lowering of ethylene levels and promotion of root elongation in canola seedlings under gnotobiotic conditions. To test a part of the previous model proposed for ACC deaminase producing bacteria with Methylobacterium, ACC levels and various enzyme activities were monitored in canola. Lower amounts of ACC were present in the tissues of seeds treated with M. fujisawaense strains than in control seeds treated with MgSO4. Though the increased activities of ACC synthase in the tissue extracts of the treated seedlings might be due to bacterial indole-3-acetic acid, the amount of ACC was reduced due to bacterial ACC deaminase activity. The activities of ACC oxidase, the enzyme catalyzing conversion of ACC to ethylene remained lower in M. fujisawaense treated seedlings. This consequently lowered the ethylene in plants and prevented ethylene inhibition of root elongation. Our results collectively suggest that Methylobacterium commonly found in soils, as well as on the surfaces of leaves, seeds, and in the rhizosphere of a wide variety of plants could be better exploited to promote plant growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The gaseous hydrocarbon ethylene, most popularly associated with ripening, plays a major role regulating seed germination, seedling growth, leaf and petal abscission, organ senescence, stress, and pathogen responses throughout the entire life of the plant (Schaller and Kieber 2002). Interestingly, ethylene can promote and inhibit growth depending on the cell type and plant species (Lehman et al. 1996). Low levels of ethylene appear to enhance root extension; higher levels of ethylene, produced by fast growing roots, can lead to inhibition of root elongation (Mattoo and Suttle 1991; Ma et al. 1998). In general, shoot and root elongation is normally inhibited by ethylene (Abeles et al. 1992) and high levels of ethylene are known to cause proliferation of small lateral roots (Mayak et al. 1999). Ethylene promotes rooting only at a narrow range of concentrations and the role of ethylene in rhizogenesis is still not been clearly understood.
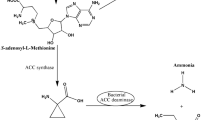
Glick et al. (1998) suggested that plant growth promoting rhizobacteria (PGPR) can stimulate plant growth by lowering the ethylene levels. In ethylene synthesis, methionine is converted to S-adenosyl methionine (SAM), 1-aminocyclopropane-1-carboxylate (ACC), and ethylene in three consecutive reactions catalyzed by the enzymes SAM-synthetase, ACC-synthase (ACS), and ACC-oxidase (ACO), respectively (Bleecker and Kende 2000). It was postulated that much of the ACC produced by this latter reaction may be exuded from seeds or plant roots along with other small molecules normally present in seed or root exudate (Bayliss et al. 1997). The ACC in the exudate may be taken up by bacteria and subsequently be hydrolyzed by the enzyme, ACC deaminase to ammonia and α-ketobutyrate. In order to maintain the equilibrium between internal and external ACC levels, more ACC is exuded by the plant and drawn away from the ethylene synthesis pathway (Glick et al. 1998; Penrose and Glick 2001); this mechanism effectively reduces the amount of ethylene evolved by the plant. Thus, the ability to promote root elongation by PGPR is a direct consequence of the presence of ACC deaminase and more and more experimental evidence indicates that ACC deaminase is one of the key mechanisms by which rhizobacteria promote the growth of plants, especially root elongation.
The presence of ACC deaminase has been studied in various plant growth promoting bacteria like Enterobacter cloacae (Penrose and Glick 2001), Rhizobium (Ma et al. 2003), and Pseudomonas, Variovorax, Alcaligenes, Bacillus (Belimov et al. 2001), etc. The ACC deaminase containing bacteria may be found on leaves and flowers as well as on seeds and roots, and the model based on the interaction of bacteria with seeds and roots is likely applicable to the entire plant (Glick 2004). Bacteria of the genus Methylobacterium (PPFMs, pink-pigmented facultative methylotrophic bacteria) are strict aerobic, Gram-negative rods, able to grow on C1 compounds (Green 1992; Trotsenko et al. 2001). Several aspects of plant growth promotion by Methylobacterium have also been investigated, such as the production of urease enzyme (Holland and Polacco 1992), stimulation of seed germination and promotion of root growth and morphology (Holland 1997; Freyermuth et al. 1996), and induced systemic resistance (Madhaiyan et al. 2004).
In this study, we surveyed Methylobacterium strains for ACC deaminase activity and the various steps involved in lowering the ethylene levels as proposed in the model for plant growth promotion by ACC deaminase containing PGPR. Three of the five Methylobacterium strains were found to possess ACC deaminase activity and were further considered for growth pouch assays and for testing plant growth promoting traits in canola. Though previous studies present a detailed scenario of the steps involved in the process of lowering ethylene by bacteria containing ACC deaminase, the role of indole-3-acetic acid (IAA) of microbial origin in reducing the ethylene levels requires more study. So we also made an attempt to know the role of IAA produced by Methylobacterium inoculation on the enzyme activities for ethylene production.
