Abstract
Light environments can influence variation in plant morphology, development and susceptibility to herbivores. Our research interest was to investigate the patterns of herbivore damage and developmental stability in dioecious understory forb Mercurialis perennis in contrasting light habitats, located at 1700 m a.s.l. on Mt. Kopaonik. Male and female plants from two light habitats, open (a sun-exposed field) and shaded (a spruce forest) were examined with respect to: herbivore damage (percentage of leaf area loss), fluctuating asymetry (FA) as a measurement of developmental stability, plant morphological and, specifically, leaf size traits, as well as biochemical traits relating to nutritional quality and defence, taking into account the possible presence of intersexual differences. Our results show that herbivore damage was significantly higher in open habitat, as well as one out of four univariate FA indices and the multivariate index. Morphological and biochemical traits, apart from defensive compounds, had higher values in the shade, pointing to sun-exposed habitat being more stressful for this species. Intersexual differences were observed for foliar damage, defensive compounds (phenolics and tannins), all leaf size traits, total leaf area, and protein content. Contrasting light habitats affected most of the analysed traits. Both foliar damage and FA were higher in a more stressful habitat; within habitats, no positive correlations were found. Herbivore damage was significantly male biased in open habitat. The analysis of intersexual differences in developmental stability measured by leaf asymmetry levels provided no evidence that female plants were more sensitive to environmental stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During their lifetime, plants experience many abiotic and biotic environmental stress factors, which directly influence developmental stability and morphology (Elemans 2004). Environmental variation can be translated into intraspecific variation in morphological traits, as well as the production of secondary metabolites, physical resistance and nutritive value, conferring susceptibility to herbivores (Hakes and Cronin 2012). Under the pressure of herbivore attacks, plants have evolved numerous morphological and chemical defences in attempt to reduce the extent of damage (Johnson 2011).
Light, as one of key environmental factors, can modify the outcome of plant–herbivore interactions (Valladares et al. 2016). Light differential herbivory has been recorded on numerous occasions (e.g., Moura et al. 2017). Plants suffered higher damage at sun-exposed habitats (Salgado-Luarte and Gianoli 2010, 2012; Takafumi et al. 2010) or, inversely, in the shade (Niesenbaum and Kluger 2006; Muth et al. 2008). Explanations of observed folivory patterns include the differential effects of light availability on plant traits. Leaves produced in the shade are thinner, softer, with higher nitrogen and water content; therefore, they are expected to be more attractive to herbivores than leaves exposed to full sunlight (Roberts and Paul 2006; Valladares and Niinemets 2008). Additionally, plants in shaded habitats are expected to have lower production of carbon-based secondary metabolites and, consequently, lower levels of chemical defence (Roberts and Paul 2006; Barbehenn and Constable 2011).
One of the key research topics in the field of plant evolutionary ecology is the influence of suboptimal environmental conditions (i.e., stress) on developmental stability (Palmer and Strobeck 2003). Developmental stability reflects the ability of individual organisms to maintain the phenotype constant under different environmental conditions. Stress activates mechanisms of developmental stability to produce the optimal phenotype, alleviating the consequences of random deviations during the development of the organism. Contrary to this, developmental instability represents the inability of organisms to control developmental processes and amortize random fluctuations (Nijhout and Davidowitz 2003; Graham et al. 2010).
The way to determine levels of developmental instability under environmental stress is to estimate fluctuating asymmetry (Miljković 2012; Telhado et al. 2017). Fluctuating asymmetry (FA) is defined as small, random deviations from perfect symmetry in bilateral characters, such as leaves (Møller and Shykoff 1999; Palmer and Strobeck 2003). Increased FA is usually considered a signal of organisms’ inability to buffer different types of stress originating from environmental and biotic factors. Suboptimal habitat light conditions can also act as stress factor; for example, in shade-tolerant plant species, high light intensities can have a detrimental effect.
The relation between herbivory and developmental stability is an important issue, and FA can be used to analyze it. FA is often considered to be an indicator of stress (since stressed plants tend to attract herbivores) or can represent the consequence of herbivory (when herbivores induce asymmetry) (Alves-Silva and Del-Claro 2016). Higher FA values could indicate higher leaf-nutritive value and lower levels of chemical defense (Lempa et al. 2000) so herbivores may use departures from perfect symmetry as a sign that a plant represents better quality food (Cornelissen and Stiling 2005a). Alternatively, herbivores themselves can act as a stress factor during leaf development and cause higher levels of FA (Zvereva et al. 1997; Santos et al. 2013). Thus, positive correlations between FA and herbivory are expected. Cornelissen and Stiling (2005a) have found that more asymmetric leaves sustain higher levels of damage. While some studies also found positive FA—herbivory relations (e.g., Cuevas-Reyes et al. 2011), others found the opposite (Bañuelos et al. 2004; Telhado et al. 2010).
Assuming that FA is an indicator of sustained stress (Graham et al. 2010) and that stressed plants are more attractive to herbivores (Cornelissen and Stiling 2005a), plants with higher FA levels should, logically, face higher herbivore pressure (Lempa et al. 2000). Therefore, we expected that shade-adapted plants, such as Mercuriallis perennis, in sun-exposed habitats would have higher FA values, and hence experience more damage.
In dioecious species, plant sex is another factor possibly influencing size, defence levels and the ability to buffer environmental stress, all of which can have an impact on the ultimate result of plant–herbivore interactions (Obeso 2002; Harris and Pannell 2008). Sexual dimorphism in morphological and biochemical traits can result in sex-biased herbivory, with male bias being suggested as a rule (Cornelissen and Stilling 2005b). Male and female plants were reported to differ in secondary biochemical defense traits (Malonado-Lopez et al. 2014), aspects of foliar nutritional quality (Cornelissen and Stilling 2005b) and size (Barrett and Hough 2013), all of which can influence susceptibility to herbivores.
Females and males draw different amounts of resources and different metabolic currencies into reproduction, growth and defense (Harris and Pannell 2008). The high energy-demanding process of reproduction in females is usually traded off by lower vegetative growth accompanied by higher defense levels (Barrett and Hough 2013). Nevertheless, females appear to be more sensitive and perform worse compared to males when exposed to abiotic stresses, such as water or nutrient deficiency [reviewed in Juvany and Munné-Bosch (2015)]. If so, one would expect that FA, as the measurement of inability to control for environmental disturbances during development, would be higher in females.
