Abstract
Estuaries are particularly susceptible to climate change and drought resulting in atypical changes to freshwater flows. How such changes in flow impact on the ecology of estuarine fishes may depend on how a species moves in response to changing flow conditions. Acoustic telemetry was used to interpret fine-scale movements of two co-inhabiting estuarine fish species, black bream, Acanthopagrus butcheri and estuary perch, Macquaria colonorum in relation to freshwater flows, season and moon phase. We found black bream to be highly mobile, regularly travelling the length of the estuary and into the neighbouring estuaries. In contrast, estuary perch had particular home ranges and made occasional, upstream or downstream movements. Possibly influenced by freshwater flows, estuary perch moved at greater rates in the Tambo compared to fish in the Mitchell. Black bream resided in the upper estuary during winter and spring and the lower estuary during summer and autumn, whereas estuary perch remained in the upper estuary throughout the year, with occasional downstream movements in winter and spring. This study revealed 1) significantly large increases in freshwater flows result in mass downstream movements in both species, 2) fish moved upstream during full moons and 3) there are contrasting spatio-temporal patterns in movement between species. The results from this study highlight that estuarine fishes are likely to show differential sensitivity to the impacts of drought and climate change and illustrate how acoustic telemetry methods can be used to determine the environmental needs of fishes and help efforts to conserve and manage estuaries worldwide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding how animals move and interact in their changing environment is an important component of ecological science (Peterson et al. 2001; Nathan et al. 2008; Patterson et al. 2008; Izzo et al. 2016). Characterising movements and behaviour of animals facilitates insight into the ecology of a species, which allows for better management and conservation practices (Peterson et al. 2001; Jonsen et al. 2003; Patterson et al. 2008; Izzo et al. 2016). Modern technology and statistical methods now provide a mechanism to track the location, movement and behaviour of animals over extended periods of time and on fine spatial scales. In the aquatic environment, there are many capture independent and capture dependent methods to study the spatial behaviours of fishes at a range of temporal scales (Lucas and Baras 2000). A popular method, acoustic telemetry, can record the presence of up to hundreds of tagged animals over defined temporal and spatial scales (Heupel et al. 2006). Acoustic telemetry has been widely applied in marine, freshwater and estuarine environments to establish patterns of habitat use, behaviour, migration and distances moved by fish (Arendt et al. 2001; Childs et al. 2008; Hindell et al. 2008; Crook et al. 2010;). More recently, researchers have been linking acoustic telemetry data with environmental data to establish interactions between species and their environment (Childs et al. 2008; Crook et al. 2010; Sakabe and Lyle 2010; Bendell et al. 2011).
Estuarine environments are highly dynamic and variable with large vertical and horizontal gradients in salinity, temperature, dissolved oxygen, turbidity and chlorophyll a (Kurup et al. 1998; Whitfield 1999; Gillanders and Kingsford 2002). How a species interacts with its habitat within an estuary is dependent on life-stage, prey, predators and environmental conditions (Blaber and Blaber 1980; Whitfield 1999; Secor and Rooker 2005). A predominant factor that explains much of an estuary’s dynamic nature is variability in freshwater flows (Gillanders and Kingsford 2002). Therefore, it is likely that freshwater flows will have an influence on the spatial distribution of fishes within estuaries (Whitfield 1999). Interpreting the link between freshwater flows, environmental gradients and movement of fish within estuaries is crucial to the management and conservation of estuaries as they undergo change due to climate change, particularly in regulated river systems (Hughes 2003; Bates et al. 2008). Conservation and management of estuarine fish species is particularly important as many species of environmental, social and economic significance, including important recreationally and commercially targeted species, utilise estuaries at some stage during their life cycle.
Two fish species of temperate Australian estuaries are the black bream, Acanthopagrus butcheri (Sparidae) and estuary perch, Macquaria colonorum (Percichthyidae). Although the ecology of black bream has been well studied (Newton 1996; Hindell 2007; Hindell et al. 2008; Sakabe et al. 2011), very little is known about the ecology of estuary perch (McCarraher and McKenzie 1986; Walsh et al. 2010; Walsh et al. 2013), particularly in relation to movement patterns. Black bream is an estuarine resident species, completing its entire life cycle within an estuary (Newton 1996; Potter and Hyndes 1999). They can tolerate a wide range of salinities but are commonly found in salinities of 15–25. In contrast, estuary perch is a euryhaline species and along with Australian bass, Macquaria novemaculeata are the only two known catadromous percichthyids worldwide. Both species share similar morphology, habitat and geographical distribution (Harris 1986; Jerry et al. 1999; Trnski et al. 2005). There is a degree of spatial overlap between the two species and it is known that hybridisation between the species can occur (Jerry et al. 1999).
Black bream have been recorded moving large distances up and down estuaries in excess of 1000 km in cumulative distance in a year (Hindell 2007; Hindell et al. 2008; Sakabe and Lyle 2010). In the case of the Gippsland Lakes, south-eastern Australia, a large temperate estuarine-marine lake system, black bream have been recorded moving throughout the entire system (lakes and tributary estuaries) (Butcher and Ling 1958, 1962; Ling 1958). Recent evidence suggests that when freshwater flows are reduced, black bream move into the tributary estuaries prior to the spawning season and remain within the tributary estuary until post-spawning (Hindell et al. 2008). Environmental factors have a significant influence on the movement of black bream during the spawning season, in spring and early summer, as they seek preferred salinities and water temperatures (Butcher 1945; Sherwood and Backhouse 1982; Haddy and Pankhurst 1998; Sarre and Potter 1999; Nicholson et al. 2008; Sakabe et al. 2011; Williams et al. 2012).
Relatively little is known about the movement and habitat utilisation of estuary perch. Adult fish have been caught in freshwater through to coastal waters, but are more commonly found in brackish estuarine water (McCarraher and McKenzie 1986). Adult fish are thought to make multiple downstream migrations throughout the year to spawn in the middle to lower sections of an estuary. Furthermore, it is hypothesised that increases in freshwater flows and displacement of the salt-wedge estuary trigger these spawning migrations (Harris 1986). Spawning occurs during the winter months from July to August in New South Wales, eastern Australia, at latitudes of 34–36°S and from July to November in western Victoria, south-eastern Australia, at latitude of 38°S (McCarraher and McKenzie 1986; Newton 1996). Eggs and larvae of estuary perch have also been found in coastal waters (McCarraher and McKenzie 1986).