Materials and methods
Bacterial strains and growth conditions
The Methylobacterium strains used are listed in Table 1. The isolates from rice (stem, leaf, and rhizosphere soil) were obtained previously by plating the aliquots onto ammonium mineral salts (AMS) minimal medium, with 0.5% methanol as the sole carbon source, as described by Holland and Polacco (1992). The 16S rDNA gene sequence analysis of the isolated bacterial strains placed them under Methylobacterium. The Methylobacterium fujisawaense type strain KACC10744 (DSM 5686T) was obtained from the Korean Agricultural Culture Collection (KACC), Suwon, Republic of Korea. The ACC deaminase knockout mutant E. cloacae UW4/AcdS − was kindly provided by Bernard R. Glick, Department of Biology, University of Waterloo, Ontario, Canada and was cultured in tryptic soy broth (Difco, Laboratories, Detroit, MI, USA) containing 15 μg tetracycline ml−1 at 30°C.
ACC deaminase assay
The isolates were checked for their ability to utilize ACC as a N source by assessing their growth in DF minimal salts medium (Dworkin and Foster 1958) supplemented with 3 mM ACC. ACC deaminase activity of the isolates was measured using a spectrophotometer that measured the absorbance at 540 nm, the α-ketobutyrate produced from the enzymatic cleavage of ACC as described previously by Shah et al. (1998), and a modified procedure of Honma and Shimomura (1978). The α-ketobutyrate produced was estimated by comparing the absorbance at 540 nm of a sample to a standard curve of α-ketobutyrate (Sigma-Aldrich Co., St Louis, MO, USA) ranging between 0.1 and 1.0 mmol. A stock solution of α-ketobutyrate was prepared in 0.1 M Tris–HCl (pH 8.5) and stored at 4°C. To measure specific activity of the cultures protein estimation was carried out according to Lowry method (Lowry et al. 1951).
Plant growth promoting characteristics of Methylobacterium strains
The production of indoles in the culture supernatants of the bacteria was measured spectrophotometrically at 530 nm after incubation of the bacteria for 5 days on liquid minimal medium (composition in g l−1: 2 g KH2PO4, 2 g (NH4)2SO4, 0.025 g MgSO4·7H2O, and 0.002 g FeSO4·7H2O, pH 7.2) supplemented with 0.5% methanol (v/v) and with l-tryptophan (100 mg l−1). The reaction consisted 2 ml of cell free suspension to which 100 μl of 10 mM orthophosphoric acid added and 4 ml of Salkowski’s reagent (1 ml of 0.5 M FeCl3 in 50 ml of 35% HClO4) followed by incubation at room temperature for 25 min. The assay was calibrated by generating a standard curve for samples containing IAA. The culture extracts for analyzing cytokinins were prepared according to Koenig et al. (2002) and the immunoassays were performed as described later to determine the amounts of the two cytokinins, isopentenyladenosine (iPA) and trans-zeatin riboside (t-ZR), present in the culture supernatants.
Ethylene production by seedlings treated with ACC deaminase producing Methylobacterium strains
Surface sterilized canola (Brassica campestris) seeds (Hungnong seeds, Seminis Korea Inc., Republic of Korea) were imbibed in 0.03 M MgSO4 (control) or a bacterial suspension (A600 nm=0.15) for 2 h. Thirty seeds were placed in 120-ml vials on sterile filter paper before 2 ml of water was added. The ethylene production was measured on day 7, after removing the excess water. The vials were capped with a rubber septum and following 4-h incubation, 1 ml of headspace was sampled from each vial and the ethylene content measured in a gas chromatograph (GC) (DS 6200, Donam Instruments Inc., Republic of Korea) packed with Poropak-Q column at 70°C, equipped with a flame ionization detector. The carrier gas was N2 at the flow rate of 60 ml min−1 and the combustion gas was H2 at the flow rate of 50 ml min−1 with the combustion-supporting gas air at the flow rate of 500 ml min−1. The capacity of the column for ethylene trapping was checked in a model experiment in which air containing 0.2 nmol ethylene l−1 was passed through the column for 60 min; under these conditions, 100% of the ethylene was trapped.
