Abstract
Fluctuating asymmetry (FA), a widely used measure of developmental instability in plants and animals, which describes random differences in size and/or shape between the two sides of a bilateral character. We used FA as a tool to detect stress in three mangrove species (Avicennia germinans, Laguncularia racemosa, Rhizophora mangle), growing in both disturbed and conserved habitats in the Atlantic coast of Mexico. In this region, disturbed habitats are the result of deforestation, livestock, tourism and agriculture activities. Twenty plants of each species were sampled in each of four sites (two disturbed and two conserved) and levels of FA, proportion of individuals with herbivory, proportion of leaves with damage, and leaf area removed by herbivores were evaluated. In disturbed habitats, regardless of plant species, more plants were attacked by insects, more leaves were damaged, and more leaf area was removed by herbivores, indicating higher overall damage to plants. We detected that FA levels varied significantly amongst mangrove species, they were higher in disturbed compared to conserved habitats, indicating the importance of FA as a monitoring tool of mangrove stress. A positive relationship between FA and herbivory levels also indicates that herbivores might be a source of stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants have to constantly deal with a suite of abiotic and biotic stressing factors such as available nitrogen and water deficit, herbivory and pathogen incidence (Suzuki et al. 2014), which might infer into different physiological costs (Heil and Bostock 2002). Despite that plants have molecular, physiological, and cellular modifications to adapt to stressful events (Heil and Bostock 2002; Rozendaal et al. 2006), in some instances they are unable to perform regulatory processes, which can result in developmental instability of different organs (Escós 1997). Developmental instability is the inability of a genotype to consistently produce the same phenotype in a particular environment, and it has been suggested as an indicator of environmental and/or genetic stress (Møller and Swaddle 1997). Fluctuating asymmetry (FA) is a widely used measure of developmental instability in plants and animals. It describes the random differences in size and/or shape between the two sides of a bilateral character (Palmer and Strobeck 1986; Møller and Shykoff 1999). Individuals that develop in a stress-free environment are capable of buffering most of the random errors in their development (Freeman et al. 2005), but as environmental stress increases, the resilience of such organisms to recover from disturbances decreases, resulting in higher FA (Leamy and Klingenberg 2005; Cuevas-Reyes et al. 2013). FA is therefore predicted to increase in organisms that experience increased levels of environmental stress and is often considered an integral component of individual fitness, revealing perturbations of genetic or environmental origins (Leamy and Klingenberg 2005; Cuevas-Reyes et al. 2011a).
Fluctuating asymmetry in plants can be influenced by abiotic factors such as pollution, altitude, temperature and soil fertility (Cornelissen et al. 2003; Freeman et al. 2004; Beasley et al. 2013), as well as biotic factors such as hybridization, parasitism and herbivory (Freeman et al. 2004; Albarrán-Lara et al. 2010; Cuevas-Reyes et al. 2011a, b, 2018). Particularly, the relationship between FA and herbivore susceptibility is still ambiguous. In some cases, leaf FA is positively related with the levels of herbivory (Cornelissen and Stiling 2005; Cuevas-Reyes et al. 2011a, 2018), but in other cases, no relationship has been found (Telhado et al. 2010). In the same way, several studies have documented the effects of habitat disturbance on leaf FA in plants of different terrestrial ecosystems such as tropical and temperate forests (Freeman et al. 2004; Nagamitsu et al. 2004; Beasley et al. 2013), but little information is yet available for plant species of coastal areas and estuaries (but see Torrez-Terzo and Pagliosa 2007; Constantino et al. 2009).
Mangroves are one of the most productive ecosystems in the world. The high availability of nutrients from rivers and land runoffs, and their effective recycling through microbial mineralization, contribute to coastline protection (Bouillon et al. 2008; Nagelkerken et al. 2008). Mangroves are considered ‘‘key-ecosystems’’ because they provide a wide variety of environmental services and denote habitat, shelter and food for numerous organisms (Mumby et al. 2004; Nagelkerken et al. 2008). However, it is also one of the most seriously threatened environments with global losses exceeding 35% as a result of human activities that are related to land use conversion of mangroves to mariculture, agriculture, forestry, urbanization, oil exploration and tourism (Campagna et al. 2011; Valiela et al. 2001). Particularly, in Mexico, the main threats to mangroves are habitat destruction, pollution and over-exploitation of resources. The lack of urban, industrial and tourist development planning, as well as agricultural, livestock and aquaculture development, have displaced and reduced considerable extensions of mangroves (CONABIO 2009). Finally, another source of mangrove disturbance are the urban solid waste, industrial pollutants, pesticides and agricultural fertilizers, which have had a great negative impact on mangroves communities.
Herbivory is one of the most important biotic interactions that affects the functioning of mangrove ecosystems (Elster et al. 1999). Specifically, leaf chewers (i.e. folivores), including crabs, birds and insect species, have high leaf consumption rates of mangrove leaves (Feller 1995; Kathiresan 2003). The general pattern of herbivory by this insect guild in the mangrove species shows a range of 0.3% to 35% of the leaf area consumed. However, these values of leaf consumption are highly variable between species, individuals and/or sites (Robertson et al. 1992). For example, the levels of herbivory in Rhizophora mangle varies from 4 to 25%, while in Avicennia germinans are from 8 to 36% (Farnsworth and Ellison 1991). According to some authors, leaf chewers require consuming a great amount of mangrove leaves, since they generally have low nutritional quality and therefore, represent a poor source for this insect guild (Feller 1995; Kathiresan 2003).