Given our current knowledge of the ecology and biology of black bream and estuary perch, we hypothesised that freshwater flows are an important factor in influencing the location of fish within an estuary, particularly during the spawning season. Therefore, the aim of this study was to use acoustic telemetry to determine the location and movements of black bream and estuary perch over a 16-month period in the Mitchell and Tambo estuaries within the Gippsland Lakes. This study focussed on these two estuaries as previous research has indicated they encompass important spawning habitat for black bream (Williams et al. 2012, 2013), and we have previous knowledge that black bream move throughout the lakes during non-spawning periods (Hindell et al. 2008). The upper estuaries also provide habitat for the more resident estuary perch (Harris 1986).
The passive acoustic telemetry technology used in this study can establish approximate locations of tagged fished for up to 16 months, if individuals remain within the array of receivers. In the present study, acoustic receivers were used to determine the locations and fine-scale movement patterns (<2 km) of both fish species in relation to changes in freshwater flows in the two estuaries. To incorporate broad temporal patterns, this study investigates seasonal and monthly locations of tag fish in relation to distance from the estuary mouth. Moon phase was also included in the analyse as we hypothesised that fish would move more or less during a full moon when compared to the new moon.
Materials and methods
Study sites
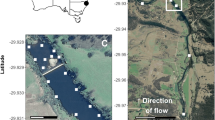
This study was conducted from August 2008 through to December 2009 in the estuarine reaches of the Mitchell and Tambo Rivers, two major rivers that flow into the Gippsland Lakes in south-eastern Australia (Fig. 1). The Mitchell River is classified as the longest unregulated river in the state of Victoria with a catchment size of 4778 km2. The estuary section is long (~25 km), narrow and slightly winding and has a manmade rock barrier at ~25 km upstream that restricts further movement of saline water upstream and potentially acts as a barrier to fish movement (Fig. 1). The estuary has an average depth of ~3 m with a deeper 8 m pool of water between 12 and 14 km upstream. The Tambo River catchment is smaller (2867 km2) and has a slightly shorter estuary (15 km in length). The estuary has an average depth of 2–3 m but contains a deeper pool of water ~14 km upstream. A constantly changing natural sand barrier prevents further saline intrusion upstream (Fig. 1). Though this study focuses on the Mitchell and Tambo Estuaries, we included data loggers at the mouth of the Nicholson River to detect any movements into this neighbouring estuary (Fig. 1). The Nicholson River is significantly smaller with a catchment of only 530 km2 and an average depth of 2–3 m. All three estuary mouths are within close proximity and flow into Lake King (Fig. 1). The Gippsland Lakes are Australia’s largest estuarine-marine lagoon system and cover approximately 340 km2 (Fig. 1). The lakes consist of three large shallow coastal lagoons 69 km long and 10 km at the widest point. The entire system is connected to the ocean through a single, narrow, permanently open entrance to the far east of the system. The lake system is recognised under the Ramsar Convention as a site of international importance, supporting rare, endangered and threatened species of flora and fauna.
Map of the study site showing the location of individual acoustic receivers (●) that were placed in the extent Mitchell Estuary (labelled M1 upstream to M12) and Tambo Estuary (labelled T1 upstream to T9) estuaries and at the mouth of the Nicholson Estuary (labelled N1 upstream to N2). M12 marks the upper estuarine extent in the Mitchell River and T9 marks the upper estuarine extent in the Tambo River
Rainfall and freshwater flow in the Gippsland Lakes has been reduced considerably since 2000 due to an extended period of drought affecting south-eastern Australia. Average annual daily discharge in 2008 for the Mitchell River had decreased by 40% from the long-term average (Since August 1937; 17.43 m3.s−1 compared to 28.62 m3.s−1; www.vicwaterdata.net) and in the Tambo River had decreased by 56% (3.98 m3.s−1compared to 8.94 m3.s−1; www.vicwaterdata.net). Monitoring stations within the lakes have recorded an increase in salinities from 20 to 30 and there has been further movement of the salt-wedge inland along the major tributaries, including the Mitchell and Tambo Estuary (unpublished data, Environmental Protection Agency).
Setup of acoustic receiver array
Acoustic receivers (Vemco, VR2) were placed at approximately 2 km intervals with 12 receivers in the Mitchell (M1 – M12) and 9 receivers in the Tambo Estuary (T1 – T9; Fig. 1). Two receivers were placed at the mouth of the Nicholson Estuary (N1 – N2) to detect movements in and out of this neighbouring estuary (Fig. 1). The receivers were strategically placed to maximise detection ranges by attaching them to solid structures such as pier pylons, woody debris and navigational markers at approximately 1–2 m depth on straight sections of the rivers. These estuaries are narrower than the detection range of the receivers and therefore a receiver acts as a detection gate. The receivers work by detecting and recording information from ultrasonic signals (69 kHz) emitted by acoustic transmitters implanted in fish. Range testing within this system, and reported in other studies, found that the detection range for transmitters was up to 400 m despite the effects of possible interferences from wind and turbulence (Hindell 2007). Therefore, if a fish is within 400 m of the receiver a ‘detection’ will be recorded each time a tag transmits. Due to the 2 km spacing there were small 200 m gaps in detectability of fish between receivers.
Receivers were deployed from July 2008 until December 2009 to allow sampling over two spawning seasons as the tags used have a lifespan of 16 months. Each receiver was downloaded in the field on three occasions, late February 2009, July 2009 and December 2009. The final download took place after the life expectancy of the tags had expired. Receivers at M4 and M5 were not initialised until 23 October 2008. Receiver and battery failure led to several gaps in the data set at T2 from 4 March until 30 July 2009 and M7 from 4 November 2009 until the end of the study. The receiver at T4 had failed completely until replaced with another receiver on 29 July 2009 (Fig.1).