Over the past decades, much research has been devoted to exploring asymmetry levels in plant modules [reviewed in Graham et al. (2010), Klingenberg (2015)], and the influence of various stressors, such as light availability, on FA (Silva et al. 2016; Nikiforou and Manetas 2017). However, studies rarely included plant sex as a factor contributing to the observed patterns of developmental instability in dioecious species, even less seldom in combination with herbivory. Thus, the aims of this study were to explore and compare relations between developmental stability, herbivore damage, morphological and biochemical traits in dioecious forb Mercurialis perennis between two contrasting light habitats, and to explore sexual dimorphism in the analysed traits.
Materials and methods
Study species and contrasting light habitats
The wind-pollinated dioecious geophyte M. perennis is a common member of the ground flora of Euro-Caucasian temperate forests. The leaves are simple, elliptic, with serrated edge. Different habitat conditions can alter foliage shape, dentation and pendunculus length. However, the main factor affecting leaf structure and morphology is light; sun-exposed leaves are elongated, tougher and thicker (Jefferson 2008). This perennial preferentially colonizes ancient woodlands, and is rarely associated with disturbed habitats (Vandepitte et al. 2010; Vujić et al. 2016). It typically grows in dense patches in the deep shade of beech and oak forests and rarely occurs in completely open sites. High light fluxes were found to cause reductions in shoot height and leaf area (Elemans 2004).
The study was conducted on Mt. Kopaonik (Serbia) samples that were taken from adjacent habitats located at 1700 m a.s.l. (N 43°18′42″ E 20°50′32″). The first one, designated as ‘open’ habitat, is in an open field of alpine grassland (class Festuco–Seslerietea), exposed to full sunlight throughout the growing season, where M. perennis plants grow in small, distant clumps. The other, ‘shaded’ habitat, is in a spruce wood (class Vaccinio–Piceetea), where M. perennis grows in larger, denser stands under the canopy of trees.
Sample collection and measurements
The field study included 120 individuals, 30 males and 30 females per habitat. All plants were collected on the same day in mid-June 2016. We collected shoots at random from distinct individual clones, visually distinguishable and separated more than 50 cm [based on our previous field observations and the estimates given in Vandepitte et al. (2009), and also occasionally checked by performing excavations]. In case of neighbouring clones, only plants of opposite sexes were collected. Immediately after sampling, cut stems were submerged in water. After being fully rehydrated, plant height (H) was measured; leaves were separated from the stem and placed in a flatbed scanner, in the same order as they had been arranged on the shoot, with the abaxial side facing the glass (Fig. 1a). After scanning, leaves were dried for 48 h at 65 °C, grounded in liquid nitrogen and underwent further biochemical analysis. The first pair of fully expanded leaves was weighed fresh, reweighed after drying and used to calculate specific leaf area (SLA).
Total leaf area (TLA), leaf blade surface area (BSA), leaf area consumed (LAC) and fluctuating asymmetry (FA) were determined using leaf scans processed in ImageJ (Abràmoff et al. 2004). All scans were calibrated to the nearest 0.01 mm before measurements were taken, and the software resolution did not allow for measurement error greater than 1%. Leaf area consumed by herbivores (LAC) was calculated for each leaf as follows: (removed leaf area/total leaf area) × 100, and averaged out per plant.
Fluctuating asymmetry analyses
For two leaves of the same node we calculated fluctuating asymmetry (matching symmetry as a type of symmetry; see axes on Fig. 1b) (Klingenberg et al. 2002). Four leaf traits were measured: petiole length (PL), midvein length (MVL), leaf blade width (BW) and leaf blade surface area (BSA) of both left and right leaf, designated as PLL, MVLL, BWL, BSAL and PLR, MVLR, BWR, BSAR, respectively (Fig. 1b).
To ensure uniform measurements, we placed a reference grid, using the landmarks on the top and base of each leaf. The grid, made in MakeFan program of IMP package (Sheets 2003), consisted of eight evenly spaced lines (“comb” structure). Leaf width was always measured along the fourth line of the grid counting from the leaf base up (Fig. 1b). Absolute asymmetry was calculated as the unsigned difference between signed left- and right-side values of the trait. To correct the fact that asymmetry may be size dependent (Cuevas-Reyes et al. 2013, and references therein), we calculated size-scaled FA index for each trait as |Rj−Lj| divided by ((Rj + Lj)/2), where Rj and Lj represent the measurements of right- and left-side values of leaf trait j. Examining simultaneously FA in multiple traits can increase the probability of detecting the effects of stress on FA (Leung et al. 2000). As a multivariate measure of FA, we employed multivariate index FAIND, which represents asymmetry on individual level, i.e., mean deviation from symmetry of all examined traits (Palmer and Strobeck 2003). It was calculated as Σ|ln(Rj)−ln(Lj)|/T (T is number of traits per individual).
To explore repeatability of measurements, a total of 480 leaves from 80 randomly selected individuals were remeasured by the same researcher (SS), without reference to previous measurements. Two sets of measurements were considered as replicates; ANOVA with individual as a factor and repeated measurement of R−L nested in individual was performed to estimate repeatability following the equation: \({\text{ME4}}\,=\,{{({\text{M}}{{\text{S}}_{{\text{IND}}}} - {\text{M}}{{\text{S}}_{{\text{ERR}}}})} \mathord{\left/ {\vphantom {{({\text{M}}{{\text{S}}_{{\text{IND}}}} - {\text{M}}{{\text{S}}_{{\text{ERR}}}})} {\left[ {{\text{M}}{{\text{S}}_{{\text{IND}}\,}}+\,(n - {\text{1}}){\text{ M}}{{\text{S}}_{{\text{ERR}}}}} \right]}}} \right. \kern-0pt} {\left[ {{\text{M}}{{\text{S}}_{{\text{IND}}\,}}+\,(n - {\text{1}}){\text{ M}}{{\text{S}}_{{\text{ERR}}}}} \right]}}\) (ME4—repeatability; MSIND—variation among individuals; MSERR—variation within individuals). To explore the potential presence of directional asymmetry, two-way mixed model ANOVA with main effects side (as fixed factor) and the individual (as random factor), and their interaction (side × individual) was employed (Palmer and Strobeck 2003).
