Abstract
Microcystins (MCs) are produced during the growth and proliferation of some species of cyanobacteria, mainly Microcystis aeruginosa, which has massive growth in eutrophic water bodies. Microcystins are highly toxic metabolites derived from some cyanobacteria species that exert its main effect in the liver through the inhibition of protein phosphatase (PP1 and PP2A). However, other damages in fish species are less documented and could be unexpected. The aim of the current study was to evaluate the effects of Microcystis aeruginosa extract (MaE) into the central nervous system (CNS) of the Nile tilapia. The MaE was normalized by MCs content (MC-LR). We include a positive control for protein phosphatase inhibition, norcantharidin intraperitoneally dosed at sublethal levels. On the eighth day, measurement of neurotransmission biomarkers (AChE, BChE, CbE, and GABA) were measured, as well as levels of mitochondrial calcium and the mitochondrial membrane potential by flow cytometry in the brain and spinal cord were assessed, in addition to the PP1/PP2A activity in the liver. The MCs elicited mortality at 5 µg/L. The positive control and MCs at sublethal levels inhibited the PP1/PP2A activity in the liver and induced alterations in the neurotoxicity biomarkers evaluated in the CNS. This response is probably due to the disruption of transport ions, dependent and independent of ATP because of alterations in the mitochondrial membrane potential and mitochondrial calcium. The findings of this study suggest that pollutants capable of inducing cyanobacterial blooms are able, in an indirect way, to exert neurotoxic effects in fish species through MC levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microcystins (MCs) are toxins (secondary metabolites) produced by cyanobacteria (blue-green algae), primarily Microcystis aeruginosa, forming blooms worldwide, particularly in tropical and temperate environments. To date, more than 240 different MCs with rich structural variety have been identified using various technologies (Li et al., 2021; Zhang et al., 2022) released into the water during or on senescence of cyanobacterial blooms (Harada & Tsuji, 1998; Malbrouck & Kestemont, 2006). MCs are the most common of the cyanobacterial toxins found in freshwater and are being often responsible for poisoning terrestrial animals, including livestock, wildlife, and humans who are exposed to MC-polluted water (Carmichael et al., 2001; Chen et al. 2009). A critical example is the death of more than 330 African elephants in Botswana, probably by exposure to cyanotoxins, highlighting the need to develop more comprehensive and systematic (re)assessments of the risks of cyanotoxins (Wang et al., 2021). By these reasons, there is an increasing concern all over the world due to the acute and chronic effects on humans and wildlife from the MCs linked with water pollution. MCs are potent and highly specific hepatotoxins due to the inhibition of type 1 (PP1) and type 2A (PP2A) protein phosphatases (Gupta et al., 2003; Harada & Tsuji, 1998; Mikhailov et al., 2003). A large number of studies have documented the hazardous potential of MCs in aquatic organisms as well (e.g. Chorus & Bartram, 1999; Landsberg, 2002; Olivares-Rubio et al., 2015; Wiegand & Pflugmacher, 2005).
However, some studies have also indicated behavioural changes and neurotoxic effects induced by MCs on fish species. In embryo and adult zebrafish (Danio rerio) and moderlieschen (Leucaspius delineatus), there is evidence of alterations in swimming activity and spontaneous locomotor behaviour after MC-LR exposure (Baganz et al., 1998; Baganz et al., 2004; Kist et al., 2012). This was also seen in embryo zebrafish exposed to Planktothrix agardhii containing MC-YR and MC-LR (Jonas et al., 2015) and treated with pure MC-LR (Wu et al., 2016). Other studies on Jenynsia multidentata reported changes in swimming activity by acute MC-LR exposure (100 μg/L), as well as anxiogenic effects with a decrease of almost 63% in swimming distance and an increase in the immobility time (Cazenave et al., 2008). Interestingly, the parental MC-LR exposure of zebrafish induced larvae hypoactivity related to alterations in neurotransmitter systems and neuronal development by transfer of this toxin to the offspring (Wu et al., 2017). The neurotoxicity biomarkers showed that the M. aeruginosa extract containing MC-LR decreased the AChE activity in the rainbow trout (Gélinas et al., 2012) as in newly hatched zebrafish (Qian et al., 2018) or embryo zebrafish treated with MC-LR alone (Wu et al., 2016). In contrast, a significant increase in the metabolism of this enzyme in zebrafish by MC-LR-waterborne exposure was reported (Kist et al., 2012). In spite of preceding reports, there are no available studies in the Nile tilapia (a commercially important fish), as in many other fish species. Therefore, the study aim was to evaluate the neurotoxic effects of a wild strain of Microcystis aeruginosa extract at sublethal concentrations in the brain and spinal cord of juvenile Oreochromis niloticus through measurement of neurotransmission biomarkers (AChE, BChE, CbE, and GABA), levels of mitochondrial calcium, and mitochondrial membrane potential. We will also compare these damages to those induced by a specific inhibitor of the PP1/PP2A activity, norcantharidin.