Fish tagging
Fish were caught using either otter trawl, seine or mesh nets of various mesh sizes. Tagging of fish occurred from late July to early September 2008 prior to the spawning season, which is between September and December (Williams et al. 2012). The justification for tagging fish so close to the start of the spawning season was to allow for the transmitters, with a battery life of up to 450 days, to be active over two spawning seasons. Fish were predominately collected from the upper estuary, in the vicinity of deeper pools, as this is where large numbers of fish occurred and could easily be caught and released. Previous research indicates that black bream are highly mobile and collecting fish from only a couple of sites would not bias the fish in regards to size, sex or reproductive state. Only fish larger than 150 mm were tagged, as we were particularly interested in movements of mature fish. Fish that were to be tagged were immediately removed from the nets and placed in a 0.75 m3 holding pen until surgery. Fish were individually anaesthetised to stage III sedation (Ross and Ross 1999) with Benzocaine (2 g in 10 L estuarine water). Once fish were sedated, fork length and weight were recorded. A small incision was made 2–3 cm off-centre ventral into the peritoneal cavity and a single, individually coded, acoustic transmitter (VEMCO V9-2 L coded with a random signal delay of 50–130 s; 9 × 21 mm in size and 1.6 g in weight) was inserted. The incision was closed with 2–3 simple sutures (Braided Polyglycolic Acid Suture, 3/8 circle, USP 3/0). Post-surgery the wounds on the fish were swabbed with antiseptic and then the fish was placed into a holding pen to monitor recovery. Once fish had recovered and were able to maintain balance they were released at the site of capture. Total time of surgery, including time in holding pens, varied from 5 to 15 min.
Environmental data
Freshwater flow (discharge in m3.s−1) is recorded daily for each river and stored at the Victorian Water Resources Data Warehouse (www.vicwaterdata.net). Daily freshwater flow data were extracted from this database.
Moon phase, sunrise and sunset data were extracted from www.timeanddate.com. Day length was calculated by subtracting the sunrise time from the sunset time to give the length of daylight hours. The percentage of moon exposed was used as the moon phase.
Data analysis
Descriptive data analysis was used to characterise the spatial and temporal patterns of movement for tagged black bream and estuary perch. Minimum distance travelled was calculated for individual fish by adding together the distance between receivers that the fish was detected. Detection days were calculated as the number of days individual fish were detected and the detection period was calculated as the number of days between first and last detection. A detection index was calculated by dividing the detection period by detection days. The detection index gives the percentage of days that each fish was detected within its detection period.
A Bayesian state-space model was used to estimate species-specific detection probabilities at individual receivers, movement rates for each species and locations between detection events (refer Appendix 2 Tracey et al. (2011) for model description). Uninformative priors were used for each of the parameters. For this analysis each estuary were divided into 1 km segments and time was considered in ten-minute intervals. Consequently the movement rates estimated are in the natural units of the model, which give the probability of a fish moving from its current location to an adjacent segment in the next ten-minute interval. Similarly the detection rates are the probability of a fish being detected by the receiver (if one is present) in its current location in a ten-minute interval.
The relationship between season, freshwater flows, moon phase and fish location in the estuary was explored using generalised linear models (GLM). The daily median fish location (as estimated by the state space model) was regressed against season (categorical), freshwater flows, moon phase, with coefficients estimated separately for each species and estuary. The GLM allowed the individual factors influencing fish location to be considered separately. To consider seasonal signals in more detail a separate GLM was conducted with month (categorical) instead of season. Using these models we tested the hypotheses that; a) the median location of fish within each estuary is different between seasons, b) increasing freshwater flow changes the location of fish within each estuary; and c) the location of fish changes with moon phase. Analyses were conducted in R Project Software (R: A Language and Environment for Statistical Computing 2010).
Results
Environmental context
During this study freshwater flows in both the Mitchell and Tambo estuaries were well below the long-term averages with the exception of a few flow events that lasted no longer than a month and occurred between September and January in both years (Fig. 2). The lowest flows occurred between February and July (Fig. 2). Freshwater flows in the Mitchell Estuary varied from 0.12 to 127.92 m3·s−1 and in the Tambo estuary between 0.29 to 215.90 m3·s−1 (Fig. 2).
Spatio-temporal patterns in black bream movement
During this study 19 black bream were tagged in the Mitchell Estuary and 22 in the Tambo Estuary over two tagging sessions approximately five weeks apart (Table 1). Fish fork lengths ranged from 220 to 390 mm FL with a mean size of 262 mm and 257 mm for the Mitchell and Tambo Estuary’s respectively (Table 1). All fish were detected post release and the total detection period ranged from 4 to 454 days with all but two fish being detected at more than two receivers. In total the receiver array made 739,264 detections for black bream throughout this study, with 294,686 detections in the Mitchell Estuary, 437,003 detections in the Tambo Estuary and 7575 detections at the mouth of the Nicholson Estuary. The receiver 10 km from the estuary mouth (M6) received the highest number of detections in the Mitchell Estuary and the receiver 16 km from the estuary mouth (T9) received the highest number of detections in the Tambo Estuary.
The mean daily distance travelled by black bream was estimated to be 1.7 km·d−1 and 2.1 km·d−1 in the Mitchell and Tambo Estuary, respectively, with some fish covering over 1000 km·y−1 (Table 1). In general, black bream were highly mobile, regularly moving up and down each estuary and on occasions into Lake King (Fig. 3). At least 34 (83%) tagged black bream moved into the Lake King during this study and at least 12 (30%) tagged black bream moved between estuaries. Black bream displayed strong estuary fidelity with the majority of fish returning to the estuary in which they were tagged. Despite being highly mobile, tagged fish tended to return regularly and spend considerable time (>12 h) within the area they were tagged.
The detection profiles provide a visual snapshot of the number of detections (logged) across all sites (Fig. 3). The number of detections across sites was highly variable for black bream with some degree of seasonality present (Fig. 3). Based on the number of detections, fish appear to be moving to the upper estuary between June and November (Fig. 3). Between November and the following June there are several noticeable up- and down- stream migrations of fish with more detections occurring at the estuary mouth (M1 and T1).
The median location of tagged fish was calculated using both raw and modelled data that were de-trended for the significant effects of freshwater flow and moon phase. Plotting the median locations of fish showed a very similar temporal pattern in both estuaries with the median location of fish between December and April being in the lower half of the estuaries (0–7 km from the estuary mouth), while from May to November the median location of fish was in the upper half of the estuaries (>7 km from the estuary mouth; Fig. 4).