Biochemical analyses
Approximately 50 mg of grounded sample was extracted over 12 h at room temperature using 80% acetone and 1% acetic acid solution. The extracts were used to evaluate total soluble protein content (TSPC) in leaves according to Bradford (1976), with bovine serum albumin as a standard. Absorbance was measured at λ = 280 nm using Ultrospec 3200 pro GE, Helthcare spectrophotometer. Following the method of Singleton and Rossi (1965), acetone extracts were incubated with Folin–Ciocalteu reagent and 20% Na2CO3 at room temperature for 2 h. The absorbance was measured at λ = 765 nm to estimate total phenolic content (TPhC). Finally, we conducted a colorimetrical method modified by Xu and Chang (2007) to measure total condensed tannin content (TCT). Sample acetone extracts were incubated with 4% vanilin solution and concentrated HCl for 2 h at room temperature, followed by absorption reading at λ = 500 nm.
Statistical analyses
Statistical analyses were performed in SAS Statistical package (SAS Institute, Inc. 2011). For descriptive statistics, tests of normality, mean value differences and a posteriori comparisons, we performed SAS procedures MEANS, UNIVARIATE, and TTEST. Data were transformed where necessary, using the transformation log (TLA, SLA, TCT, PL, MVL and BSA), arcsine square root (LAC and LWC) and square root (H and TSPC). To explore the effects of habitat and sex on herbivory levels and plant traits, two-factorial ANOVAs with habitat and sex as fixed factors were performed, while for the effects on leaf FA and leaf size traits, the additional factor was individual (nested in sex and habitat) as random factor. The analyses were performed using SAS GLM procedure. CORR procedure was used to analyse correlation patterns between the analysed traits of female and male plants in open and shaded habitat.
Results
Plant traits and foliar damage
The effect of habitat was significant for all analysed plant traits except phenolic and tannin contents (Table 1). Plants from the open habitat were smaller, and had lower values of TLA, SLA, LWC and TSPC, while the values of TPhC, as well as TCT, were similar in both habitats (Fig. 2).
Differences in mean values (mean ± SE) of M. perennis plant traits: H (height), TLA (total leaf area), SLA (specific leaf area), LWC (leaf water content), TSPC (total soluble protein content), TPhC (total phenolic content), TCT (total condensed tannin content) and LAC (leaf area consumed), for females (clear bars) and males (bars with lines) in open (light bars) and shaded habitat (dark bars). The asterisks represent statistically significant differences between habitats and between sexes within habitat (t test, * P < 0.05, ** P < 0.01, *** P < 0.001)
Prominent differences in foliar damage were found between habitats (Table 1). Individuals from open habitat suffered greater damage, i.e., higher percent of leaf area loss (P < 0.001, Fig. 2). Although the degree of damage at individual leaf level was rather low, rarely exceeding 10%, it was nevertheless approximately 5.2 times higher in open than in shaded habitat (open: mean 3.03%, range 0.02–10.68%; shaded: mean 0.58%, range: 0–5.26%).
The effect of habitat was also significant for all leaf-size traits: PL, MVL, BW and BSA (Table 2), with plants in the shade consistently exhibiting higher trait values (Fig. 3). We also observed high levels of intraindividual variation in all measured leaf traits (Table 2, effect of individual nested in sex and habitat, P < 0.001 in all cases).
Differences in mean values (mean ± SE) of M. perennis leaf-size traits PL (petiole length), MVL (midvein length), BW (leaf blade width) and BSA (leaf blade surface area), for females (clear bars) and males (bars with lines) in open (light bars) and shaded habitat (dark bars). The asterisks represent statistically significant differences between habitats and between sexes within habitat (t test, *** P < 0.001)
Plant sex had a significant effect on TLA, TSPC, TPhC and TCT (Table 1). The intersexual differences in TLA and TSPC were significant in the shade, with females exhibiting higher values (Fig. 2). In the case of TPhC and TCT, the effect of sex was significant in both habitats, with consistently higher trait values in females (Fig. 2). Male and female plants also showed significant differences in four leaf-size traits (Table 2): females had wider leaves with longer midveins and greater blade surface areas; exceptionally, petioles were significantly shorter in females from the open habitat only (Fig. 3).
Intersexual differences in herbivore damage were significant in the open habitat, where males suffered greater leaf area loss. In shaded habitat, LAC values were low and did not differ significantly; habitat × sex interaction was significant (Table 1; Fig. 2).
Fluctuating asymmetry
The analysis of the remeasured dataset confirmed that two sets of measurements were not statistically different (all P > 0.05). The repeatability measure (ME4) for all traits ranged from 0.494 to 0.646 (data not presented). Nonsignificant effect of factor side in two factors ANOVA showed the absence of directional asymmetry.
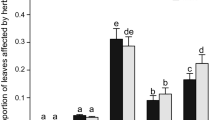
The effect of habitat was significant for two indices, FAPL and FAIND with higher values exhibited in the sun-exposed habitat. We recorded significant effect of plant sex only for FAMVL, which had lower values in females (Table 3; Fig. 4).
Mean values (± SE) of univariate size-scaled FA indices for: petiole length (FAPL), midvein length (FAMVL), leaf blade width (FABW) and leaf blade surface area (FABSA), and multivariate index for analysed leaf traits on individual level (FAIND); females—clear bars, males—bars with lines, open habitat—light bars, shaded habitat—dark bars. The asterisk represents statistically significant difference between habitats (t test, * P < 0.05, ** P < 0.01)
Correlation patterns
In the shaded habitat, the number of statistically significant correlations, as well as their magnitude, was higher compared to the open habitat for both sexes (Fig. 5). The level of herbivore damage was not significantly correlated with the majority of FA indices, the only exception being negative correlation with FABSA in males from the open habitat. With regard to other traits, LAC was negatively correlated with specific leaf area in both habitats and with water content in shade, but only for females. No significant relationship was detected between LAC and plant size, protein content or secondary metabolites—total phenolic and condensed tannin content. FA indices showed overall moderate to strong positive correlations with each other. This pattern was more pronounced in shaded habitat. Correlations with other traits were generally not observed, except for negative correlations between plant height and two indices (FAMVL and FABSA) but only in males in shaded habitat. Not surprisingly, plant size traits (H and TLA) were positively correlated. In addition, strong positive correlations were found between SLA and LWC, as well as between TPhC and TCT.