2 Materials and Methods
2.1 Obtaining Microcystis aeruginosa Strain and Its Crude Extract
The algae were attained from the upper layer of a freshwater body with a visible degree of eutrophication, being careful to take part of the top creams of green tones. The obtained sample was taken from Tezozomoc Park in Mexico City, bordering to the north with the municipality of Tlalnepantla and to the northwest with the municipality of Naucalpan into the State of Mexico, between the coordinates 19° 29′05″ N and 99° 12′36″ W. The biomass was cultured by cross streak in petri dish containing agar dissolved with sterile f/2 medium (Guillard, 1975). Permanent slides were prepared of isolated specimens using standard techniques for microalgae. Observations were made with a Zeiss optical microscope equipped with a Canon PowerShot G6 digital camera and identified by Komárek and Anagnostidis (2005) and Guiry in Guiry and Guiry (2020) criteria. Once the cyanobacterium was identified as Microcystis aeruginosa, stock cultures were grown in sterile f/2 medium in 4-L glass bottles at 22 ± 2 °C supplied with continuously bubbling air and irradiated with white fluorescent tubes (516 ± 50 lx) with nonstop light for at least 3 weeks to reach its exponential growth and its begin in the decay phase. The biomass was centrifuged at 2,500 rpm, and the extract was obtained with methanol at 65 °C using a Soxhlet extractor for 8 h. The methanolic extract was concentrated with a rotary evaporator. The quantification of MCs in the methanolic extract was performed using Shimadzu high-performance liquid chromatography (HPLC) with an Agilent Technologies column (4.6 × 250 mm). The reference standard was purchased from Sigma-Aldrich (Sulpeco®, cat. 33,578,) certified as microcystin RR-YR-LR solution (5 µg/mL in methanol, each) following analytic procedures previously documented (Olivares-Rubio et al., 2015). The Microcystis aeruginosa methanolic extract rendered a value of 1.65 ± 0.07 µg/mL of MC-LR plus MC-RR and 0.09 ± 0.001 µg/mL of MC-YR.
2.2 Short-Term Exposure
Juvenile specimens of Nile tilapia (Oreochromis niloticus) were obtained from a fish farm and were maintained under laboratory conditions in agreement with Article 38 and Chapter V of the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 for the protection of animals used for scientific purposes (https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063). The Nile tilapia (n = 8/treatment) of 10.54 ± 0.17 cm (mean ± SEM) were subjected to a short-term exposure of 8 days. The test MC concentration was established considering the value proposed by the World Health Organization (WHO) of 1 µg/L as the maximum permissible limit in water for human consumption. Higher values (5 and 10 µg/L of MCs) were also included. The control group for MC water exposure was methanol with a concentration of 0.001% (v/v) and semi-hard synthetic water as the absolute control. The levels of positive control for PP1/PP2A inhibition (norcantharidin, Sigma-Aldrich®, cat. N8784) were established based on acute tests (25 to 2.5 mg/kg). The DL10 (1.0 mg/kg) was selected as the higher dose for short-term exposure; additionally, in a logarithmic way, two doses below (0.1 mg/kg and 0.01 mg/kg) were intraperitoneally administrated using a saline solution as a vehicle. During the time exposure, fish were fed every 3 days with pellets (2% w/bw). Due to the nature of the biomarkers under study, it was not possible to use a fish anaesthetic; for this aim, the specimens of O. niloticus were anaesthetized on ice and euthanized by fast freeze to obtain the brain, spinal cord, and liver after the exposure. These tissues samples were weighed and stored in buffer solution according to the needs of each biomarker and homogenized in a Glas-Col™ homogenizer under an ice bath. For enzymatic evaluations (PP1/PP2A, AChE, BChE, CbE, and GABA levels), PBS1X was used, and the homogenate was centrifuged at 9,000 rpm/4 °C/15 min to obtain the S9 fraction. The fractions were stored at − 80 °C. The total content of proteins was determined using a 2-D Quant Kit GE Healthcare™, using a BSA standard curve from 10 to 50 µg.
2.3 Biomarkers Evaluation: PP1/PP2A Activity
A volume of 200 μL of S9 fraction was added to 800 μL of buffer pH 7.2 (50 mM imidazole pH 7.2, 2 mM dithiothreitol, 0.5 mg/ml bovine serum albumin, 5 mmol theophylline, 0.2 mmol MnC12) plus 10 μL of 20 mM p-nitrophenyl phosphate. The mixture was incubated at 30 °C for 20 min in the dark. The reaction was stopped with 200 μL of 0.5 M Na2CO3, and the absorbance at 410 nm against reagent blank was determined. Enzymatic activity of PP1/PP2A [EC3.1.3.16] was calculated by the molar extinction coefficient of p-nitrophenol of 18,300 mM−1 cm−1 (Silberman et al., 1984). Results are shown as mmol/min/mg protein.