The high rates of herbivory can cause lethal injuries to mangrove trees (Kathiresan and Bingham 2001), but there is a lack of information regarding data on FA as a monitoring tool of mangrove stress and their potential relationship with the herbivory patterns. Therefore, we used a multi-species approach to determine herbivory susceptibility in three mangroves species and the importance of fluctuating asymmetry as an indicator of environmental stress in disturbed habitats in Mexico. We addressed the following questions: (1) Do herbivory levels differ between mangrove species? (2) Are mangrove species more susceptible to herbivory in disturbed compared to conserved habitats? (3) Are levels of leaf FA affected by habitat disturbance in mangrove species? (4) Do FA patterns correlate with the levels of herbivory? We hypothesize that in disturbed habitats, mangroves will be more stressed and will have higher levels of leaf FA, and accordingly higher herbivory susceptibility.
Materials and methods
Study site
This study was conducted in the northwest limit of La Mancha Lagoon in the state of Veracruz, Atlantic coast of Mexico (96°22′W, 19°35′N). The climate is warm, sub-humid with a cyclone influence; the mean annual precipitation is 1234 mm. In this area, Laguncularia racemosa (L.) Gaertn F. (Combretaceae), Rhizophora mangle (L.) Linnaeus (Rhizophoraceae) and Avicennia germinans (L.) Linnaeus (Acanthaceae) occur in sympatric conditions along a mosaic of conserved and disturbed habitats as result of deforestation, livestock, tourism and agriculture activities. These mangrove species are used in Mexico for local housing construction, fences, poles to catch fish, tool handles, carpentry, telegraph poles, electrical wiring, railroad ties, framed in boats, as well as for fuel (Utrera-López and Moreno-Casasola 2008).
Study system
Laguncularia racemosa (white mangrove): is a common component of mangrove forests along the Pacific and Atlantic coasts of Mexico (Rzedowski 1978). It is a perennial tree that can reach 10 m in height and occupies the medium to high intertidal zone of mangrove forests and colonizes disturbed sites, where it can form pure stands (Tomlinson 1986; Sobrado 2005).
Avicennia germinans (black mangrove): it occurs in most mangroves of the Atlantic and the Pacific coasts. It is a perennial shrub, usually 2 to 8 m high, characteristic of flooded areas of salt or brackish water.
Rhizophora mangle (red mangrove): is an evergreen tree or shrub, from 1.5 to 15 m (up to 30 m). This species has a broad distribution in Mexico and occurs along of the Gulf Coast, the Pacific and the Caribbean, in such extreme latitudes as Isla San Esteban in Baja California. This species is distributed on the Gulf side, from Tamaulipas to Veracruz, Yucatan and Quintana Roo. At the Pacific coast from Baja California to Sonora and Chiapas. These mangrove species have an associated herbivore fauna that includes dipteran and lepidopteran larvae, mites, hemipterans, crabs and even birds (Piyakarnchana 1981; Kathiresan 2003). We did not identify the herbivores that cause leaf damage in our mangrove species studied. Therefore, we only quantified the total herbivory, independently of the causative agent.
Data sampling
The central coast of the Gulf of Mexico has a considerable proportion of wetlands (3.8%) (Calles et al. 1998). Particularly, two-thirds of the wetlands in Veracruz are freshwater, and most of them are found in the coastal plain. Historically, these lands of the Gulf of Mexico have been used intensively for agriculture (corn crops) during the last thousand years, and currently around 70% of the territory has been transformed into pastures for cattle ranching and agricultural fields of sugarcane (Doolittle 1987; Moreno-Casasola et al. 2009).
To examine the differences in leaf area consumed by herbivores and FA levels, we selected two intact areas “conserved” and two disturbed areas where R. mangle, L. racemose, and A. germinans occur in La Mancha Lagoon, Mexico. The conserved areas are located within a private reserve of conservation of the Institute of Ecology A.C., where there are no human activities and the mangrove species represent a closed canopy belt near the banks of the lagoon system. The disturbed areas occur outside of the reserve and are characterized by the presence of human activities such as livestock, fishing, logging and even urbanization. Some studies indicate that these conserved habitats have mean interstitial salinity ranging from 27.0 to 32.5 ppt, and annual leaf litter production ranging between 6.92 t/ha/year and 13.5 t/ha/year (Utrera-López and Moreno-Casasola 2008; Moreno-Casasola et al. 2009). In our knowledge, there is no data of physicochemical parameters of mangrove communities in disturbed habitats that occur outside of the reserve La Mancha, Veracruz. However, in both habitat conditions, a continuous flood occurs and the sediment is inundated during at least six to eight continuous months per year, mainly during the dry season (from October to April) (Moreno-Casasola et al. 2009).
