Abstract
Mass fish mortality occurred in November 2006 in a water hole in the Great Ruaha River, Ruaha National Park, and was due to the lack of shade by riparian vegetation or fringing wetlands. Nearby water holes with shade or fringing wetlands suffered no fish mortality. Despite being vulnerable to intense birds’ predation, fish stayed in the top few centimeters due to hypoxia in deeper water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Great Ruaha River (GRR) in Tanzania drains an area of about 68,000 km2 and used to flow throughout the year (Fig. 1; Mtahiko et al. 2006). Due to water mismanagement and unsustainable use of water upstream of the Ruaha National Park (RNP), the GRR now stops flowing for several months per year in the dry season. It degrades then into a series of small, stagnant water pools that are heavily eutrophicated by animal dung. This water is important because except for five small springs in the RNP, and except for some elephants and zebras that dig for groundwater in the GRR riverbed, this is the only drinking water available for wildlife in RNP in the dry season. Poor water quality prevails throughout the dry season as long as the water stagnates, until the river flows again at the start of the wet season. No study has been undertaken of the water quality and fish survival in these water holes in RNP. Studies in similar stagnant water holes in the Seronera River in the Serengeti National Park show that these holes have poor water quality, with hypoxia and anoxia occurring routinely, and that the poor water quality may contribute to mortality of wildlife drinking the water (Mduma et al. 1999; Wolanski and Gereta 1999; Mnaya et al. 2006). Few studies, and none in East Africa, have been undertaken on how fish is affected by poor water quality characterized by high temperature and occasional periods of hypoxia due to aerobic respiration in the water column and anaerobic processes in the sediment (Stefan and Fang 1994; Addy and Green 1997; Carpenter 1998). These water holes are the only water available for fish in the RNP in the dry season, yet they are obviously very stressful to fish because mass fish mortality has been observed in such stagnant water holes in the GRR in RNP. For instance during the 2003 dry season nearly 3,000 fish of different species including, Sulusulu (Marcusenius macrolepidotus), Gala Dagaa (Brycinus affinus), Tiger fish (Hydrocynus sp.), Mbalame (Barbus macrolepis), Red eyed mudsucker (Labeo cylindricus), Rufiji tilapia (Oreochromis urolepis) and Katoga (Bagrus orientalis) died in about five water holes in the GRR in RNP; mortality was presumably due to low dissolved oxygen in GRR water holes (Mtahiko and Ngumbi, pers. com.).
This paper presents a study on water quality in three water holes in the GRR in the dry season. At this site, mortality of hundreds of fish, mainly Rufiji tilapia (Oreochromis urolepis), occurred in about 10 days in one of the water holes, while there was no fish mortality at the other two water holes.
Methods
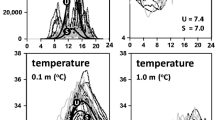
Water quality was sampled in three water holes in the GRR at Msembe (Fig. 1) at two hours intervals for 48 h on 13–14th November 2006 at the peak of the dry season. The pools were about 30–40 m apart and were in similar muddy sand soils. Each water hole had a maximum depth of about 0.4 m. As shown in Fig. 2, hole 1 was about 1.5 m wide and 5 m long and was in the shade of a tree on the river bank; hole 2 was about 1.5 m wide and 5 m long and had a small fringing wetland on one side covering half of the hole; hole 3 was about 1 m wide and 10 m long and was exposed to direct sunlight without wetland or shade.
Dissolved oxygen (DO) concentration, temperature, pH, and salinity, were measured at three depth (0.05, 0.2 and 0.4 m) using a 400 cc Niskin bottle to draw water. Salinity and temperature were measured using EC/TDS/TEMP COM-100 salinity-temperature meter, accuracy was 0.1 μS/cm and 0.1°C respectively. DO was measured using Hanna model HI 9142 DO meter, accuracy was 0.1 ppm. The pH was measured using a Sharp pH meter WP model, accuracy 0.01. The instruments were calibrated just before the field study.
Results
Temperature varied widely in the range 22.7–34.6°C, with the minimum temperature at night and maximum during the daytime (Table 1). The diurnal fluctuation of temperature was much larger (11.8°C) at hole 3 than at holes 2 (7.5°C) and 1 (8.2°C). Bottom waters were at times slightly warmer than surface waters.
The water was highly stratified in DO in hole 2 and much less so in holes 1 and 3 (Fig. 3). The DO was extremely low at night and the DO concentration fluctuated widely between daytime and night in all three pools (Fig. 3). The DO in hole 2 was variable between different depths, such that values for the surface were higher compared to the bottom, although they exhibited a similar trend like the other two holes. Hole 3 had extended periods of very low DO concentration (<1.5 ppm, i.e. hypoxia occurred) with occasional periods of high DO (>20 ppm). The DO concentration changed markedly at sunrise and sunset, it was stable during the night and it increased continuously in daytime, peaking at 1500 h and it then progressively decreased until sunset. The waters in all holes were nearly anoxic at night, with a minimum DO concentration of 0.4, 0.8 and 0.2 ppm at 0.1 m depth for holes 1, 2, and 3 respectively. The maximum daytime DO values were about 42.5% saturation in hole 2 and 250% saturation in holes 1 and 3.
Salinity exhibited only small fluctuations; the highest value was 0.22 ppt in hole 1 while the lowest salinity was 0.11 ppt in hole 2.
The waters at the three holes were alkaline with pH range 7.4–10.3 (not shown). The pH pattern exhibited substantial diurnal fluctuations with pH of 7.6 at sunset and sunrise, and highest pH of 10.3 during late afternoon at hole 3. The pH values for hole 1 and 2 ranged 7.8–8.9 and 7.4–7.9 respectively.