The monthly median (+/− SE) location of tagged black bream (n = 42) in the Mitchell (solid red lines) and Tambo estuaries (dashed green line). Locations are de-trended for the effects of freshwater flow and moon phase and are shown for median flow and 50% moon phase. (Refer to section: Predictions of Fish Movements)
Spatio-temporal patterns in estuary perch movement
During this study eight estuary perch were tagged in the Mitchell Estuary and 17 in the Tambo Estuary over two tagging sessions approximately five weeks apart (Table 2). Fish fork length ranged from 200 to 305 mm with a mean length of 241 mm and 278 for the Mitchell and Tambo Estuary, respectively (Table 2). All fish were detected post release with the exception of two fish that were presumed not to have survived the first month post-release. The detection period ranged from 35 to 450 days. The receiver array made 570,006 detections for estuary perch throughout this study, with 285,523 detections in the Mitchell Estuary, 284,483 detections in the Tambo Estuary and no detections at the mouth of the Nicholson Estuary. The receiver 14 km upstream from the estuary mouth (M8) received the most number of detections in the Mitchell Estuary and the receiver 16 km upstream from the estuary mouth (T9) received the most number of detections in the Tambo Estuary.
The mean daily distance travelled by tagged estuary perch was estimated to be 0.5 km·d−1 and 1.8 km·d−1 in the Mitchell and Tambo Estuary, respectively, with some fish moving over 200 km·y−1 (Table 2). At least 15 (60%) tagged estuary perch moved to the lakes during this study and no tagged fish visited or moved to another estuary.
The detection profiles provide a visual snapshot of the number of detections (logged) for estuary perch across all sites (Fig. 5). The profiles demonstrate that there was a high degree of site fidelity for estuary perch (Fig. 5). Fish spent extended periods of time within the vicinity of where they were caught, tagged and released (Fig. 5). Fish in the Mitchell Estuary moved considerably less than fish in the Tambo Estuary (Fig. 5). In the Mitchell Estuary, fish made occasional short-distance movements upstream or downstream over short periods of time. In the Tambo Estuary fish made more regular and longer trips downstream throughout the study.
The median location of tagged fish was calculated using both raw and modelled data that were de-trended for the significant effects of flow and moon phase. Plotting the median location of fish showed that estuary perch were located further upstream than black bream (Fig. 6). In the Mitchell Estuary, fish remained in the upper estuary (>10 km from the estuary mouth) throughout this study (Fig. 6). In comparison, estuary perch in the Tambo Estuary occurred in the upper estuary (>10 km from the estuary mouth) with the exception of July, December and January where the median location of fish was in the lower estuary (<10 km form the estuary mouth; Fig. 6).
The monthly median (+/− SE) location of tagged estuary perch (n = 25) in the Mitchell (solid red lines) and Tambo Rivers (dashed green lines). Data are from both raw and modelled data that have been de-trended for the effects of freshwater flow and moon phase. Locations are de-trended for the effects of freshwater flow and moon phase and are shown for median flow and 50% moon phase
State-space model of movement and detection predictions
The Bayesian state-space model suggests movement rates for black bream were similar in both the Mitchell and Tambo Estuary (Fig. 7a). Estuary perch had a lower movement rate than black bream, particularly in the Mitchell Estuary (Fig. 7b).
Movement rates for (a) black bream and (b) estuary perch as predicted by the state-space model (solid red line = Mitchell Estuary, dashed green line = Tambo Estuary). The y-axis gives the probability density at the movement rates shown on the x-axis. Movement rates (indicated on the x axis) are the probability of moving into an adjacent 1 km region of the river in each ten-minute time step. The height and spread of the curves show how certain the model is about the detection probability for each species in each river
The Bayesian state-space model also showed detection rates were high, typically around 40% per ten minute interval, with a single receiver having a lower detection rate of 20% for both in species in both estuaries (Figs. S1 and S2).
Predictions of fish movements
Results from the GLM support a temporal pattern of movement that is more significant for black bream when compared to estuary perch (Fig. 8a). Interestingly, the temporal patterns for black bream were similar for both estuaries with the median location of tagged fish occurring higher upstream in winter and spring compared to the median location of fish in autumn and further downstream in summer when compared with autumn (Fig. 8a). The median location of estuary perch in the Mitchell Estuary did not display any temporal variability. The median location of estuary perch in the Tambo Estuary occurred significantly further downstream in summer when compared with the median location of fish in autumn.
Plots of the model estimates (+/− SE) for the GLM coefficients for both black bream (n = 41) and estuary perch (n = 25). Coefficients are for (a) season, relative to autumn, (b) freshwater flows (c) moon phase. Negative numbers refer to a downstream change and a positive numbers refer to an upstream change in location and are in km. For example in winter, Black beam in the Mitchell River are expected to be 3.8 km further upstream than in autumn
The GLM also suggests that there were significant effects of freshwater flow on the median location of black bream and estuary perch in both estuaries with the median location of tagged fish moving downstream when flow rates were above the median flow rate detected during this study (Fig. 8b). The influence of freshwater flows and a downstream shift in the median location of black bream and estuary perch was greater in the Mitchell Estuary compared to the Tambo Estuary (Fig. 8b).
The GLM also supported a significant effect of moon phase (Fig. 8c). Black bream were located further upstream during a full moon when compared to their location during a new moon in both the Mitchell and Tambo Estuary (Fig. 8c). Estuary perch in the Mitchell Estuary were located further upstream during a full moon compared to their location during a new moon. There was no effect of moon phase for estuary perch in the Tambo Estuary (Fig. 8c).
Discussion
An acoustic telemetry approach was used to monitor the movements of the well-studied black bream Acanthopagrus butcheri (Butcher 1945; Morrison et al. 1998; Sarre and Potter 1999; Walker and Neira 2001; Jenkins et al. 2010; Williams et al. 2013) and the poorly understood estuary perch Macquaria colonorum (McCarraher and McKenzie 1986; Walsh et al. 2013) during a period of below average freshwater flows. The movements observed for black bream were very similar to that of previous studies, with defined seasonal movements that reflect their reproductive ecology (Hindell et al. 2008; Sakabe and Lyle 2010). Overall, black bream were a more itinerant species covering several kilometres of estuary within a single day. This reflects the transient grazing behaviour and it is likely that fish are searching for prey such as barnacles, mussels, and crabs (Chuwen et al. 2007; Sarre 1999) that are more abundant in the lower estuary and the lakes (J. Williams pers. obs.). In comparison, estuary perch were highly site attached and remained in the upper estuary for the majority of the year. Both species moved large distances (>5 km) downstream in response to increased pulses of freshwater flows (>58 m3·s−1 in the Mitchell Estuary and >11 m3·s−1 in the Tambo Estuary). The results from this study highlight that estuarine fishes are likely to show differential sensitivity to the impacts of drought and climate change and illustrate how acoustic telemetry methods can be used to determine the environmental needs of fishes (Gillanders and Kingsford 2002; Barletta et al. 2010; Jenkins et al. 2015; Izzo et al. 2016) and help guide efforts to conserve and manage rivers and estuaries worldwide.