We randomly selected 20 individuals of each mangrove species at each study site. From each individual, we randomly collected 50 fully expanded mature leaves, sampling on superior, intermediate and inferior canopy strata (Cuevas-Reyes et al. 2011a, b).
Fluctuating asymmetry measurements
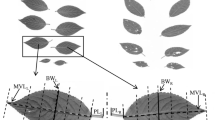
Fluctuating asymmetry was calculated in 25 fully expanded intact mature leaves of each individual. A digital image was obtained for each leaf. We measured the distance from the right side (Rw) and left side (Lw), from the leaf edge to the midrib at the midpoint of the leaf corresponding to its widest part. Fluctuating asymmetry was calculated as the absolute value of the difference between the distances from the midrib to the left and right margins of the leaf (|Ai − Bi|), divided by the average distance (Ai + Bi/2), to correct for the fact that asymmetry may be size-dependent (Cornelissen and Stiling 2005; Cuevas-Reyes et al. 2011a, 2018). Additionally, 10 leaves were blindly re-measured, without reference to previous measurements to control the measurement error in FA. We then evaluated the degree of significance of FA relative to measurement error using a two-way mixed-model ANOVA. The significance of the interaction (individual × leaf × side) indicated that variation in FA was greater than expected by measurements error (F9, 25 = 33.9; P < 0.0001).
According to Palmer and Strobeck (1986), there are three types of asymmetry, each characterized by a different combination of mean and variance of the distribution of right-minus-left (R−L) differences. Fluctuating asymmetry is found when the R-minus-L differences are normally distributed with a mean value of zero. Directional asymmetry is found when the R-minus-L differences are also normally distributed, but with a mean that is significantly different from zero. Antisymmetry is characterized by a platykurtic or bimodal distribution of R-minus-L differences about a mean of zero. To determine whether our data fitted only FA and no other types of asymmetry, we performed a Student’s t test and Lilliefors’ normality test to test whether mean values of signed right-minus-left values differed significantly from zero (Telhado et al. 2010; Alves-Silva and Del-Claro 2016). We found that R-minus-L measurements did not differ from zero (t = 1.1; P > 0.05), and therefore, we discarded the presence of directional asymmetry in our data. In the same way, we also rejected the presence of antisymmetry because our data (R-minus-L) exhibited a normal distribution (P > 0.05).
Herbivory levels
The herbivory levels were estimated in 25 leaves selected randomly per individual plant. We took a digital image of each leaf to calculate the total leaf area and the area removed by herbivores, using the Image analysis software for plant disease quantification (Assess Image). The proportion of leaf area removed by herbivores was calculated for each leaf by dividing the leaf area consumed by the total leaf area. To control for plant size, we measured plant height and diameter at breast height (DBH) (Cuevas-Reyes et al. 2013).
Statistical analyses
A generalized linear model applying the GENMOD procedure was performed to evaluate the frequency of leaves damaged by herbivorous insects in each habitat condition. The model used mangrove species as independent variables. The number of leaves with herbivory divided by the total number of leaves was used as the dependent variable. We used a Poisson error distribution and a Logit link function (SAS 2000; Stokes et al. 2000).
We performed a two-way ANOVA to compare herbivory levels amongst the three plant species in each habitat condition. Mangrove species and habitat condition were considered as independent variables and mean leaf area removed by herbivores used as the response variable in each case. An LSMeans test was performed as post hoc comparison (P < 0.05).
The differences in FA between the three-mangrove species in each habitat condition were evaluated using a logistic regression analysis, using GENMOD (SAS 2000; Stokes et al. 2000). The model considers mangrove species and habitat condition as independent variables. FA was used as response variable (SAS 2000; Stokes et al. 2000). Finally, we used Spearman’s rank correlation analysis to determine the relationship between FA, herbivory, total leaf area, plant height and DBH, for each mangrove species in each habitat condition. Since these tests involved multiple comparisons, a Bonferroni correction was applied to adjust p-values.
Results
We found higher herbivory levels in disturbed habitats for all mangrove species (Table 1). Particularly, R. mangle was the species with higher proportion of leaves damaged (85.4% ± 3.3%) in comparison with L. racemosa (77.3% ± 2.1%) and A. germinans (67% ± 3.5%). Conversely, we found in conserved habitats that the proportion of damaged leaves was lower than undamaged leaves in the three-mangrove species (Table 1). The average percentage of leaves with damage in A. germinans was 29.5.3% ± 2.8%, in R. mangle was 22.3% ± 1.8% and in L. racemosa was 20.3% ± 1.8%.
Leaf area removed by herbivores was different between mangroves species (Table 1). The leaf area removed was higher in R. mangle (8.7% ± 0.23) followed by L. racemosa (5.7% ± 0.18) and A. germinans (3.8% ± 0.35). In addition, the leaf area removed by herbivores was higher in disturbed than in conserved habitats in the three-mangrove species (Table 1).