Gnotobiotic root elongation assay
Gnotobiotic root elongation assay was performed with canola to study effects of seed treatment with ACC deaminase producing methylobacteria. The bacterial strains were grown aerobically to late log phase in liquid AMS medium and then transferred to DF salts medium containing ACC (3.0 mM) as the sole source of nitrogen. The culture conditions and the procedure for gnotobiotic growth pouch assay followed Penrose and Glick (2003). Surface sterilized canola seeds were incubated for 1 h at room temperature with the appropriate treatment: sterile 0.03 M MgSO4 (used as a negative control), 10−4 M l-α-(2-aminoethoxyvinyl) glycine hydrochloride (AVG) (Sigma, USA) dissolved in 0.03 M MgSO4 (used as a positive control), or bacterial suspensions in sterile 0.03 M MgSO4 (absorbance of 0.15 at 600 nm). E. cloacae UW4 AcdS − is a mutant strain that lacks ACC deaminase activity and was used as a negative control in this study. The root lengths of the canola seedlings were measured following 5 days of growth at 20±1°C beginning with a cycle of 12 h of dark followed by 12 h of light (18 μmol m−2 s−2). The seedlings were assayed for ACC levels, in vitro ACO, ACS activity, and for immunoassays estimating IAA and cytokinins.
Measurement of ACC level in plant tissue
Plant tissues frozen in liquid nitrogen, stored at −20°C were used. One gram of frozen tissue was extracted in 5 ml 80% methanol containing butylated hydroxytoluene as antioxidant (BHT, 2 mg l−1) and incubated at room temperature for 45 min. Samples were centrifuged at 2,000g at 20°C for 15 min, the pellet resuspended in 4 ml methanol and centrifuged. The combined supernatants were evaporated to dryness under vacuum. Residues were resuspended in 2 ml distilled water and ACC levels were determined by the method of Wachter et al. (1999) using the protocol of Lizada and Yang (1979). In brief, the upper aqueous phase (0.5 ml) obtained by extracting with dichloromethane was mixed with 0.1 ml HgCl2 (80 mM) in test tubes and sealed with rubber septa. 0.2 ml NaOCl solution (40 parts NaOH, 80 parts 12.5% NaOCl solution, 30 parts distilled water) was injected into the tubes, shaken, and incubated for 8 min. One milliliter of the gaseous portion was removed and assayed for ethylene with a GC as described above. The efficiency of ACC oxidation, on average 60%, was estimated by analyzing replicate samples containing internal standards of ACC.
In vitro ACO and ACS activity
The protein extracts for measuring the in vitro ACO activity were prepared according to Petruzzelli et al. (2000). The frozen tissues were pulverized in liquid nitrogen and homogenized in 2 ml g−1 of extraction buffer consisting of 100 mM Tris–HCl (pH 7.2), 10% (w/v) glycerol, and 30 mM sodium ascorbate. The homogenate was centrifuged at 28,000g for 15 min at 4°C. The supernatant was used for the in vitro ACO assays (Malerba et al. 1995). The enzyme activity was assayed at 30°C for 15 min in 10-ml screwcap tubes fitted with a Teflon-coated septum containing 1.5 ml of supernatant, 50 μM FeSO4, 1 mM ACC, and 5% (v/v) CO2. At the end of this time period, the quantity of ethylene released into the headspace was determined by GC.
Enzyme extracts for the ACS activity were obtained by homogenizing a 1 g sample of pulverized tissues in 4 ml of 100 mM Na phosphate (pH 9.0) containing 5 μM pyridoxal phosphate (PLP), 4 mM 2-mercaptoethanol (2-ME), 1 mM EDTA, and 10% glycerol in the presence of 1 g polyvinylpolypyrrolidone (PVPP). The precipitate obtained between 35 and 75% saturation after ammonium-sulfate fractionation was resuspended in 2.5 ml of a solution containing 100 mM Na phosphate (pH 7.8), 5 μM PLP, 0.5 mM 2-ME, and 10% glycerol. The preparation of the enzymes was carried out at 4°C (Satoh et al. 1997). Protein content was determined according to the method of Lowry et al. (1951) with BSA as a standard. ACS activity was assayed by incubating a 100-μl enzyme solution (1.29-mg protein) with 100 mM 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES)–KOH (pH 8.5), 5 μM PLP, 100 μM S-adenosyl-l-methionine, and test chemicals at given concentrations in a total volume of 400 μl. After incubation for 15 min at 30°C, the amount of ACC produced was determined as described above.