All tagged black bream were considered mature, based on the length of fish at the time of tagging, allowing us to relate seasonal movements to spawning strategies. Black bream aggregated in the upper estuary in winter and throughout the spring spawning period, consistent with previous research (Newton 1996; Hindell et al. 2008; Sakabe and Lyle 2010). Black bream often spawn in the upper estuary at the freshwater interface, an area of the estuary that becomes highly stratified during spring (Newton 1996; Nicholson et al. 2008; Williams et al. 2012; Williams et al. 2013). This study corroborates the findings of Williams et al. (2013), who demonstrated a close spatial coupling between the eggs, larvae and the halocline at 9–21 km upstream from the estuary mouth, as tagged fish were present at the same location when this occurred. By early summer, black bream had moved downstream and were residing in the lower estuary; several tagged fish had even left the estuary and were most likely residing in Lake King. Hindell et al. (2008) recorded the same broad scale movements of fish residing in the lakes during summer and the river estuaries during winter and spring.
There is little knowledge on the movement and spawning ecology of estuary perch and there is much contradiction in the literature (Harris 1986; McCarraher and McKenzie 1986; Trnski et al. 2005; Walsh et al. 2013). The current study supports the theory that estuary perch reside in the upper estuary at the freshwater interface throughout the year, with downstream spawning migrations triggered by increased freshwater flows (Walsh et al. 2013). Like the estuary perch, both tupong, Pseudaphritis urvillii and mulloway, Argyrosomus japonicas used pulse freshwater flows as triggers to migrate downstream to the estuary mouth and/or ocean to spawn (Crook et al. 2010; Taylor et al. 2014). In this instance the ‘home’ of tagged estuary perch in both estuaries occurred within the vicinity of the deepest pool of the upper estuary (>4 m) towards the toe of the salt-wedge. Estuary perch are a nocturnal ambush predator feeding on shrimp, crabs and other fish (McCarraher and McKenzie 1986) and therefore the steeper banks of the pools are likely to provide optimal shelter and prey. However, estuary perch tagged in the Tambo Estuary were far more active, moving larger distances, than fish in the Mitchell Estuary. These differences between estuaries could have been, at least in part, due to fish being in poorer condition, as while tagging the estuary perch, we noted that fish in the Mitchell Estuary were thinner and had large parasites. Why this might be the case is unknown and beyond the scope of this study.
Striped bass, Morone saxatilis and white perch, Morone americana are two very well studied species that reside and migrate between the tidal freshwater region and the brackish waters of their natal river (Secor 1999; Wingate and Secor 2007; Kerr et al. 2009). A combination of acoustic telemetry and otolith microchemistry studies has revealed that both species exhibit partial or divergent migration behaviours, wherein one portion of the population migrates and the other is sedentary (Secor 1999; Secor et al. 2001; Wingate and Secor 2007; Kerr et al. 2009). Partial migration is often associated with avian ecology and is a fundamental behaviour associated with the evolution of migration (Kerr et al. 2009, Gillanders et al. 2015). Wingate and Secor (2007) and Kerr et al. (2009) both suggest that partial migration could be widespread among other fish species and that it has important implications for population dynamics. Gillanders et al. (2015) found evidence of both resident and migratory contingents in the black bream population of the Murray River estuary and went on to suggest that there was variation in the growth and fitness between the two proportions of the population. While this study did not test for intra-specific variability, it is highly plausible that there are resident and migratory proportions to the population of black bream in the Gippsland Lakes. In comparison the population of tagged estuary perch showed evidence of being solely resident. However, for both species, a significant pulse of freshwater flow was an environmental cue or a physical driver of population scale migration downstream.
Freshwater flow was a significant factor influencing the movement and location of tagged fish in this study. Freshwater flows account for much of the biotic and abiotic variability within estuaries (see Gillanders and Kingsford 2002; Barletta et al. 2005; Barletta et al. 2008; Gillson 2011; Jenkins et al. 2015 for reviews), particularly in microtidal systems such as the Gippsland Lakes. Physicochemical variability can be an important driver of the life histories of estuarine species (Kerr et al. 2009; Dantas et al. 2012). One example of this is salinity, which is widely recognised as being a key factor influencing movement and spawning of fish in estuaries (Marshall and Elliott 1998; Barletta et al. 2005; Selleslagh and Amara 2008). In this instance, the Mitchall and Tambo estuaries form as highly stratified salt-wedge estuaries for most of the year (Kurup et al. 1998; Williams et al. 2013). It is only during significantly high freshwater flow periods that the salt-wedge is flushed out of the estuary. This occurred twice during this study and it is the timing and size of these events that could have a significant impact on the ecology of these two species.
Black bream move upstream in winter to spawn in spring. If a large flow event occurred mid-spawning season, this would result in fish moving out of their preferred spawning habitat and the likely flushing of eggs and larvae out of the upper estuary, which is the most important nursery habitat for black bream larvae (Sakabe et al. 2011; Williams et al. 2013). In contrast, estuary perch rely on these flows to trigger spawning migrations. In south-eastern Australia freshwater flows follow a temperate seasonal cycle; however, there has been a recent shift in the timing and decreasing annual freshwater flows with increased storm events (Poloczanska et al. 2007). If climate change results in fewer freshwater flow events and an increase in intensity when they do occur, this suggests that the reproductive success of estuary perch is also likely to be compromised. This highlights the need to take a multi-species approach to better understand how fish move in response to natural and anthropogenic environmental change and their likely population-level consequences. This is of great importance as climate change predictions suggest further decreases in freshwater flows with more severe storm events across large parts of the world (Hughes 2003; Pittock 2003; Bates et al. 2008).