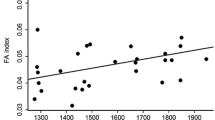
The results of the analysis using the GENMOD procedure indicated that fluctuating asymmetry levels differed between mangrove species according to the logistic regression analysis (Table 1). In addition, FA was higher in disturbed (0.17 ± 0.03) than in conserved (0.04 ± 0.005) habitats (Table 1). In the case of L. racemosa, after a Bonferroni correction, Spearman’s rank correlation showed that leaf area removed was negatively correlated with the total leaf area, whereas total leaf area showed a positive relationship with plant height and DBH in conserved habitats (Table 2a). In disturbed habitats, we found a positive relationship between FA and leaf area removed, total leaf area, plant height and DBH (Table 2a). In R. mangle, under conserved habitat conditions, a similar set of relationships was found, as leaf area removed was negatively correlated with the total leaf area, while positive relationships were found between total leaf area and DBH, plant height and DBH (Table 2b). In disturbed habitats, FA and leaf area removed, plant height, total leaf area and DBH, were positively correlated (Table 2b). A positive relationship was found between plant height and DBH in A. germinans under conserved habitat conditions, while in disturbed habitats, positive relationships were found between FA and leaf area removed, plant height, total leaf area and DBH (Table 2c).
Discussion
Mangroves have been perceived as a highly resilient ecosystem to disturbance, as they have specific adaptations that allow them to resist natural disasters such as a tsunami or climatic changes (Lugo 1980; Alongi 2002). However, some studies have demonstrated that under disturbance conditions such as urbanization, industrialization, pollution (i.e. heavy metal presence), the levels of environmental stress increase in some species of mangroves (Alongi 2002; Feng-Qin et al. 2007). In our study, we demonstrated that individuals of R. mangle, L. racemosa and A. germinans exhibited significantly higher levels of fluctuating asymmetry in disturbed than in conserved habitats, corroborating our hypothesis that mangrove species experience higher levels of environmental stress in disturbed habitats, and therefore, have higher levels of FA (Møller and Swaddle 1997; Lens et al. 2000; Cuevas-Reyes et al. 2011a, 2018).
Habitat disturbance affects species composition, abundance and distribution of herbivores in different ways, increasing resources for some herbivorous or reducing resources for others (Barberena-Arias and Aide 2002; Cuevas-Reyes et al. 2018). Herbivore abundance and herbivory levels may be reduced, as a result of changes in environmental conditions, plant nutritional quality and/or concentration of defensive compounds of plants (bottom-up effects) (Cuevas-Reyes et al. 2013; Maldonado-López et al. 2015). For example, high temperatures and deficiency of water availability, which is characteristic of disturbed habitats, may accelerate different biochemical processes that alter herbivore growth and development, resulting in the reduction of body size and alterations in their life history and patterns of reproduction (Angilletta 2009; Lee and Roh 2010). Conversely, habitat disturbance might cause increased insect abundance and herbivore damage through top-down effects, when population decline of higher trophic levels such as parasitoids and natural enemies positively affect herbivore density and herbivory levels (Arnold and Asquit 2002; Tscharntke et al. 2007). For oak forests, Maldonado-López et al. (2016) showed that both plant quality (leaf, bud and petiole production) and herbivory by insects were higher in isolated plants and smaller forest fragments in comparison with larger fragments of oak forests. Our findings for the mangroves are in accordance with this idea considering that plants can be influenced by the changes in abiotic conditions associated with habitat disturbance such as increased temperature, salinity and irradiation, which in turn, indirectly affect the interactions between mangrove species and their herbivorous insects via “bottom-up effects” (i.e. changes in plant nutritional quality and/or concentration of defensive compounds of plants) (Cuevas-Reyes et al. 2013; Maldonado-López et al. 2015), increasing the proportion of damaged leaves by herbivores. In addition, leaf area removed by insects was higher in disturbed than in conserved habitats for all mangrove species evaluated. Because FA has been considered as an indicator of environmental stress in different groups such as birds, mammals, fishes, amphibians insects, humans and plants (Wauters et al. 1996; Blackenhorn et al. 1998; Allenbach et al. 1999; Hansen et al. 1999; Møller and Shykoff 1999; Rikowski and Grammer 1999; Anciles and Marini 2000; Söderman et al. 2007; Cuevas-Reyes et al. 2011a, b, 2013), we propose that higher levels of fluctuating asymmetry recorded in mangrove species in disturbed habitats, suggest more environmental stress and therefore, more susceptibility to insect herbivore attack in this habitat conditions.
Our results can be explained by “The Plant Stress Hypothesis” (White 1984; Mattson and Haack 1987) that states that environmental stress negatively affect plant resistance to herbivory by altering biochemical source-sink relationships and the chemical composition of leaves, leading to a more attractive and palatable food to herbivores (Rhoades 1983; Mattson and Haack 1987; Cornelissen and Stiling 2005). The mechanism that support this hypothesis is that plants under stress conditions increase the levels of amino acids and decrease the production of secondary metabolites (such as tannins and phenols) in their tissues, which in turn, increase insect performance, the opportunity of offspring survival and susceptibility to herbivory (Torrez-Terzo and Pagliosa 2007).