Discussion
Contrasting light habitats
Plant habitus, physical and biochemical traits, as well as leaf size and shape are dictated by physiological demands and developmental constraints, all of which are under the strong influence of environmental conditions (Cuevas-Reyes et al. 2011). The light regime has been detected as one of the key factors shaping plant structural and functional responses to folivory (Salgado-Luarte and Gianoli 2012).
Contrasting light habitats affected analysed plant and leaf traits in M. perennis, except for defensive compounds. Plants from the sun-exposed habitat were smaller, with smaller and narrower leaves. This points to an overall negative effect of high light fluxes on this species, a result in accordance with previous findings (Elemans 2004; Jefferson 2008). In addition, shade-adapted species (such as M. perennis), carpeting woodland understory, usually have canopies comprised of thinner, softer leaves, richer in water and nitrogen, compared to plants exposed to full sunlight (Barbehenn and Constable 2011). The longer petioles in shaded habitat observed in this study can be explained by the previous finding that, in shade-tolerant herbs, this trait is results of selective pressure, likely to reduce the effects of competition for light (Weijschedé et al. 2006).
Although plants in shade had, as expected, softer leaves with higher SLA values, water and protein contents, the extent of herbivore damage was greater (approx. 5.2 times) in plants from the open habitat. This difference was highly significant. Environmental conditions can alter the outcome of plant–herbivore interactions, ultimately leading to differential herbivore pressures (Hakes and Cronin 2012). Our findings of shade-tolerant forb M. perennis being more damaged in the sun-exposed habitat are in line with recent results on other understory species (Takafumi et al. 2010; Salgado-Luarte and Gianoli 2012; Suárez-Piña et al. 2016). Plant size did not prove to be a significant factor in susceptibility to herbivores; within habitat correlations showed that height and TLA were positively mutually correlated, but not with LAC. Although for M. perennis the sun-exposed habitat was found to be more stressful compared to deep shade, and LAC was higher in the open field, we found no evidence that greater damage was the consequence of stressed plants being a more attractive food source. In fact, sun-exposed plants had a lower mean level of soluble proteins. Still, we detected no significant correlations between LAC and TSPC, a finding consistent with the conclusion of meta-analysis by Huberty and Denno (2004) that stress-induced changes in foliar nitrogen content did not affect feeding preference of chewing insects. Leaf area loss was also not significantly correlated with analysed defensive compounds; contrary to expectations, TPhC and TCT did not differ between the habitats. However, it should be kept in mind that other compounds, such as alkaloids, can also have a defensive role (e.g., Sanches-Vilas and Pannell 2011).
One of the possible explanations for the observed pattern of herbivory variation is that the excess damage to sun-exposed leaves could be attributed to the higher compensatory consumption of a lower quality food source, as pointed out in some other cases (Cornelissen and Stiling 2005b; Lusk et al. 2010). It should be noted, however, that the observed differences in analysed traits between contrasting light habitats might also be partly due to other factors, abiotic and biotic, that were not specifically investigated in this research, such as soil characteristics, folivore abundance or indirect effects mediated by plant neighbours (e.g., Castagneyrol et al. 2013).
Fluctuating asymmetry
Although the effects of light intensity on plant traits represent fairly explored topic, until recently less research effort had been made in investigating and discerning the influence of light on fluctuating asymmetry (Miljković 2012; Venâncio et al. 2016; Moura et al. 2017). In light-demanding species, such as Quercus pyrenaica, low-light conditions can lead to increased leaf developmental instability (Puerta-Piñero et al. 2008). Furthermore, experimental covering of Cucurbita pepo leaves resulted in higher leaf FA values (Freeman et al. 2003).
In our study, developmental stability was mildly affected by light environment—one univariate FA index (FAPL), as well as the multivariate index, had higher values for the open habitat. Assuming that elevated levels of environmental stress lead to higher FA values (Graham et al. 2010), this indicates that for M. perennis as a shade-adapted species, an open habitat is more stressful. However, this effect was not very strong.
Several studies have found that the effects of stress on FA levels can be weak. For example, in a recent study of Sandner and Matthies (2017), which included experimental manipulation of light intensity and measurement of FA in Silene vulgaris leaves, FA generally was not higher under elevated stress levels. Venâncio et al. (2016) pointed out that the natural history of the species in question (i.e., whether it is light demanding or shade tolerant) should be considered when using FA values as a proxy for determining levels of stress imposed by habitat light conditions and setting a sustainable hypothesis on the FA stress relations.
Since M. perennis is a typical understory perennial, overall FA as an indicator of light-induced environmental stress levels was lower than expected. It has been suggested that the weak effect of stress on FA levels, or lack of it (Costa et al. 2013; Wadhwa et al. 2017), could be explained by adaptation to long-term exposure to certain stress factors (Anne et al. 1998). In plants, apart from adaptation, plastic responses (especially in leaf shape) may affect the relationship between developmental instability and FA (Palmer and Strobeck 2003). In addition, Freeman et al. (2003) proposed that, according to the type of growth, it is possible for FA levels to decrease in mature leaves.
Recently, a fairly large body of literature on the relationship between FA and herbivory has accumulated. Numerous experimental and field studies have found a positive relationship between the two phenomena (Cuevas-Reyes et al. 2011, 2013; Cornelissen and Stiling 2011; Ribeiro et al. 2013; Santos et al. 2013; Fernandes et al. 2016), while others detected no significant relationship (Auslander et al. 2003; Bañuelos et al. 2004; Hagen et al. 2008; Telhado et al. 2010; Costa et al. 2013).
In our study, both herbivore damage (expressed through LAC) and developmental instability (expressed through FA) were higher in the more stressful open habitat, although the overall FA level was lower than expected. Within habitats, leaf damage and FA were not significantly correlated, except for one negative correlation in males from the open habitat, indicating that herbivores did not use FA as an adequate indicator of the overall host plant state. It has been noticed that a positive FA—herbivory relationship is more commonly found in model systems in which host plant and herbivores have a very tight connection, such as in the case of leaf gall formers and miners (Cornelissen and Stiling 2011).