Immunoanalysis of IAA and cytokinins in plant tissues
The IAA and cytokinins concentrations in plant extracts were determined using immunoassay by enzyme linked immunosorbant assay (ELISA). Extracts were prepared by homogenizing the seedlings (1 g) with TBS buffer (per liter, 3.03 g Trizma base, 5.84 g NaCl, 0.2 g MgCl2·6H2O, 0.2 g sodium azide, pH 7.2) in a ratio of 1:4 (w/v) in sterile 50 ml centrifuged tubes. Supernatants of the extracts (collected by centrifuging the extract at 3,885g for 5 min, and recentrifuging at 3,885g for 3 min) were used for immunoassays. All cytokinins and IAA were purchased from Sigma, and ELISA test kits were from AGDIA (AGDIA Inc., Indiana, USA). ELISA tests were performed according to kit instructions. Stock solutions of IAA and cytokinins (10 mM) were prepared in absolute methanol and stored at 4°C. Dilutions were made with sterile AMS media as diluent to build the standard curves. Samples were diluted as needed to obtain accurate estimates within the ranges of standard concentrations. The absorbance was read at 405 nm using an ELISA plate reader (BIO-RAD Model 550, Japan).
Statistical analysis
All experiments were repeated twice with three replicates each and the extractions were conducted in triplicate. Standard curves were generated by regression analysis (Excel, Microsoft) for every enzyme assay. The data were subjected to analysis of variance and testing of means by Duncan’s Multiple Range Test (DMRT) at P≤0.05 using SAS, Version 8.2 (SAS 2001).
Results
Identification and plant growth promoting characteristics of Methylobacterium strains
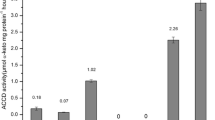
The characteristics and the results of taxonomic identification of the Methylobacterium strains are given in Table 1. The strains were identified up to the species level based on 16S rRNA gene sequence similarity (99%) and it was established that the studied bacteria belonged to M. fujisawaense and M. extorquens. The Methylobacterium strains varied in their ability to utilize ACC and significant differences could be observed in the ACC deaminase activity of cell free extracts. No ACC deaminase activity was detected in cell free extracts of the M. extorquens strains CBMB120 and CBMB130. The activities were higher in the extracts of M. fujisawaense strains, CBMB20 and CBMB110, than in the type strain M. fujisawaense producing 94.48, 24.74, and 5.23 nmol α-ketobutyrate mg−1 of protein h−1, respectively (Table 2). Other properties of Methylobacterium, which could be considered characteristics that are of concern in plant PGPR interactions, were studied. The quantitative analysis with the Salkowski reagent of the culture liquids of methylobacteria grown in defined medium with l-tryptophan and incubated for 5 days produced significantly different amounts of IAA (P=0.05). The five Methylobacterium strains accumulated the phytohormone in amounts ranging from 2.33 to 9.04 μg ml−1 in the presence of l-tryptophan (Table 2). When l-tryptophan was not supplemented to the culturing medium, the production of IAA was in the range of 0.51–1.91 μg ml−1. Immunoassays using ELISA kits were performed to determine whether the Methylobacterium produced cytokinins. The cytokinins t-ZR and iPA were present at detectable and replicable levels in all of the cultures tested. t-ZR was present in smaller quantities than iPA (Table 2). The levels of cytokinins recovered from the cultures were variable and the strains CBMB20 and CBMB110 produced higher amounts of cytokinins compared to the other strains. Since the strains CBMB120 and CBMB130 showed no growth on ACC containing media, we could not proceed with these strains and selected only CBMB20 and CBMB110 for the gnotobiotic assays.
Gnotobiotic root elongation assay
The germination percentage of the Methylobacterium treated seeds was comparatively greater when compared to control. CBMB20 recorded the highest percentage of germination (96.67) followed by CBMB110 (91.67%). But the germination percentage in E. cloacae UW4/AcdS − and AVG treated seeds showed significantly lower values compared to control (Table 3). Following 5 days in gnotobiotic growth pouches, canola roots from seeds treated with Methylobacterium strains CBMB20 and CBMB110 showed significant increases in root length when compared with either the control or the AcdS − knockout mutant treated seeds. The average root length of the M. fujisawaense strains CBMB20, CBMB110 treated plants were 8.50 and 7.67 cm, respectively, when compared with those treated with E. cloacae UW4/AcdS − (4.98 cm) and the untreated seeds, i.e. control (4.76 cm) (Table 3). The roots of the M. fujisawaense strains including reference strain treated canola were, on average, nearly twice as long as either untreated plants or plants treated with the mutant strain. Seeds treated with AVG, an ethylene synthesis inhibitor, produced an increase of 30.88% in root length compared to control.