We found differences in the herbivory levels between mangrove species, A. germinans had the lowest level of leaf area consumed, followed by L. racemosa and R. mangle. Differences in plant chemistry, leaf palatability, local microclimate have been suggested as possible causes for these differences (Joern and Mole 2005; Bauerfeind and Fischer 2013). Particularly, mangrove species can survive in stressful environments with hypersaline conditions, due to evolution of physiological and anatomical mechanisms to conserve water and eliminate salts, as is the case mainly of A. germinans, and to a lesser extent in L. racemosa and R. mangle (Sobrado 2005; Parida and Jha 2010). As a consequence, these plant species have variation in the levels of leaf sclerophilly (Feller 1995; Gonçalves-Alvim and Fernandes 2001), which are characterized by having a low nutritional quality, higher concentrations of chemical compounds, great thickness and hardness that in turn, reduce the probability of abscission and the herbivore incidence (De lacerda et al. 1985; Fernandes and Price 1988; Tavares de Menezes and Peixoto 2009). Therefore, it is possible expected that the levels of damage caused by herbivores of free-living such as leaf chewers decrease in plant species with scleromorphic leaves in comparison with others herbivory guilds such as gall-inducing insects and leaf miners (Price et al. 1998; Feller 2002).
A positive relationship between foliar FA and leaf area removed by herbivores was found only in disturbed habitats for the three-mangrove species evaluated. Because habitat disturbances change some environmental conditions (i.e. air quality, soil fertility, temperature and humidity) that may affect not only the patterns of plant growth but also plant responses to herbivory, increasing nutritional quality and/or decreasing secondary chemistry compounds production (Cornelissen and Stiling 2011), resulting in greater susceptibility to herbivory (Cornelissen and Stiling 2005; Lempa et al. 2000). In particular, human activities such as logging, fishing, livestock and urbanization have strong impacts on mangrove communities through their effect on abiotic factors and biodiversity. In particular, it has been shown that mangrove disturbances affect fluvial sedimentation patterns by increasing pollution by hydrocarbons, organochlorine pesticides and other chemical compounds produced by agricultural effluents (Lewis et al. 2011). In addition, mangrove disturbance affect different soil biochemical parameters, decreasing microbial biomass C, microbial biomass N, N flush, basal respiration, metabolic ratio (qCO2), ATP N mineralization rates and dehydrogenase and catalase activities (Dinesh et al. 2004). Finally, logging of mangrove forests results in an increase of CO2 emissions due to the oxidation of carbon in mangrove peat. This additional oxidation can occur if peat is disturbed and air contact increases, as would be the case when shrimp ponds are built on peat soils and peat is pushed into banks or dams (Lovelock et al. 2011). These abiotic changes in mangroves communities as result of disturbances can affect de ecology and therefore, the antagonistic interactions of mangroves species. Our results suggest that habitat disturbance causes a reduction of habitat suitability for Laguncularia racemosa, Rhizophora mangle and Avicennia germinans, which is expressed in higher foliar FA levels, influencing nutritional quality and/or defensive chemical compounds making them more susceptible to herbivore incidence (Cuevas-Reyes et al. 2013). Finally, our results highlight the importance of FA as a valuable biomarker for habitat disturbance and provides a quick, cheap and valuable cue to evaluate the early stages of environmental disturbance (Torrez-Terzo and Pagliosa 2007) and its effects on the dynamics of plant–insect interactions through herbivory.
References
Albarrán-Lara AL, Mendoza-Cuenca L, Valencia-Avalos S, González-Rodríguez A, Oyama K (2010) Leaf fluctuating asymmetry increases with hybridization and introgression between Quercus magnoliifolia and Quercus resinosa (Fagaceae) through an altitudinal gradient in Mexico. Int J Plant Sci 171:310–322. https://doi.org/10.1086/650317
Allenbach DM, Sullivan KB, Lydy MJ (1999) Higher fluctuating asymmetry as a measure of susceptibility to pesticides in fishes. Environ Toxicol Chem 18:899–905
Alongi DM (2002) Present state and future of the world’s mangrove forests. Environ Conserv 29:331–349. https://doi.org/10.1017/S0376892902000231
Alves-Silva E, Del-Claro K (2016) On the inability of ants to protect their plant partners and the effect of herbivores on different stages of plant reproduction. Austral Ecol 41:263–272. https://doi.org/10.1111/aec.12307