It was proposed that stressed plants exhibiting more asymmetric leaves have higher amounts of available nitrogen combined with lower levels of defensive compounds (Lempa et al. 2000). However, it is possible that certain herbivore guilds may not use FA as an indicator of plant state [review by Cornelissen et al. (2008)]. We found no significant relations between asymmetry and aspects of plant nutritive value or defensive compounds (phenolics and tannins). Similarly, Bañuelos et al. (2004) found no relationship between leaf FA and content of defensive compound anthraquinone in Rhamnus alpinus. Therefore, we conclude that in M. perennis, leaf FA could not be used by herbivores as a reliable indicator of levels of plant defense or nutritive value, i.e., as a proxy for finding suitable host plants.
The effects of plant sex
One of the aims of this study was to estimate intersexual differences in analysed traits. In all traits that exhibited significant sexual dimorphism, it was female-biased, except for male-biased sexual dimorphism in LAC and PL in the open habitat. In dioecious plants, sexual dimorphism in size can be manifested, as a consequence of trade-offs between growth, reproduction and defence (Cornelissen and Stiling 2005b). In our study, plant height exhibited no significant sexual dimorphism; this is in line with previous findings in this species that sexual dimorphism in height decreased with increasing altitude (Cvetković and Jovanović 2007). It has been noticed that in more stressful habitats, intersexual differences in vegetative allocation might tend to decrease; a trend previously described in sexual dimorphism patterns relating to altitude (Sakai et al. 2006) and light availability (Labouche and Pannell 2016). However, three leaf size traits (MVL, BW and BSA), as well as TLA were greater in females (though the difference in TLA was significant in shade only). Female plants that allocate more to reproductive structures tend to invest more into resource acquisition structures (Malonado-Lopez et al. 2014) such as leaves, especially under low-light conditions.
Females also exhibited higher TSPC values; sexual dimorphism was significant in shade. Within plant source–sink translocations with respect to timing of reproduction (Wright and Dorken 2014) can explain differences in protein levels. During flowering, males allocate significant amounts of nitrogen from leaves to producing pollen (Harris and Pannell 2008). Since the plants were sampled in reproductive stage, males had, as expected, lower TSPC; this is in accordance with previous findings on congeneric M. annua (Harris and Pannell 2008; Sánchez-Vilas and Pannell 2011).
In dioecious species, the morph-bearing female function is usually considered better defended (Obeso 2002). Our results underpin this claim, since we found females in both habitats having higher values of defensive compounds TPhC and TCT; similar results were previously recorded in other species (Cepeda-Cornejo and Dirzo 2010; Zhang et al. 2016). Sexual dimorphism in morphological and biochemical traits can result in sex-differential herbivory, male bias being suggested as a rule (Cornelissen and Stilling 2005b). Sexes can differ in aspects of leaf nutritional quality (Cornelissen and Stilling 2005b), secondary biochemical defence traits (Malonado-Lopez et al. 2014) and size (Barrett and Hough 2013), all of which may be associated with susceptibility to herbivores.
Over the years, studies on this problem have grown in number, reporting male bias in herbivore damage (Cornelissen and Stilling 2005b), female bias (Maldonado-López et al. 2014) or no bias at all (Espírito-Santo et al. 2012; Buckley and Avila-Sakar 2013). Here, we found evidence of sex-biased herbivory in the open habitat, where males experienced higher leaf damage. This finding can be interpreted in terms of females being a better defended sex due to higher contents of secondary defensive compounds, phenolics and tannins. Sex-biased herbivory in shade was not found, possibly because overall levels of damage were very low (mean 0.58%).
Few studies so far have simultaneously tackled the problems of possible effects of plant sex on developmental stability and herbivore damage. Bañuelos et al. (2004), working on dioecious Rhamnus alpinus, did not find any relationship between the degree of asymmetry and sex or herbivore loads. In contrast, the results of Inbar and Kark (2007) on Pistacia atlantica across the environmental gradient show evidence of lower levels of FA in females, and thus greater developmental stability. In our study, sexual dimorphism in developmental stability was detected only for index FAMVL which had higher values in males. Thus, there was no evidence that female plants are more sensitive to environmental stress as measured by FA leves. FA indices for BW, MVL and BSA were strongly positively correlated in both habitats and sexes, and could indicate that path of development of these traits is not influenced by light environment or sex.
We found differential patterns of herbivore damage with respect to light habitat and sex in the field study on understory forb M. perennis. Contrasting light habitats affected most of the analysed traits. Leaf damage was significantly higher in the open habitat, as well as one univariate and the multivariate FA index. All analysed morphological and biochemical traits, except for phenolics and tannin contents, had significantly higher values in shade. The obtained results point to sun-exposed habitats being more stressful for this species. Since there was no evidence that greater damage was the consequence of stressed plants being more attractive food source, the observed pattern could be explained by higher compensatory consumption of a lower quality food source. Both leaf area loss and developmental instability were higher in the more stressful open habitat, although the phenolic and tannin contents did not differ, and the overall FA was lower than expected. Within habitats, foliar damage was not positively correlated with FA. We found evidence of male-biased herbivory in the open habitat; male plants also had lower contents of analysed defensive compounds. Sex-biased herbivory in the shade was not found, probably because the overall level of damage was very low. Intersexual differences were also observed for leaf size traits and protein content. Sexual dimorphism analyses provided no evidence that female plants are more sensitive to environmental stress as measured by FA levels.