Discussion
The water quality conditions were stressful to fish in all the three holes. Although the fish were at risk of predation by numerous fish-eating birds in the area, they stayed near the water surface, particularly so at night and early morning (Fig. 2). This can be explained by hypoxic conditions lower in the water column (Fig. 3). The oxygen stress was measurably lessened in the wetland and tree shade water holes. During daytime the water at hole 3 was the warmest (34.6°C) and very stressful to fish (Wilcock et al. 1995). The fish mortality in hole 3 was apparently due to a combination of extremely high daytime temperature as well as very low DO concentration. The presence of shading in hole 1 and a wetland in hole 2 prevented these two stresses to occur simultaneously, and thus enabled fish to survive.
The presence of slightly warmer water at the bottom than at the surface may be due to heating from the decay of animal dung on the bottom (Wolanski and Gereta 2001).
The pH values for holes 1 and 2 were lower than those in hole 3 because the rate of carbon dioxide uptake by the plants and algae for photosynthesis was decreased by shade from trees and wetland vegetation. During daytime aquatic plants and algae utilized carbon dioxide from the water column faster than could be replenished from the air above, which raised pH levels. At night they released carbon dioxide and pH returned to equilibrium.
DO fluctuated in a diurnal cycle in all three water holes, and the key processes controlling the diurnal fluctuations were apparently photosynthesis/respiration of algae near the water surface and the decomposition of organic matter near the bottom (Horne and Goldman 1994; Marzolf et al. 1994; Wolanski and Gereta 1999; Mnaya et al. 2006). Our study reveals that these models need to add the effect of shading by riparian vegetation to be applicable to fish survival and mortality.
High pH combined with high temperature during the daytime may result in the conversion of the ammonia to highly toxic free ionized (dissolved) form (NH4+) that is harmful to fish and plants (Azov and Godman 1982; Arter 2004). Although there are no ammonia data for the GRR, this hypothesis warrants further studies.
Even though the fauna and flora are effectively protected in RNP, the misuse of water upstream is leading to habitat fragmentation in the GRR in RNP, fish mortality, and the loss of biodiversity in RNP; remediation measures are needed (Sokile et al. 2003; Mtahiko et al. 2006).
References
Addy K, Green L (1997) Natural Resources Facts, Fact Sheet No. 96 –3, Dissolved Oxygen and Temperature. University of Rhodes Island. Department of Natural Resources. Cooperative Extension
Arter BS (2004) Sheepscot River Water Quality Monitoring Strategic Plan: A guide for coordinated water quality monitoring efforts in an Atlantic salmon watershed in Maine. Prepared for the Project SHARE: Research and Management Committee. 84 pp. KRIS Bibliographic Resources. Sheepscot River Basin
Avoz Y, Goldman JC (1982) Free ammonia inhibition of algal photosynthesis in intensive cultures. Appl Environ Microbiol 43(4):735–739
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with Phosphorus and Nitrogen. Ecol Appl 8(3):559–568
Horne JA, Goldman CR (1994) Limnology, 2nd edn. McGraw-Hill, Inc, New York
Marzolf ER, Mulholland PJ, Steinman AD (1994) Improvements to the diurnal upstream-downstream dissolved oxygen change technique for determining whole-stream metabolism in small streams. Can J Fish Aquat Sci 51:1591–1599
Mduma SAR, Sinclair ARE, Hilborin R (1999) Food regulates the Serengeti wildebeest—a 40—year record. J Anim Ecol 68:1101–1122
Mnaya B, Mwangomo E, Wolanski E (2006) The influence of wetlands, decaying organic matter, and stirring by wildlife on the dissolved oxygen concentration in eutrophicated water holes in the Seroneara River, Wetlands Ecol Manage Serengeti National Park, Tanzania 14:421–425
Mtahiko MGG, Gereta E, Kajuni AR, Chiombola EAT, Ng’umbi GZ, Coppolillo P, Wolanski E (2006) Towards an ecohydrology-based restoration of the Usangu wetlands and the Great Ruaha River, Tanzania. Wetlands Ecol Manage 14:489–503
Sokile CS, Kashaigili JJ, Kadigi RMJ (2003) Towards an integrated water resource management in Tanzannia: the role of appropriate institutional framework in Rufiji basin. Elsevier J Spl Edn Phy Chem Earth, A/B/C 28(20–27):1015–1023
Stefan G H, Fang X (1994) Model simulations of dissolved oxygen characteristics of Minnesota lakes: past and future. Envirn Manage 18:73–92
Wilcock RJ, McBride GB, Nagels JW, Northcott GL (1995) Water quality in a polluted lowland stream with chronically depressed dissolved oxygen: causes and effects. N Z J Mar Freshw Res 29:277–288
Wolanski E, Gereta E (1999) Oxygen cycle in hippo pool. Serengeti National Park, Tanzania. Afr J Ecol 37:419–423
Wolanski E, Gereta E (2001) Water quantity and quality as the factors driving the Serengeti ecosystem, Tanzania. Hydrobiologia 458:169–180
Acknowledgements
We thank the Director General of Tanzania National Parks (TANAPA), The Ruaha National Park (RUNAPA) authority and staff especially Mr. Jafari Ramadhani for his tireless efforts and assistance during the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Epaphras, A.M., Gereta, E., Lejora, I.A. et al. The importance of shading by riparian vegetation and wetlands in fish survival in stagnant water holes, Great Ruaha River, Tanzania. Wetlands Ecol Manage 15, 329–333 (2007). https://doi.org/10.1007/s11273-007-9033-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-007-9033-y