Anciles M, Marini MA (2000) The effects of fragmentation on fluctuating asymmetry in passerine birds of Brazilian tropical forests. J Appl Ecol 37:1013–1028
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford
Arnold AE, Asquit NM (2002) Herbivory in a fragmented tropical forest: patterns from islands at Lago Gatún, Panama. Biodiver Conserv 11:1663–1680. https://doi.org/10.1023/A:1016888000369
Barberena-Arias MF, Aide TM (2002) Variation in diversity and species composition of insect communities in Puerto Rico. Biotropica 34:357–367. https://doi.org/10.1646/0006-3606(2002)034%5b0357:VISATC%5d2.0.CO;2
Bauerfeind S, Fischer K (2013) Testing the plant stress hypothesis: stressed plants offer better food to an insect herbivore. Entomol Exp Appl 149:148–158. https://doi.org/10.1111/eea.12118
Beasley DE, Bonisoli-Alquati A, Mousseau TA (2013) The use of fluctuating asymmetry as a measure of environmentally induced developmental instability: a meta-analysis. Ecol Indic 30:218–226. https://doi.org/10.1016/j.ecolind.2013.02.024
Blackenhorn WU, Reusch T, Muehlhauser C (1998) Fluctuating asymmetry, body size and sexual selection in the dung fly Sepsis cynipsea: testing the good genes assumptions and predictions. J Evol Biol 11:735–753
Bouillon S, Borges AV, Castañeda-Moya E et al (2008) Mangrove production and carbon sinks: a revision of global budget estimates. Glob Biogeochem Cycles 22:1–12. https://doi.org/10.1029/2007GB003052
Calles A, Castillo G, Garcia I, Hernández H, Legaria L, Márquez W, Moreno-Casasola P, Moreno R, Morosini F, Portilla E, Silva-López G, Vargas JM, Vázquez G (1998) Humedales en Veracruz. In: Abarca F, Herzig M (eds) Manual para el Manejo y Conocimiento de los Humedales. Textos Adicionales. SEMARNAP-Arizona Fish and Wildlife, Mexico
Campagna C, Short FT, Polidoro BA, Mcmanus R, Collette BB, Pilcher NJ, De Mitcheson YS, Stuart SN, Carpenter KE (2011) Gulf of Mexico oil blowout increases risks to globally threatened species. Bioscience 61:393–397. https://doi.org/10.1525/bio.2011.61.5.8
CONABIO (2009) Manglares de México: Extensión y distribución, 2nd edn. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México, p 99
Constantino PAL, Monteiro RF, Wilson MD (2009) Gall midge attack intensity and host-plant response in a neotropical coastal ecosystem. Rev Bras Entomol 53:391–397. https://doi.org/10.1590/S0085-56262009000300013
Cornelissen T, Stiling P (2005) Perfect is best: low leaf fluctuating asymmetry reduces herbivory by leaf miners. Oecologia 142:46–56. https://doi.org/10.1007/s00442-004-1724-y
Cornelissen T, Stiling P (2011) Similar responses of insect herbivores to leaf fluctuating asymmetry. Arthropod Plant Int 5:59–69. https://doi.org/10.1007/s11829-010-9116-1
Cornelissen T, Stiling P, Drake B (2003) Elevated CO2 decreases leaf fluctuating asymmetry and herbivory by leaf miners on two oak species. Glob Change Biol 10:2736. https://doi.org/10.1111/j.1365-2486.2003.00712.x
Cuevas-Reyes P, Oyama K, González-Rodríguez A, Fernandes GW, Mendoza-Cuenca L (2011a) Contrasting herbivory patterns and leaf fluctuating asymmetry in Heliocarpus pallidus between different habitat types within a Mexican tropical dry forest. J Trop Ecol 27:383–391. https://doi.org/10.1017/S026646741100006X
Cuevas-Reyes P, Fernandes GW, González-Rodríguez A, Pimenta M (2011b) Effects of generalist and specialist parasitic plants (Loranthaceae) on the fluctuating asymmetry patterns of rupestrian host plants. Basic Appl Ecol. https://doi.org/10.1016/j.baae.2011.04.004
Cuevas-Reyes P, Gilberti L, González-RodríguezA Fernandes GW (2013) Patterns of herbivory and fluctuating asymmetry in Solanum lycocarpum St. Hill (Solanaceae) along an urban gradient in Brazil. Ecol Indic 24:557–561. https://doi.org/10.1016/j.ecolind.2012.08.011
Cuevas-Reyes P, Novais PGC, Gélvez-Zúñiga I, Fernandes GW, Venâncio H, Santos JC, Maldonado-López Y (2018) Effects of ferric soils on arthropod abundance and herbivory on Tibouchina heteromalla (Melastomataceae): importance of fluctuating asymmetry as indicator of environmental stress. Plant Ecol 219:69–78. https://doi.org/10.1007/s11258-017-0778-y
De Lacerda LD, Jose DV, de Rezende CA, Francisco MCF, Wasserman JC, Martins JC (1985) Leaf chemical characteristics affecting herbivory in a New World mangrove Forest. Biotropica 18:350–355
Dinesh R, Chaudhuri SG, Ganeshamurthy AN, Pramanik SC (2004) Biochemical properties of soils of undisturbed and disturbed mangrove forests of South Andaman (India). Wetl Ecol Manag 12:309–320
Doolittle WE (1987) Las Marismas to Panuco to Texas: the transfer of open range cattle ranching from Iberia through Northeastern Mexico. Conf Lat Am Geogr Yearb 13:3–11