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11:36–42. https://imagescience.org/meijering/publications/download/bio2004.pdf
Alves-Silva E, Del-Claro K (2016) Herbivory-induced stress: Leaf developmental instability is caused by herbivore damage in early stages of leaf development. Ecol Indic 61:359–365. https://doi.org/10.1016/j.ecolind.2015.09.036
Anne PF, Mawri S, Gladstone DC (1998) Is fluctuating asymmetry a reliable biomonitor of stress? A test using life history parameters in soybean. Int J Plant Sci 159:559–565. https://doi.org/10.1086/297573
Auslander M, Nevo E, Inbar M (2003) The effects of slope orientation on plant growth, developmental instability and susceptibility to herbivores. J Arid Environ 55:405–416. https://doi.org/10.1016/S0140-1963(02)00281-1
Bañuelos MJ, Sierra M, Obeso JR (2004) Sex, secondary compounds and asymmetry: effects on plant–herbivore interaction in a dioecious shrub. Acta Oecol 25:151–157. https://doi.org/10.1111/j.1600-0706.2005.14075.x
Barbehenn RV, Constable CP (2011) Tannins in plant-herbivore interactions. Phytochemistry 72:1551–1565. https://doi.org/10.1016/j.phytochem.2011.01.040
Barrett SC, Hough J (2013) Sexual dimorphism in flowering plants. J Exp Bot 64:67–82. https://doi.org/10.1093/jxb/ers308
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Buckley NE, Avila-Sakar G (2013) Reproduction, growth, and defense trade-offs vary with gender and reproductive allocation in Ilex glabra (Aquifoliaceae). Am J Bot 100:357–364. https://doi.org/10.3732/ajb.1200603
Castagneyrol B, Giffard B, Péré C, Jactel H (2013) Plant apparency, an overlooked driver of associational resistance to insect herbivory. J Ecol 101(2):418–429. https://doi.org/10.1111/1365-2745.12055/full
Cepeda-Cornejo V, Dirzo R (2010) Sex-related differences in reproductive allocation, growth, defense and herbivory in three dioecious neotropical palms. PLoS One 5:e9824. https://doi.org/10.1371/journal.pone.0009824
Cornelissen T, Stiling P (2005a) Perfect is best: low leaf fluctuating asymmetry reduces herbivory by leaf miners. Oecologia 142:46–56. https://doi.org/10.1007/s00442-004-1724-y
Cornelissen T, Stiling P (2005b) Sex-biased herbivory: a meta-analysis of the effects of gender on plant-herbivore interactions. Oikos 111:488–500. https://doi.org/10.1111/j.1600-0706.2005.14075.x/full
Cornelissen T, Stiling P (2011) Similar responses of insect herbivores to leaf fluctuating asymmetry. Arthropod Plant Interact 5:59–69. https://doi.org/10.1007/s11829-010-9116
Cornelissen T, Wilson Fernandes G, Vasconcellos-Neto J (2008) Size does matter: variation in herbivory between and within plants and the plant vigor hypothesis. Oikos 117:1121–1130. https://doi.org/10.1111/j.0030-1299.2008.16588.x/full
Costa FVD, Pinheiro de Azevedo IF, Braga LDL, Perillo LN, Neves FDS, Leite LO, Cuevas-Reyes P (2013) Fluctuating asymmetry and herbivory in two ontogenetical stages of Chamaecrista semaphora in restored and natural environments. J Plant Interact 8:179–186. https://doi.org/10.1080/17429145.2012.657253
Cuevas-Reyes P, Oyama K, González-Rodríguez A, Fernandes GW, Mendoza-Cuenca L (2011) Contrasting herbivory patterns and leaf fluctuating asymmetry in Heliocarpus pallidus between different habitat types within a Mexican tropical dry forest. J Trop Ecol 27:383–391. https://doi.org/10.1017/S026646741100006X
Cuevas-Reyes P, Gilberti L, González-Rodríguez A, Fernandes GW (2013) Patterns of herbivory and fluctuating asymmetry in Solanum lycocarpum St. Hill (Solanaceae) along an urban gradient in Brazil. Ecol Indic 24:557–561. https://ac.els-cdn.com/S1470160X12003020/10.1016/j.ecolind.2012.08.011
Cvetković D, Jovanović V (2007) Altitudinal variation of the sex ratio and segregation by gender in the dioecious plant Mercurialis perennis L.(Euphorbiaceae) in Serbia. Arch Biol Sci 59:193–198. https://doi.org/10.2298/ABS0703193C
Elemans M (2004) Light, nutrients and the growth of herbaceous forest species. Acta Oecol 26:197–202. https://doi.org/10.1016/j.actao.2004.05.003
Espírito-Santo MM, Neves FS, Fernandes GW, Silva JO (2012) Plant phenology and absence of sex-biased gall attack on three species of Baccharis. PloS One 7:e46896. https://doi.org/10.1371/journal.pone.0046896
Fernandes GW, de Oliveira SCS, Campos IR, Barbosa M, Soares LA, Cuevas-Reyes P (2016) Leaf fluctuating asymmetry and herbivory of Tibouchina heteromalla in restored and natural environments. Neotrop Entom 45:44–49. https://doi.org/10.1007/s13744-015-0342-1
Freeman DC, Brown ML, Dobson M, Jordan Y, Kizy A, Micallef C, Emlen JM (2003) Developmental instability: measures of resistance and resilience using pumpkin (Cucurbita pepo L.). Bio J Linn Soc 78:27–41. https://doi.org/10.1046/j.1095-8312.2003.00123.x/full
Graham JH, Raz S, Hel-Or H, Nevo E (2010) Fluctuating asymmetry: methods, theory, and applications. Symmetry 2:466–540. http://www.mdpi.com/2073-8994/2/2/466
Hagen SB, Ims RA, Yoccoz NG, Sørlibråten O (2008) Fluctuating asymmetry as an indicator of elevation stress and distribution limits in mountain birch (Betula pubescens). Plant Ecol 195:157–163. https://doi.org/10.1007/s11258-007-9312-y
Hakes AS, Cronin JT (2012) Successional changes in plant resistance and tolerance to herbivory. Ecology 93:1059–1070. http://www.jstor.org/stable/23225222
Harris MS, Pannell JR (2008) Roots, shoots and reproduction: sexual dimorphism in size and costs of reproductive allocation in an annual herb. Proc R Soc B Biol Sci 275:2595–2602. http://rspb.royalsocietypublishing.org/content/275/1651/2595
Huberty AF, Denno RF (2004) Plant water stress and its consequences for herbivorous insects: a new synthesis. Ecology 85:1383–1398. https://doi.org/10.1890/03-0352/full