Monitoring ethylene production
Measurements of ethylene emission by GC detected considerable amounts of ethylene emitted, both from treated and untreated plants from 0.204 and 2.40 nmol g−1 FW h−1. Methylobacterium treated canola seedlings emitted lower levels of ethylene when compared to the untreated seedlings (Fig. 1a). The ethylene emitted from the seedlings treated with a suspension of M. fujisawaense strains CBMB20 and CBMB110 was equivalent to that from seedlings treated with the ethylene inhibitor AVG and that from E. cloacae UW4/AcdS −, a negative ACC mutant, is equal to that of untreated canola seedlings.
Effect of Methylobacterium inoculation on ethylene emissions (a) and ACC accumulations (b) in canola. For ethylene emission measurements, seedlings were grown on vials for 7 days, sealed, and following 4-h incubation, ethylene in the headspace sample was measured on a GC. Each value represents the mean ± SE, n=3. AVG included as a positive control and the UW4/AcdS − as a negative control. T Methylobacterium fujisawaense type strain
Enzyme activities regulating ethylene production
ACC, the immediate precursor of ethylene was quantified in the tissues of 8-day-old canola seedlings and compared between the treated and untreated seeds, showed significant differences (P=0.05). The ACC accumulated in the plant tissues was significantly reduced in treatments with M. fujisawaense strains, CBMB20, CBMB110, and M. fujisawaense type strain with a reduction of 37.38, 61.50, and 13.61% respectively, when compared to control. Seedlings from canola seeds treated with the ethylene inhibitor, AVG, showed the lowest level of ACC, with 62.01% reduction. But those treated with the ACC negative mutant E. cloacae UW4/AcdS − showed equal amounts of ACC as that of control (Fig. 1b).
The activity of ACS, the enzyme catalyzing the conversion of SAM to ACC could be measured on a GC by measuring the amount of ACC. The in vitro ACS activity measured from the extracts remained significantly higher in the Methylobacterium treated seedlings (Fig. 2a). The enzyme activity of ACO, which converts ACC into ethylene could be measured in vivo, i.e. ethylene production of intact tissues incubated with a saturating ACC concentration, and in vitro, i.e. ethylene production in assays with protein extracts (Fluhr and Mattoo 1996). The in vitro ACO activity measured in canola seedlings treated with Methylobacterium strains decreased when compared with the untreated seedlings (Fig. 2b). In contrast the seedlings treated with E. cloacae UW4/AcdS − had higher amounts of ACO, since this bacterial strain lacked the ACC deaminase gene.
Effects of Methylobacterium inoculation on the in vitro activity of ACC synthase (a) and ACC oxidase (b) obtained from canola seedlings. ACC synthase activity was determined from the amount of ACC formed with 100 μM S-adenosyl-l-methionine as a substrate. ACC oxidase was determined by supplying 1 mM ACC as a substrate. Each value represents the mean ± SE, n=3. T M. fujisawaense type strain
IAA and cytokinins in plant tissues
IAA and cytokinins in plant extracts were detected using ELISA test immunoassays. The amount of IAA and cytokinins increased with bacterial treatments compared to AVG treatment which inhibits ethylene production. The IAA concentrations varied from 32.21 to 83.36 pmol g−1 FW with significant differences between the treatments, and in Methylobacterium treated plants it ranged from 50.15 to 83.18 pmol g−1 FW (Fig. 3a). The total cytokinins (iPA + t-ZR) in bacterial treatments varied from 1.18 to 2.50 nmol g−1 FW with variations within them. Maximum amount of cytokinins could be seen in the extracts of plants treated with M. fujisawaense strains CBMB20 and CBMB110 (Fig. 3b).
IAA (a) and total cytokinins (b) (iPA and t-ZR) levels in canola plants treated with Methylobacterium strains. Seedlings were grown for 10 days in 12 h light/12 h dark at 20±1°C in a growth pouch, after which the whole seedlings were collected. Each value represents the mean ± SE, n=3. T M. fujisawaense type strain
Discussion
The Methylobacterium strains used in this study produced considerable amounts of IAA and cytokinins. The phytohormones produced by Methylobacterium stimulate seed germination and plant development (Madhaiyan et al. 2005a, b) and the cytokinins produced by them are physiologically meaningful to the host plants or alternatively, they induce greater cytokinins in the host (Butler et al. 2000). But out of the five strains used, only three strains utilized ACC revealing the presence of ACC deaminase and also possessed considerable ACC deaminase activity. Similar results were also obtained in Rhizobium where only five of the 13 strains surveyed for ACC deaminase activity were positive (Ma et al. 2003).