Elster C, Perdomo L, Polania J, Schnetter M-L (1999) Control of Avicennia germinans recruitment and survival by Junonia evarete larvae in a disturbed mangrove forest in Colombia. J Trop Ecol 15:791–805
Escós J (1997) Grazing impact on plant fractal architecture and fitness of a Mediterranean shrub (Anthyllis cytisoides). Funct Ecol 11:66–78
Farnsworth EJ, Ellison A (1991) Patterns of herbivory in Belizean mangrove swamps. Biotropica 23:555–567
Feller IC (1995) Effects of nutrient enrichment on growth and herbivory of dwarf red mangrove (Rhizophora mangle). Ecology 65:477–505
Feller IC (2002) The role of herbivory by wood-boring insects in mangrove ecosystems in Belize. Oikos 97:167–176
Feng-Qin Z, You-Shao W, Zhi-Ping L, Jun-De D (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50
Fernandes GW, Price PW (1988) Biogeographical gradients in galling species richness: tests of hipotheses. Oecologia 76:161–167
Freeman DC, Brown ML, Duda JJ, Graham JH, Emlen JM, Krzysik AJ, Balbach H, Kovacic DA, Zak JC (2004) Developmental instability in Rhus Copallinum L.: multiple stressors, years, and responses. Int J Plant Sci 165:53–63. https://doi.org/10.1086/380986
Freeman DC, Brown ML, Duda JJ, Graraham JH, Emlen JM, Krzysik AJ, Balbach H, Kovaci DA, Zak JC (2005) Leaf fluctuating asymmetry, soil disturbance and plant stress: a multiple year comparison using two herbs, Ipomoea pandurata and Cnidoscolus stimulosus. Ecol Indic 5:85–95. https://doi.org/10.1016/j.ecolind.2004.05.002
Gonçalves-Alvim SJ, Fernandes GW (2001) Biodiversity of galling insects: historical, community and habitat effects in four tropical savannas. Bio Conserv 10:79–98
Hansen LTT, Amundsen T, Forsgren E (1999) Symmetry: attractive not only to females. Proc R Soc Lond B 266:1235–1240
Heil M, Bostock RM (2002) Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann Bot 89:503–512. https://doi.org/10.1093/aob/mcf076
Joern A, Mole S (2005) The plant stress hypothesis and variable responses by blue grama grass (Bouteloua gracilis) to water, mineral nitrogen, and insect herbivory. J Chem Ecol 9:2069–2090. https://doi.org/10.1007/s10886-005-6078-3
Kathiresan K (2003) Insect folivory in mangroves. Indian J Mar Sci 32:237–239
Kathiresan K, Bingham BL (2001) Biology of mangroves and mangrove ecosystems. Adv Mar Biol 40:81–251. https://doi.org/10.1016/S0065-2881(01)40003-4
Leamy LJ, Klingenberg CP (2005) The genetics and evolution of fluctuating asymmetry. Annu Rev Ecol Evol Syst 36:1–21. https://doi.org/10.1146/annurev.ecolsys.36.102003.152640
Lee KP, Roh C (2010) Temperature-by-nutrient interactions affecting growth rate in an insect ectotherm. Entomol Exp Appl 136:151–163. https://doi.org/10.1111/j.1570-7458.2010.01018.x
Lempa K, Martel J, Koricheva J, Haukioja K, Ossipov V, Ossipova S, Pihlaja K (2000) Covariation of fluctuating asymmetry, herbivory and chemistry during birch leaf expansion. Oecologia 122:354–360
Lens L, Van Dongen S, Galbusera P, Schenck T, Matthysen E, Van de Casteele T (2000) Developmental instability and inbreeding in natural bird populations exposed to different levels of habitat disturbance. J Evol Biol 13:889–896
Lewis M, Pryor R, Wilking L (2011) Fate and effects of anthropogenic chemicals in mangrove ecosystems: a review. Environ Pollut 159:2328–2346
Lovelock CE, Bennion V, Grinham A, Cahoon DR (2011) The role of surface and subsurface processes in keeping pace with sea-level rise in intertidal wetlands of Moreton Bay, Queensland, Australia. Ecosystems 14:745–757
Lugo AE (1980) Mangrove ecosystems: successional or steady state? Biotropica 12:65–72. https://doi.org/10.2307/2388158
Maldonado-López Cuevas-Reyes P, González-Rodríguez Pérez-López G, Acosta-Gómez Oyama K (2015) Relationships among plant genetics, phytochemistry and herbivory patterns in Quercus castanea across a fragmented landscape. Ecol Res 30:133–143. https://doi.org/10.1007/s11284-014-1218-2
Maldonado-López Y, Cuevas-Reyes P, Oyama K (2016) Diversity of gall wasps (Hymenoptera:Cynipidae) associated with oak trees (Fagaceae: Quercus) in a fragmented landscape in Mexico. Arthropod Plant Interact 10:29–39. https://doi.org/10.1007/s11829-015-9404-x
Mattson WJ, Haack RA (1987) The role of drought in outbreaks of plant-eating insects. Bioscience 37:110–118. https://doi.org/10.2307/1310365
Møller AP, Shykoff JA (1999) Morphological developmental stability in plants: patterns and causes. Int J Plant Sci 160:S135–S146. https://doi.org/10.1086/314219
Møller AP, Swaddle JP (1997) Asymmetry, developmental stability, and evolution. University Press, Oxford