Inbar M, Kark S (2007) Gender-related developmental instability and herbivory of Pistacia atlantica across a steep environmental gradient. Folia Geobot 42:401–410. https://doi.org/10.1007/BF02861702
Jefferson RG (2008) Biological flora of the British isles: Mercurialis perennis L. J Ecol 96:386–412. https://doi.org/10.1111/j.1365-2745.2007.01348.x/full
Johnson MT (2011) Evolutionary ecology of plant defences against herbivores. Func Ecol 25:305–311. https://doi.org/10.1111/j.1365-2435.2011.01838.x/full
Juvany M, Munné-Bosch S (2015) Sex-related differences in stress tolerance in dioecious plants: a critical appraisal in a physiological context. J Exp Bot 66:6083–6092. https://doi.org/10.1093/jxb/erv343
Klingenberg CP (2015) Analyzing fluctuating asymmetry with geometric morphometrics: concepts, methods, and applications. Symmetry 7:843–934. http://www.mdpi.com/2073-8994/7/2/843
Klingenberg CP, Barluenga M, Meyer A (2002) Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution 56:1909–1920. https://doi.org/10.1111/j.0014-3820.2002.tb00117.x/full
Labouche AM, Pannell JR (2016) A test of the size-constraint hypothesis for a limit to sexual dimorphism in plants. Oecologia 181:873–884. https://doi.org/10.1007/s00442-016-3616-3
Lempa K, Martel J, Koricheva J, Haukioja E, Ossipov V, Ossipova S, Pihlaja K (2000) Covariation of fluctuating asymmetry, herbivory and chemistry during birch leaf expansion. Oecologia 122:354–360. https://doi.org/10.1007/s004420050041
Leung B, Forbes MR, Houle D (2000) Fluctuating asymmetry as a bioindicator of stress: comparing efficacy of analyses involving multiple traits. Am Nat 155:101–115. http://www.jstor.org/stable/10.1086/303298
Lusk CH, Onoda Y, Kooyman R, Gutiérrez-Girón A (2010) Reconciling species-level vs plastic responses of evergreen leaf structure to light gradients: shade leaves punch above their weight. New Phytol 186:429–438. https://doi.org/10.1111/j.1469-8137.2010.03202.x/full
Maldonado-López Y, Cuevas-Reyes P, Sánchez-Montoya G, Oyama K, Quesada M (2014) Growth, plant quality and leaf damage patterns in a dioecious tree species: is gender important? Arthropod Plant Interact 8:241–251. https://doi.org/10.1007/s11829-014-9314-3
Miljković D (2012) Developmental stability of Iris pumila flower traits: a common garden experiment. Arch Biol Sci 64:123–133. https://doi.org/10.2298/ABS1201123M
Møller AP, Shykoff JA (1999) Morphological developmental stability in plants: patterns and causes. Int J Plant Sci 160:S135–S146. https://doi.org/10.1086/314219
Moura RF, Alves-Silva E, Del-Claro K (2017) Patterns of growth, development and herbivory of Palicourea rigida are affected more by sun/shade conditions than by Cerrado phytophysiognomy. Acta Bot Bras 31:286–294. https://doi.org/10.1590/0102-33062016abb0446
Muth NZ, Kluger EC, Levy JH, Edwards MJ, Niesenbaum RA (2008) Increased per capita herbivory in the shade: necessity, feedback, or luxury consumption. Ecoscience 15:182–188. https://doi.org/10.2980/15-2-3095
Niesenbaum RA, Kluger EC (2006) When studying the effects of light on herbivory, should one consider temperature? The case of Epimecis hortaria F. (Lepidoptera: Geometridae) feeding on Lindera benzoin. L. (Lauraceae). Environ entomol 35:600–606. https://doi.org/10.1603/0046-225X-35.3.600
Nijhout HF, Davidowitz G (2003) Developmental perspectives on phenotypic variation, canalization, and fluctuating asymmetry. In: Polak M (ed), Developmental instability: causes and consequences. Oxford University Press, Oxford, pp 3–13. (ISBN: 0-19-514345-0)
Nikiforou C, Manetas Y (2017) Ecological stress memory: evidence in two out of seven species through the examination of the relationship between leaf fluctuating asymmetry and photosynthesis. Ecol Indic 74:530–534. https://doi.org/10.1016/j.ecolind.2016.11.004
Obeso JR (2002) The costs of reproduction in plants. New Phytol 155:321–348. https://doi.org/10.1046/j.1469-8137.2002.00477.x/full
Palmer AR, Strobeck C (2003) Fluctuating asymmetry analyses revisited. In: Polak M (ed) Developmental instability: causes and consequences. Oxford University Press, Oxford, pp 279–319
Puerta-Piñero C, Gómez JM, Hódar JA (2008) Shade and herbivory induce fluctuating asymmetry in a Mediterranean oak. Int J Plant Sci 169:631–635. http://www.jstor.org/stable/10.1086/533601
Ribeiro VA, Silva RND, Sousa-Souto L, Neves FDS (2013) Fluctuating asymmetry of and herbivory on Poincianella pyramidalis (Tul.) LP Queiroz (Fabaceae) in pasture and secondary tropical dry forest. Acta Bot Bras 27:21–25. https://doi.org/10.1590/S0102-33062013000100003
Roberts MR, Paul ND (2006) Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol 170:677–699. https://doi.org/10.1111/j.1469-8137.2006.01707.x/full
Sakai A, Sasa A, Sakai S (2006) Do sexual dimorphisms in reproductive allocation and new shoot biomass increase with an increase of altitude? A case of the shrub willow Salix reinii (Salicaceae). Am J Bot 93:988–992. http://www.amjbot.org/content/93/7/988.full.pdf+html
Salgado-Luarte C, Gianoli E (2010) Herbivory on temperate rainforest seedlings in sun and shade: resistance, tolerance and habitat distribution. PLoS One 5:e11460. https://doi.org/10.1371/journal.pone.0011460
Salgado-Luarte C, Gianoli E (2012) Herbivores modify selection on plant functional traits in a temperate rainforest understory. Am Nat 180:E42–E53. http://www.jstor.org/stable/10.1086/666612
Sánchez-Vilas J, Pannell JR (2011) Sex-differential herbivory in androdioecious Mercurialis annua. PloS One 6:e22083. https://doi.org/10.1371/journal.pone.0022083
Sandner TM, Matthies D (2017) Fluctuating asymmetry of leaves is a poor indicator of environmental stress and genetic stress by inbreeding in Silene vulgaris. Ecol Indic 79:247–253. https://doi.org/10.1016/j.ecolind.2017.04.030