Temperature, turbidity and dissolved oxygen can also influence the movement and location of fish within an estuary (Marshall and Elliott 1998; Barletta et al. 2008; Dantas et al. 2010). Although high-resolution data on these variables were not available for comparison to the movement data, it is likely that seasonal rise in temperature acts as a cue for black bream to move upstream to their preferred spawning habitat similar to the migration patterns of freshwater roach Rutilus rutilus (Skov et al. 2010). In microtidal estuaries, turbidity and dissolved oxygen are often regulated by freshwater flows. Periods of decreased flow can result in increased areas of hypoxia, particularly in the upper estuary where black bream and estuary perch often reside (Newton 1996; Nicholson et al. 2008). All of these water quality parameters will also be influenced by the changing climate as a result of decreased flows and this will lead to alterations in movement behaviour and spawning success.
Moon phase, had a significant effect on the location of tagged fish with fish occurring further upstream on a full moon when compared to the new moon. We hypothesise both species are more active during the full moon cycle with increased visibility allowing for feeding and spawning. Sakabe and Lyle (2010) demonstrated a link between tidal cycles and the movements of black bream, which is fairly common for other species such as spotted grunter Pamadasys cammersonnii (Childs et al. 2008). However, the Gippsland Lakes are a microtidal system and with the river estuaries being tributaries to an estuarine lake it is unlikely that tidal cycles alone influence the movements of black bream in this system.
In conclusion, the use of acoustic tag technology was beneficial in monitoring the movements of two species of fish and how they differ in response to dynamic environmental variables. Freshwater flows are the dominant factor not only in determining the physiochemical structure of the estuary but also the location of fish within an estuary. This study has highlighted stark differences in fish movement within an estuary and the need for a multi-species approach to understand the impacts of drought, flood and changing climate. These results in combination with the findings of Jenkins et al. (2015) suggest that black bream, being more mobile, would be more adaptable in regards to changes in freshwater flows due to climate change altering the physical structure of the estuary. Whereas, estuary perch are likely to be less resilient to changes in freshwater flows that will alter the physical structure of the sites of residency and remove potential spawning ques. However, there is a need for longer term studies (>2 years) to better elucidate the finer details on what is driving specific movements and how the population dynamics will change with further reductions in freshwater flow and increased flooding events.
References
Arendt MD, Lucy JA, Evans DA (2001) Diel and seasonal activity patterns of adult tautog, Tautoga onitis, in lower Chesapeake Bay, inferred from ultrasonic telemetry. Environ Biol Fish 62:379–391. doi:10.1023/A:1012266214143
Barletta M, Barletta-Bergan A, Saint-Paul U, Hubold G (2005) The role of salinity in structuring the fish assemblages in a tropical estuary. J Fish Biol 66:45–72. doi:10.1111/j.0022-1112.2005.00582.x
Barletta M, Amaral CS, Corrêa MFM, Guebert F, Dantas DV, Lorenzi L, Saint-Paul U (2008) Factors affecting seasonal variations in demersal fish assemblages at an ecocline in a tropical–subtropical estuary. J Fish Biol 73:1314–1336. doi:10.1111/j.1095-8649.2008.02005.x
Barletta M, Jaureguizar AJ, Baigun C, Fontoura NF, Agostinho AA, Almeida-Val VMF, Val AL, Torres RA, Jimenes-Segura LF, Giarrizzo T, Fabré NN, Batista VS, Lasso C, Taphorn DC, Costa MF, Chaves PT, Vieira JP, Corrêa MFM (2010) Fish and aquatic habitat conservation in South America: a continental overview with emphasis on neotropical systems. J Fish Biol 76:2118–2176. doi:10.1111/j.1095-8649.2010.02684.x
Bates BC, Kundzewicz ZW, Wu S, Palitikof JP (eds) (2008) Climate change and water: technical paper of the intergovernmental panel on climate change. IPCC Secreteriat, Geneva, p 210
Bendell B, Moore A, Maxwell D, Davison P, Edmonds N (2011) Modelling the migratory behaviour of salmonids in relation to environmental and physiological parameters using telemetry data. Fish Manag Ecol 19:475–483. doi:10.1111/j.1365-2400.2011.00811.x
Blaber SJM, Blaber TG (1980) Factors affecting the distribution of juvenile estuarine and inshore fish. J Fish Biol 17:143–162. doi:10.1111/j.1095-8649.1980.tb02749.x
Butcher AD (1945) Conservation of the bream fishery, fisheries pamphlet no. 1. Fisheries and game Department, Victoria
Butcher AD, Ling JK (1958) Bream taggin experiments in east Gippsland during April and may 1944. Report no. 17, fisheries wildlife Department, Victorian state government, Melbourne
Butcher AD, Ling JK (1962) Bream tagging experiments in east Gippsland during April and may 1944. Fisheries contribution no. 11. Fisheries and wildlife Department, Victoria
Childs AR, Cowley PD, Næsje TF, Booth AJ, Potts WM, Thorstad EB, Økland F (2008) Do environmental factors influence the movement of estuarine fish? A case study using acoustic telemetry. Estuar Coast Shelf Sci 78:227–236. doi:10.1016/j.ecss.2007.12.003
Chuwen B, Platell M, Potter IC (2007) Dietary compositions of the sparid Acanthopagrus butcheri in three normally closed and variably hypersaline estuaries differ markedly. Environ Biol Fish 80:363–376. doi:10.1007/s10641-006-9134-0
Crook D, Koster W, Macdonald J, Nicol S, Belcher C, Dawson D (2010) Catadromous migrations by female tupong (Pseudaphritis urvillii) in coastal streams in Victoria, Australia. Mar Freshw Res 61:474–483. doi:10.1071/MF09170