Under the gnotobiotic growth pouch assay, the Methylobacterium treated seedlings had longer roots and also emitted lower ethylene when compared to control or the seedlings treated with E. cloacae UW4/AcdS −. The inoculation of three ACC deaminase containing Bacillus strains stimulated root elongation in canola seedlings under gnotobiotic conditions (Ghosh et al. 2003) whereas significant reduction could be noted in the length of canola roots between seeds treated with the wild type bacterium and those treated with E. cloacae UW4/AcdS − mutant (Li et al. 2000). When the PGPR contains the enzyme ACC deaminase, the bacterial cells act as a sink for ACC, the immediate biosynthetic precursor of ethylene thereby lowering plant ethylene levels and decreasing the negative effects of various environmental stresses (Stearns et al. 2005). Treatment with AVG also resulted in increased root lengths and the promotive effects may result from a reduction in ethylene concentration or inhibition of ethylene action (Khalafalla and Hattori 2000).
Ethylene action and biosynthesis is regulated by different hormones with auxin stimulating its production by promoting the de novo synthesis of ACC synthase and ACC and cytokinins and brassinosteroid (BR) increasing auxin-induced ethylene production (Yi et al. 1999; Swarup et al. 2002). The IAA and cytokinins concentrations in the extracts of canola increased with Methylobacterium inoculations in this study. An increase of ACS activity was observed in the treated plants which might be a direct consequence of increased IAA and cytokinins. This is in accordance to the model of Glick et al. (1998) suggesting that microbial binding to the surface of seed or root exudates taken up by the plant together with endogenous plant IAA can induce ACC synthase which converts SAM to ACC.
But ACC formed from methionine, by the action of ACS remained lower in the seedlings treated with ACC deaminase producing Methylobacterium strains. It was postulated that much of the ACC produced by the action of ACS may be exuded from the seeds or plants (Bayliss et al. 1997) and might be taken up by the bacteria and subsequently hydrolyzed by enzyme ACC deaminase to ammonia and α-ketobutyrate. Supportingly, in our study the ACC present in the plants treated with E. cloacae UW4/AcdS −, a negative mutant of ACC deaminase remained higher than in those treated with ACC deaminase producing Methylobacterium. With reference to the Methylobacterium strains used the M. fujisawaense that recorded lower ACC deaminase activity than the other two strains, resulted in higher ethylene emissions and the ACC in the plant tissues also remained higher. Also the ACC in the AVG treated plants remained lower, since the conversion of methionine to ACC is strongly inhibited by AVG, an inhibitor of pyridoxal phosphate mediated reactions (Adams and Yang 1979). The ACO activity remained lower in the plants treated with Methylobacterium strains, when compared to the untreated seeds or treatment with AVG and E. cloacae UW4/AcdS −. Since ACC is the substrate for ACO, reductions in the substrate level would lead to reduced activities of ACO subsequently reducing the amount of plant ethylene, the product of ACC oxidation.
This is the first report on the existence and prevalence of ACC deaminase in Methylobacterium spp. providing evidence for promotion of root elongation by Methylobacterium as a consequence of reducing ethylene levels in the plant. The results obtained here suggests that plant growth promotion by the ACC utilizing Methylobacterium is due to the reduction of plant ethylene through the utilization of ammonia cleaved from ACC by its ACC deaminase according to the hypothesis made by Glick et al. (1998). From our results, we proposed a modification of the model of Glick et al. (1998) for how Methylobacterium promotes plant growth (Fig. 4). Inoculation of canola with Methylobacterium strains increases the IAA and cytokinin concentration of the plants resulting in increased ACS activity. If the PGPR strain contains no ACC deaminase, ACC levels in the seedlings are unaltered and ethylene is still produced which inhibits root elongation. If the PGPR strain contains ACC deaminase, ACC levels in the plant drop reducing ethylene levels and releasing root growth from the inhibitory effects of this hormone. Also the ubiquitous nature and the ability to effectively utilize methanol, the metabolic waste product of plants provides additional benefits in the use of Methylobacterium as a plant growth promoting bacteria.