Moreno-Casasola P, López-Rosas H, Infante MD, Peralta LA, Travieso-Bello A, Warner BG (2009) Wetland differentiation in the landscape of La Mancha, coastal Veracruz, Mexico. Plant Ecol 200:37–52
Mumby PJ, Edwards AJ, Arias-González JE, Lindeman KC, Blackwell PG, Gall A, Gorczynska MI, Harborne AR, Pescod CL, Renken H, Wabnitz CCC, Llewellyn G (2004) Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 427:533–536. https://doi.org/10.1038/nature02286
Nagamitsu T, Kawahara T, Hotta M (2004) Phenotypic variation and leaf fluctuating asymmetry in isolated populations of an endangered dwarf birch Betula ovalifolia in Hokkaido, Japan. Plant Species Biol 19:13–21. https://doi.org/10.1111/j.1442-1984.2004.00097.x
Nagelkerken Blaber SJM, Bouillon S et al (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89:155–185. https://doi.org/10.1016/j.aquabot.2007.12.007
Palmer RA, Strobeck C (1986) Fluctuating asymmetry: measurement, analysis, and patterns. Annu Rev Ecol System 17:391–421. https://doi.org/10.1146/annurev.es.17.110186.002135
Parida AK, Jha B (2010) Salt tolerance mechanisms in mangroves: a review. Trees 24:199–217
Piyakarnchana T (1981) Severe defoliation of Avicennia alba B1 by larvae of Cleora injectaria Walker. J Sci Soc Thailand 7:33–36. https://doi.org/10.2306/scienceasia1513-1874.1981.07.033
Price PW, Fernandes GW, Lara ACF, Brawn J, Barrios H, Wright MG, Ribeiro SP, Rothcliff N (1998) Global patterns in local number of insect galling species. J Biogeogr 25:581–591
Rhoades DF (1983) Herbivore population dynamics and plant chemistry. In: Denno FR, McClure MS (eds) Variable plants and herbivores in natural and managed systems. Academic, New York
Rikowski A, Grammer K (1999) Human body odour, symmetry and attractiveness. Proc R Soc Lond B 266:869–874
Robertson AI, Alongi DM, Boto KG (1992) Food chains and carbonfluxes. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystems. American Geophysical Union, Washington, DC
Rozendaal DMA, Hurtado VH, Poorter L (2006) Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Func Ecol 20:207–216. https://doi.org/10.1111/j.1365-2435.2006.01105.x
Rzedowski J (1978) Vegetación de México. LIMUSA, México
SAS (2000) Categorical data analysis using the SAS system. SAS Institute, Cary
Sobrado MA (2005) Leaf characteristics and gas exchange of the mangrove Laguncularia racemosa as affected by salinity. Photosynthetica 43:217–221
Söderman F, van Dongen S, Pakkasmaa S, Merila J (2007) Environmental stress increases skeletal xuctuating asymmetry in the moor frog Rana arvalis. Oecologia 151:593–604
Stokes ME, Davis CS, Koch GG (2000) Categorical data analysis using the SAS system, 2nd edn. SAS, Cary
Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 203:32–43. https://doi.org/10.1111/nph.12797
Tavares de Menezes LF, Peixoto AL (2009) Leaf damage in a mangrove swamp at Sepetiba Bay, Rio de Janeiro, Brazil. Rev Bras Bot 32:715–724
Telhado C, Esteves D, Cornelissen T, Fernandes GW, Carneiro MAA (2010) Insect herbivores of Coccoloba cereifera do not select asymmetric plants. Environ Entomol 39:849–855. https://doi.org/10.1603/EN09179
Tomlinson PB (1986) The botany of mangroves. Cambridge University Press, New York
Torrez-Terzo G, Pagliosa PR (2007) Fluctuating asymmetry as a useful biomarker of Environmental stress: a case of study with Avicennia schaueriana Stapf and Leechm. Ex moldenke (Acanthaceae). Insula 33:75–94
Tscharntke T, Bommarco R, Clough Y, Crist TO, Kleijn D, Rand TA, Tylianakis JM, van Nouhuys S, Vidal S (2007) Conservation biological control andenemy diversity on a landscape scale. Biol Control 43:294–309
Utrera-López ME, Moreno-Casasola P (2008) Mangrove litter dynamics in La Mancha Lagoon, Veracruz, Mexico. Wetlands Ecol Manag 16:11–22. https://doi.org/10.1007/s11273-007-9042-x
Valiela I, Bowen JL, York JK (2001) Mangrove forests: one of the world’s threatened major tropical environments. Bioscience 51:807–815
Wauters LA, Dhondt AA, Knothe H, Parkin DT (1996) Fluctuating asymmetry and body size as indicators of stress in red squirrel populations in woodland fragments. J Appl Ecol 33:735–740
White TCR (1984) The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 63:90–105. https://doi.org/10.1007/BF0037979
Acknowledgements
Pablo Cuevas-Reyes thanks Coordinación de la Investigación Científica, UMSNH for their generous support. Cornelissen acknowledges CNPq for funding (307210/2016-2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maldonado-López, Y., Vaca-Sánchez, M.S., Canché-Delgado, A. et al. Leaf herbivory and fluctuating asymmetry as indicators of mangrove stress. Wetlands Ecol Manage 27, 571–580 (2019). https://doi.org/10.1007/s11273-019-09678-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-019-09678-z