Dantas DV, Barletta M, Costa MF, Barbosa-Cintra SCT, Possatto FE, Ramos JAA, Lima ARA, Saint-Paul U (2010) Movement patterns of catfishes (Ariidae) in a tropical semi-arid estuary. J Fish Biol 76:2540–2557. doi:10.1111/j.1095-8649.2010.02646.x
Dantas DV, Barletta M, Lima ARA, Ramos JAA, da Costa MF, Saint-Paul U (2012) Nursery habitat shifts in an estuarine ecosystem: patterns of use by sympatric catfish species. Estuar Coast 35:587–602. doi:10.1007/s12237-011-9452-0
Gillanders BM, Kingsford MJ (2002) Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanogr Mar Biol Annu Rev 40:233–309. doi:10.1201/9780203180594.ch5
Gillanders BM, Izzo C, Doubleday ZA, Ye Q (2015) Partial migration: growth varies between resident and migratory fish. Biol Lett 11:20140850. doi:10.1098/rsbl.2014.0850
Gillson J (2011) Freshwater flow and fisheries production in estuarine and coastal systems: where a drop of rain is not lost? Rev Fish Sci 19:168–186. doi:10.1080/10641262.2011.560690
Haddy JA, Pankhurst NW (1998) Annual change in reproductive condition and plasma concentrations of sex steroids in black bream, Acanthopagrus butcheri (Munro) (Sparidae). Mar Freshw Res 49:389–397. doi:10.1071/MF97239
Harris J (1986) Reproduction of the Australian bass, Macquaria novemaculeata (Perciformes : Percichthyidae) in the Sydney Basin. Mar Freshw Res 37:209–235. doi:10.1071/MF9860209
Heupel MR, Semmens JM, Hobday AJ (2006) Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Mar Freshw Res 57:1–13. doi:10.1071/MF05091
Hindell JS (2007) Determining patterns of use by black bream Acanthopagrus butcheri (Munro, 1949) of re-established habitat in a south-eastern Australian estuary. J Fish Biol 71:1331–1346. doi:10.1111/j.1095-8649.2007.01594.x
Hindell JS, Jenkins GP, Womersley B (2008) Habitat utilisation and movement of black bream Acanthopagrus butcheri (Sparidae) in an Australian estuary. Mar Ecol Prog Ser 366:219–229. doi:10.3354/meps07519
Hughes L (2003) Climate change and Australia: trends, projections and impacts. Austral Ecol 28:423–443. doi:10.1046/j.1442-9993.2003.01300.x
Izzo C, Doubleday ZA, Grammer GL, Gilmore KL, Alleway HK, Barnes TC, Disspain MCF, Giraldo AJ, Mazloumi N, Gillanders BM (2016) Fish as proxies of ecological and environmental change. Rev Fish Biol Fisheries 26:265. doi:10.1007/s11160-016-9424-3
Jenkins GP, Conron S, Morison AK (2010) Highly variable recruitment in an estuarine fish is determined by salinity stratificaiton and freshwater flows: implications of a changing climate. Mar Ecol Prog Ser 417:249–261. doi:10.3354/meps08806
Jenkins GP, Spooner D, Conron S, Morrongiello JR (2015) Differing importance of salinity stratification and freshwater flow for the recruitment of apex species of estuarine fish. Mar Ecol Prog Ser 523:125–144. doi:10.3354/meps11147
Jerry DR, Raadik TA, Cairns SC, Baverstock PR (1999) Evidence for natural interspecific hybridization between the Australian bass (Macquaria novemaculeata) and estuary perch (M. colonorum). Mar Freshw Res 50:661–666. doi:10.1071/MF98120
Jonsen ID, Myers RA, Flemming JM (2003) Meta-analysis of animal movement using state-space models. Ecology 84:3055–3063. doi:10.1890/02-0670
Kerr LA, Secor DH, Piccoli PM (2009) Partial migration of fishes as exemplified by the estuarine-dependent white perch. Fisheries 34:114–123. doi:10.1577/1548-8446-34.3.114
Kurup GR, Hamilton DP, Patterson JC (1998) Modelling the effect of seasonal flow variations on the position of salt wedge in a microtidal estuary. Estuar Coast Shelf Sci 47:191–208. doi:10.1006/ecss.1998.0346
Ling, JK (1958) The status of the East Gippsland bream fishery, Fisheries Contribution No. 8. Fisheries and Gaem Department
Lucas MC, Baras E (2000) Methods for studying spatial behaviour of freshwater fishes in the natural environment. Fish Fish 1:283–316. doi:10.1046/j.1467-2979.2000.00028.x
Marshall S, Elliott M (1998) Environmental influences on the fish assemblage of the Humber estuary, UK. Estuar Coast Shelf Sci 46:175–184. doi:10.1006/ecss.1997.0268
McCarraher DB, McKenzie JA (1986) Observations on the distribution, growth, spawning and diet of estuary perch (Macquaria colonorum) in Victorian waters. Technicial Report Series No. 42. Arthur Rylah Institute for Environmental Research.
Morison AK, Coutin PC, Robertson SG (1998) Age determination of black bream, Acanthopagrus butcheri (Sparidae), from the Gippsland Lakes of south-eastern Australia indicates slow growth and episodic recruitment. Mar Freshw Res 49:491–498. doi:10.1071/MF97237
Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE (2008) A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci 105:19052–19059. doi:10.1073/pnas.0800375105
Newton GM (1996) Estuarine ichthyoplankton ecology in relation to hydrology and zooplankton dynamics in a salt wedge estuary. Mar Freshw Res 47:99–111. doi:10.1071/MF9960099
Nicholson G, Jenkins GP, Sherwood J, Longmore A (2008) Physical environmental conditions, spawning and early-life stages of an estuarine fish: climate change implications for recruitment in intermittently open estuaries. Mar Freshw Res 59:735–749. doi:10.1071/MF07197
Patterson TA, Thomas L, Wilcox C, Ovaskainen O, Matthiopoulos J (2008) State-space models of individual animal movement. Trends Ecol Evolut 23:87–94. doi:10.1016/j.tree.2007.10.009
Peterson AT, Sánchez-Cordero V, Soberón J, Bartley J, Buddemeier RW, Navarro-Sigüenza AG (2001) Effects of global climate change on geographic distributions of Mexican Cracidae. Ecol Model 144:21–30. doi:10.1016/S0304-3800(01)00345-3
Pittock B (2003) Climate change science: Australia. In: Pittock B (ed) Climate change: an Australian guide to the science and potential impacts. The Australian Greenhouse Office, Canberra, pp 21–70
Poloczanska ES, Babcock RC, Butler A, Hobday AJ, Hoegh-Guldberg O, Kunz TJ, Matear R, Milton DA, Okey TA, Richardson AJ (2007) Climate change and Australian marine life. Oceanogr Mar Biol 45:407–478. doi:10.1201/9781420050943.ch8