Hypothetical model illustrating the steps involved in lowering of plant ethylene levels and other activities of Methylobacterium in plant growth promotion (modified from Glick et al. 1998). The model is subdivided into two phases: the ethylene biosynthetic pathway in plants and the ACC breakdown in Methylobacterium resulting in reduced ethylene in plants. The arrows indicate a chemical or physical step in the mechanism, the block arrows indicating increases in the concentration of compounds and the symbol ⊥ indicates inhibition of root elongation by ethylene. Methylobacterium results in increased plant hormones and also cleaves ACC, the immediate precursor of ethylene preventing ethylene inhibition of root elongation in canola seedlings. ACC 1-aminocyclopropane-1-carboxylic acid, ACO ACC oxidase, ACS ACC synthase, AdoMet S-Adenosyl-l-methionine, IAA indole-3-acetic acid, iPA isopentenyladenosine, MTA 5′-methylthioadenosine, t-ZR trans-Zeatin riboside
Abbreviations
- ACC:
-
1-aminocyclopropane-1-carboxylate
- ACO:
-
ACC oxidase
- ACS:
-
ACC synthase
- AMS:
-
Ammonium mineral salts
- AVG:
-
l-α-(2-aminoethoxyvinyl) glycine hydrochloride
- CFU:
-
Colony-forming units
- IAA:
-
Indole-3-acetic acid
- iPA:
-
Isopentenyladenosine
- MTA:
-
5′-methylthioadenosine
- PGPR:
-
Plant growth promoting rhizobacteria
- PLP:
-
Pyridoxal phosphate
- PPFMs:
-
Pink-pigmented facultative methylotrophic bacteria
- PVPP:
-
Polyvinylpolypyrrolidone
- SAM:
-
S-adenosyl methionine
- t-ZR:
-
trans-Zeatin riboside
References
Abeles FB, Morga PW, Saltveit ME (1992) Ethylene in plant biology. Academic, San Diego
Adams DO, Yang SF (1979) Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA 76:170–174
Bayliss C, Bent E, Culham DE, MacLellan S, Clarke AJ, Brown GL, Wood JM (1997) Bacterial genetic loci implicated in the Pseudomonas putida GR12-2R3-canola mutualism: identification of an exudate-inducible sugar transporter. Can J Microbiol 43:809–818
Belimov AA, Safronova VI, Sergeyeva TA, Egorova TN, Matveyeva VA, Tsyganov VE, Borisov AY, Tikhonovich IA, Kluge C, Preisfeld A, Dietz KJ, Stepanok VV (2001) Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 47:642–652
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Butler HK, Dadson R, Holland MA (2000) Evidence that trans-zeatin riboside produced by a microbial symbiont is physiologically meaningful to its host plant (abstract available at http://www.abstracts.aspb.org/aspp2000/public/P43/0604.html)
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–601
Fluhr R, Mattoo AK (1996) Ethylene: biosynthesis and perception. Crit Rev Plant Sci 15:479–523
Freyermuth SK, Long RLG, Mathur S (1996) Metabolic aspects of plant interaction with commensal methylotrophs. In: Lidstrom ME, Tabita FR (eds) Microbial growth on C1 compounds. Kluwer, The Netherlands, pp 277–284
Ghosh S, Penterman JN, Little RD, Chavez R, Glick BR (2003) Three newly isolated plant growth-promoting bacilli facilitate the seedling growth of canola, Brassica campestris. Plant Physiol Biochem 41:277–281
Glick BR (2004) Bacterial ACC deaminase and the alleviation of plant stress. Adv Appl Microbiol 56:291–312
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190:63–68
Green PN (1992) The genus Methylobacterium. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds), The prokaryotes, 2nd edn. vol III. Springer, Berlin Heidelberg New York, pp 2342–2349
Holland MA (1997) Methylobacterium and plants. Rec Res Dev Plant Physiol 1:207–213
Holland MA, Polacco JC (1992) Urease-null and hydrogenase-null phenotypes of a phylloplane bacterium reveal altered nickel metabolism in two soybean mutants. Plant Physiol 98:942–948
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem 42:1825–1831
Khalafalla MM, Hattori K (2000) Ethylene inhibitors enhance in vitro root formation on faba bean shoots regenerated on medium containing thidiazuron. Plant Growth Regul 32:59–63
Koenig RL, Morris RO, Polacco JC (2002) tRNA is the source of low-level trans-Zeatin production in Methylobacterium spp. J Bacteriol 184:1832–1842
Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85:183–194
Li J, Ovakim DH, Charles TC, Glick BR (2000) An ACC deaminase minus mutant of Enterobacter cloacae UW4 no longer promotes root elongation. Curr Microbiol 41:101–105
Lizada MCC, Yang SF (1979) A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem 100:142–147