Santos JC, Alves-Silva E, Cornelissen TG, Fernandes GW (2013) The effect of fluctuating asymmetry and leaf nutrients on gall abundance and survivorship. Basic Appl Ecol 4:489–495. https://doi.org/10.1016/j.baae.2013.06.005
Sheets HD (2003) IMP— Integrated Morphometrics Package. Buffalo: Department of physics, Canisius College. http://www3.canisius.edu/~sheets/morphsoft.html
Silva HV, Alves-Silva E, Santos JC (2016) On the relationship between fluctuating asymmetry, sunlight exposure, leaf damage and flower set in Miconia fallax (Melastomataceae). Trop Ecol 57:419–427. http://tropecol.com/pdf/open/PDF_57_3/4%20Venancio%20Silva,%20Alves-Silva%20&%20Santos-f.pdf
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16:144–158. http://www.ajevonline.org/content/16/3/144
Suárez-Piña J, Rueda-Almazán JE, Ayestarán LM, Alcalá RE (2016) Effect of light environment on intra-specific variation in herbivory in the carnivorous plant Pinguicula moranensis (Lentibulariaceae). J Plant Interact 11:146–151. https://doi.org/10.1080/17429145.2016.1231851
Takafumi H, Kawase S, Nakamura M, Hiura T (2010) Herbivory in canopy gaps created by a typhoon varies by understory plant leaf phenology. Ecol Entom 35:576–585. https://doi.org/10.1111/j.1365-2311.2010.01216.x/full
Telhado C, Esteves D, Cornelissen T, Fernandes GW, Carneiro MAA (2010) Insect herbivores of Coccoloba cereifera do not select asymmetric plants. Environ Entom 39:849–855. https://doi.org/10.1603/EN09179
Telhado C, Silveira FAO, Fernandes GW, Cornelissen T (2017) Fluctuating asymmetry in leaves and flowers of sympatric species in a tropical montane environment. Plant Spec Biol 32:3–12. https://doi.org/10.1111/1442-1984.12122/full
Valladares F, Niinemets Ü (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst 39:237–257. https://doi.org/10.1146/annurev.ecolsys.39.110707.173506
Valladares F, Laanisto L, Niinemets Ü, Zavala MA (2016) Shedding light on shade: ecological perspectives of understorey plant life. Plant Ecol Divers 9:237–251. https://doi.org/10.1080/17550874.2016.1210262
Vandepitte K, Roldán-Ruiz I, Leus L, Jacquemyn H, Honnay O (2009) Canopy closure shapes clonal diversity and fine-scale genetic structure in the dioecious understorey perennial Mercurialis perennis. J Ecol 97:404–414. https://doi.org/10.1111/j.1365-2745.2009.01484.x/full
Vandepitte K, Honnay O, De Meyer T, Jacquemyn H, Roldán-Ruiz I (2010) Patterns of sex ratio variation and genetic diversity in the dioecious forest perennial Mercurialis perennis. Plant Ecol 206:105–114. https://doi.org/10.1007/s11258-009-9627-y
Venâncio H, Alves-Silva E, Santos JC (2016) Leaf phenotypic variation and developmental instability in relation to different light regimes. Acta Bot Bras 30:296–303. https://doi.org/10.1590/0102-33062016abb0081
Vujić V, Rubinjoni L, Selaković S, Cvetković D (2016) Small-scale variations in leaf shape under anthropogenic disturbance in dioecious forest forb Mercurialis perennis: a geometric morphometric examination. Arch Biol Sci 68:705–713. https://doi.org/10.2298/ABS151111011V
Wadhwa S, Gallagher FJ, Rodriguez-Saona C, Holzapfel C (2017) Exposure to heavy metal stress does not increase fluctuating asymmetry in populations of isopod and hardwood trees. Ecol Indic 76:42–51. https://doi.org/10.1016/j.ecolind.2016.12.037
Weijschedé J, Martínková J, De Kroon H, Huber H (2006) Shade avoidance in Trifolium repens: costs and benefits of plasticity in petiole length and leaf size. New Phyt 172:655–666. https://doi.org/10.1111/j.1469-8137.2006.01885.x/full
Wright VL, Dorken ME (2014) Sexual dimorphism in leaf nitrogen content but not photosynthetic rates in Sagittaria latifolia (Alismataceae). Botany 92:109–112. https://doi.org/10.1139/cjb-2013-0246
Xu BJ, Chang SKC (2007) A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci 72:S159–S166. https://doi.org/10.1111/j.1750-3841.2006.00260.x/abstract
Zhang L, Yang M, Gao J, Jin S, Wu Z, Wu L, Zhang X (2016) Seasonal variation and gender pattern of phenolic and flavonoid contents in Pistacia chinensis Bunge inflorescences and leaves. J Plant Phys 191:36–44. https://doi.org/10.1016/j.jplph.2015.11.014
Zvereva EL, Kozlov M, Niemelä VP, Haukioja E (1997) Delayed induced resistance and increase in leaf fluctuating asymmetry as responses of Salix borealis to insect herbivory. Oecologia 109:368–373. https://doi.org/10.1007/s004420050095
Acknowledgements
This study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia Grant No. 173025 title “Evolution in heterogeneous environments: mechanisms of adaptation, biomonitoring and conservation of biodiversity” and Grant No. 173005 title “Molecular mechanisms of plant response to abiotic stress—the role of transcription factors and small RNAs and analysis of genetic diversity of plant crops of interest for agriculture and biotechnology”.
Author information
Authors and Affiliations
Contributions
DM and DC conceived the idea and designed the study. SS, VV and DC conducted fieldwork. NS, SS and SR performed biochemical analyses, VV and SS performed herbivory analyses, DM performed statistical analyses. SS, DM and DC wrote the draft, DC and DM wrote the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there is no conflict of interest in relation to this article.
Rights and permissions
About this article
Cite this article
Miljković, D., Selaković, S., Vujić, V. et al. Patterns of herbivore damage, developmental stability, morphological and biochemical traits in female and male Mercurialis perennis in contrasting light habitats. Alp Botany 128, 193–206 (2018). https://doi.org/10.1007/s00035-018-0203-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00035-018-0203-8