Potter IC, Hyndes GA (1999) Characteristics of the ichthyofaunas of southwestern Australian estuaries, including comparisons with holarctic estuaries and estuaries elsewhere in temperate Australia: a review. Aust J Ecol 24:395–421
Ross L, Ross B (1999) Anaesthetic and sedative techniques for fish, 2nd edn. Blackwell Science, Oxford. doi:10.1002/9781444302264
Sakabe R, Lyle JM (2010) The influence of tidal cycles and freshwater inflow on the distribution and movement of an estuarine resident fish Acanthopagrus butcheri. J Fish Biol 77:643–660. doi:10.1111/j.1095-8649.2010.02703.x
Sakabe R, Lyle JM, Crawford CM (2011) The influence of freshwater inflows on spawning success and early growth of an estuarine resident fish species, Acanthopagrus butcheri. J Fish Biol 78:1529–1544. doi:10.1111/j.1095-8649.2011.02959.x
Sarre GA (1999) Age comparisons, growth rates, reproductive biology, and diets of the black bream Acanthopagrus butcheri in four estuaries and a coastal saline lake in south-western Australia, PhD thesis. Murdoch University
Sarre GA, Potter IC (1999) Comparisons between the reproductive biology of black bream, Acanthopagrus butcheri (Teleostei: Sparidae) in four estuaries with widely different characteristics. Int J Salt Lake Res 8:179–210. doi:10.1023/A:1009041525892
Secor DH (1999) Specifying divergent migrations in the concept of stock: the contingent hypothesis. Fish Res 43:13–34. doi:10.1016/S0165-7836(99)00064-8
Secor H, Rooker JR (2005) Connectivity in the life histories of fishes that use estuaries. Estuar Coast Shelf Sci 64:1–3. doi:10.1016/j.ecss.2005.02.001
Secor DH, Rooker JR, Zlokovitz E, Zdanowicz VS (2001) Identification of riverine, estuarine, and coastal contingents of Hudson River striped bass based upon otolith elemental fingerprints. Mar Ecol Prog Ser 211:245–253. doi:10.3354/meps211245
Selleslagh J, Amara R (2008) Environmental factors structuring fish composition and assemblages in a small macrotidal estuary (eastern English Channel). Estuar Coast Shelf Sci 79:507–517. doi:10.1016/j.ecss.2008.05.006
Sherwood JE, Backhouse GN (1982) Hydrodynamics of salt wedge estuaries- implications for successful spawning in black bream (Acanthopagrus butcheri). Warrnambool Institute of Advanced Educations, Faculty of Applied Science and Technology, Warrnambool, Victoria, Australia, p 13
Skov C, Aarestrup K, Baktoft H, Brodersen J, Brönmark C, Hansson L, Nielson EE, Nielson T, Nilsson PA (2010) Influences of environmental cues, migration history, and habitat familiarity on partial migration. Behav Ecol 21:1140–1146. doi:10.1093/beheco/arq121
Taylor MD, van der Meulen DE, Ives MC, Walsh CT, Reinfelds IV, Gray CA (2014) Shock, stress or signal? Implications of freshwater flows for a top-level estuarine predator. PLoS One 9:e95680. doi:10.1371/journal.pone.0095680
Tracey SR, Hartmann K, Forbes E, Semmens J, Lyle JM (2011) Understanding movement patterns of key recreational fish species in southeast Tasmania. Institute for Marine and Antarctic Studies, University of Tasmania. http://www.imas.utas.edu.au/__data/assets/pdf_file/0004/743242/AcousticTrackingForSubmission.pdf
Trnski T, Hay AC, Fielder DS (2005) Larval development of estuary perch (Macquaria colonorum) and Australian bass (M. novemaculeata) (Perciformes : Percichthyidae), and comments on their life history. Fish Bull 103:183–194
Walker S, Neira FJ (2001) Aspects of the reproduction biology and early life history of black bream, Acanthopagrus butcheri (Sparidae), in brackish lagoon system in southeastern Australia. Aqua 4:135–142
Walsh CT, Gray CA, West RJ, van der Meulen DE, Williams LFG (2010) Growth, episodic recruitment and age truncation in populations of a catadromous percichthyid, Macquaria colonorum. Mar Freshw Res 61:397–407. doi:10.1071/MF09080
Walsh CT, Reinfelds IV, Ives MC, Gray CA, West RJ, van der Meulen DE (2013) Environmental influences on the spatial ecology and spawning behaviour of an estuarine-resident fish, Macquaria colonorum. Estuar Coast Shelf Sci 118:60–71. doi:10.1016/j.ecss.2012.12.009
Whitfield A (1999) Ichthyofaunal assemblages in estuaries: a south African case study. Rev Fish Biol Fisher 9:151. doi:10.1023/A:1008994405375
Williams J, Hindell JS, Swearer SE, Jenkins GP (2012) Influence of freshwater flows on the distribution of eggs and larvae of black bream Acanthopagrus butcheri within a drought-affected estuary. J Fish Biol 80:2281–2301. doi:10.1111/j.1095-8649.2012.03283.x
Williams J, Jenkins GP, Hindell JS, Swearer SE (2013) Linking freshwater flows and salt-wedge dynamics with the distribution of black bream, Acanthopagrus butcheri, eggs larvae and potential prey in a drought affected estuary. Mar Ecol Prog Ser 483:273–287. doi:10.3354/meps10280
Wingate RL, Secor DH (2007) Intercept telemetry of the Hudson River striped bass resident contingent: migration and homing patterns. T Am Fish Soc 136:95–104. doi:10.1577/T06-056.1
Acknowledgements
This study was not possible without the support of D. Corrie, E. Gee, and M. Le Feuvre who provided technical help and assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this study was provided by the Australian Research Council (LP0668997), Fisheries Victoria, Department of Sustainability and Environment, and the Gippsland Coastal Board.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was completed under a Fisheries Victoria research permit and with University of Melbourne and Department of Sustainability and Environment ethics committee approval.
Rights and permissions
About this article
Cite this article
Williams, J., Hindell, J.S., Jenkins, G.P. et al. The influence of freshwater flows on two estuarine resident fish species show differential sensitivity to the impacts of drought, flood and climate change. Environ Biol Fish 100, 1121–1137 (2017). https://doi.org/10.1007/s10641-017-0632-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-017-0632-z