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin-phenol reagent. J Biol Chem 193:265–275
Ma JH, Yao JL, Cohen D, Morris B (1998) Ethylene inhibitors enhance in vitro root formation from apple shoot cultures. Plant Cell Rep 17:211–214
Ma W, Sebestianova SB, Sebestian J, Burd GI, Guinel FC, Glick BR (2003) Prevalence of 1-aminocyclopropane-1-carboxylate deaminase in Rhizobium spp. Anton Leeuw Int J G 83:285–291
Madhaiyan M, Poonguzhali S, Senthilkumar M, Seshadri S, Chung HY, Yang JC, Sundaram SP, Sa TM (2004) Growth promotion and induction of systemic resistance in rice cultivar Co-47 (Oryza sativa L.) by Methylobacterium spp. Bot Bull Acad Sin 45:315–324
Madhaiyan M, Poonguzhali S, Lee HS, Hari K, Sundaram SP, Sa TM (2005a) Pink-pigmented facultative methylotrophic bacteria accelerate germination, growth and yield of sugarcane clone Co86032 (Saccharum officinarum L.). Biol Fertil Soils 41:350–358
Madhaiyan M, Poonguzhali S, Sundaram SP, Sa TM (2005b) A new insight into foliar applied methanol influencing phylloplane methylotrophic dynamics and growth promotion of cotton (Gossypium hirsutum L.) and sugarcane (Saccharum officinarum L.). Environ Exp Bot (published online 15 September 2005)
Malerba M, Crosti P, Armocida D, Bianchetti R (1995) Activation of ethylene production in Acer pseudoplatanus L. cultured cells by fusicoccin. J Plant Physiol 145:93–100
Mattoo AK, Suttle JC (1991) The plant hormone ethylene. CRC Press, Boca Raton, 337 pp
Mayak S, Tirosh T, Glick BR (1999) Effect of wild-type and mutant plant growth-promoting rhizobacteria on the rooting of mung bean cuttings. J Plant Growth Regul 18:49–53
Penrose DM, Glick BR (2001) Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase containing plant growth-promoting bacteria. Can J Microbiol 47:368–372
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15
Petruzzelli L, Coraggio I, Leubner-Metzger G (2000) Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclo-propane-1-carboxylic acid oxidase. Planta 211:144–149
SAS Institute Inc. (2001) SAS user’s guide, version 8.2, SAS Institute Inc., Cary, North Carolina
Satoh S, Oyamada N, Yoshioka T, Midoh N (1997) 1,1-Dimethyl-4-(phenylsulfonyl)semicarbazide (DPSS) does not inhibit the in vitro activities of 1-aminocyclopropane-1-carboxylate (ACC) oxidase and ACC synthase obtained from senescing carnation (Dianthus caryophyllus L.) petals. Plant Growth Regul 23:191–193
Schaller GE, Kieber JJ (2002) Ethylene. The Arabidopsis book. American Society of Plant Biologists, USA
Shah S, Li J, Moffatt BA, Glick BR (1998) Isolation and characterization of ACC deaminase genes from two different plant growth promoting rhizobacteria. Can J Microbiol 44:833–843
Stearns JC, Shah S, Greenberg BM, Dixon DG, Glick BR (2005) Tolerance of transgenic canola expressing 1-aminocyclopropane-1-carboxylic acid deaminase to growth inhibition by nickel. Plant Physiol Biochem 43(7):701–708
Swarup R, Parry G, Graham N, Allen T, Bennett M (2002) Auxin cross-talk: integration of signalling pathways to control plant development. Plant Mol Biol 49:411–426
Trotsenko YA, Ivanova EG, Doronina NV (2001) Aerobic methylotrophic bacteria as phytosymbionts. Microbiology 70:725–736
Wachter R, Fischer K, Gabler R, Kuhnemann F, Urban W, Bogemann GM, Voesenek LACJ, Blom CWPM, Ullrich CI (1999) Ethylene production and ACC accumulation Agrobacterium tumefaciens-induced plant tumours and their impact on tumour and host stem structure and function. Plant Cell Environ 22:1263–1273
Yi HC, Joo S, Nam KH, Lee JS, Kang BG, Kim WT (1999) Auxin and brassinosteroid differentially regulate the expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in mung bean (Vigna radiata L.). Plant Mol Biol 41:443–454
Acknowledgments
This work was supported by grants from the Korea Research Foundation, under the Foreign Scientist and Engineers programme research awarded to M. Madhaiyan and Rural Development Administration (RDA), Republic of Korea. The authors wish to thank anonymous referees for their valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madhaiyan, M., Poonguzhali, S., Ryu, J. et al. Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase-containing Methylobacterium fujisawaense . Planta 224, 268–278 (2006). https://doi.org/10.1007/s00425-005-0211-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0211-y