2.4 Acetylcholinesterase Activity (AChE)
The enzymatic determination of AChE [EC3.1.1.7] was performed by the Hestrin method (1949) using a standard curve of acetylcholine chloride (Sigma-Aldrich®, cat. A6625) to determine the percent of inhibition by the presence of hydrolysed acetylcholine. Fifty microlitres of the S9 fraction was taken; 125 μL of Tris buffer pH 7 and 125 μL of standard acetylcholine (10 μmoles/mL) were added, and it was stirred and incubated at room temperature for 30 min. After that time, 250 μL of 2 M alkaline hydroxylamine was added, 125 μL of 4 N HCl, and 1250 μL of 0.34 M of FeCl3. The absorbance at 540 nm was determined in a microplate Synergy MX spectrophotometer and was divided by the incubation time to obtain the absorbance per minute, obtaining in this way, the AChE activity (µM/min/mg protein).
2.5 Activity of Carboxylesterases (CbE)
The determination of the activity of carboxylesterases (CbE) [EC3.1.1.1] was carried out in the S9 fraction through the hydrolysis of p-nitrophenol acetate to form p-nitrophenol (C6H5NO3) according to Hotta et al. method (2002) with modifications. Fifty microlitres of S9 fraction and 25 µL of Tris–HCl (100 mM and pH 8) were placed in 96-well microplates, the mixture was incubated at 37 °C by 5 min, and immediately 150 µL of reaction buffer was added. The absorbance was monitored at 405 nm each minute by 5 min in a microplate Synergy MX spectrofluorometer. The production of C6H5NO3 was calculated using the coefficient of molar extinction of 18,700 M−1 cm−1, proposed by Kumar et al. (2010) for C6H5NO3 dissolved in Tris–HCl at 100 mM and pH 8.0. Data are display as mM C6H5NO3/min/mg protein.
2.6 Activity of Butyryl cholinesterase (BChE)
For quantification of enzymatic activity of BChE [EC3.1.1.8], a volume of 10 μL of S9 fraction was placed in a 96-well microplate, and 10 μL 6 mM of S-butyrylthiocholine iodide and 180 μL of the reaction solution (50 mM at pH 7.7 of phosphate buffer and a solution of dithiobis nitrobenzoate at 0.25 mM) were added. The absorbance was evaluated at 405 nm in periods of 30 s (0, 30, 60, and 90 s) at 37 °C on a Synergy MY microplate spectrophotometer. The molar extinction coefficient of 23,460 mM−1 cm−1 was used (Knedel & Böttger, 1967). Graphs symbolize the activity of BChE as mM/min/mg protein.
2.7 Evaluation of γ-Aminobutyric Acid (GABA) Levels
A volume of 400 μL of S9 fraction and 400 μL methanol HPLC grade was placed in Eppendorf tubes, mixed, and then were centrifuged at 12,000 rpm for 10 min at 4 °C in a refrigerated centrifuge. Three hundred fifty microlitres of the supernatant, 300 µL of borax solution (g/dL in deionized water) pH 8 (Sigma-Aldrich®, Cat. HT1002), and 500 µL of derivatizing agent (4-hydroxy-1-naphthaldehyde 0.3 w/v in methanol, Aldrich®, cat. 131,067) were mixed, incubated at 80° C for 10 min, and cooled. This mix was placed in a volumetric flask, and the final volume was adjusted to 5 mL with methanol. Finally the sample was filtered through 0.45 µm syringe filters. The absorbance was evaluated at 330 nm in a microplate spectrophotometer, with 250 µL of the processed sample in each well. The GABA content was estimated with a calibration curve of 4.68–75 μg/ml of γ-aminobutyric acid-certified reference standard TraceCERT® (Sulpeco®, cat. 43,811) following the Khuhawar and Rajper (2003) method modified by Vega-López et al. (2019). Results are represented as µM GABA/mg protein.
2.8 Mitochondrial Isolation and Analysis of Membrane Potential (ΔΨm) and Mitochondrial Calcium (mCa+2) Levels
The functional mitochondria were isolated from the brain and spinal cord with 100 µL of buffer IBc, according with directions proposed by Frezza et al. (2007) for cells and liver mouse cells. The mitochondrial membrane potential (ΔΨm) was evaluated with 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3) at a final concentration of 50 nM using 100 µL of isolated mitochondria according to Chang et al. (2013). Although this method was designed for cells, DiOC6(3) is also useful for the measurement of ΔΨm in isolated mitochondria (Kataoka et al., 2005). Mitochondrial calcium (mCa+2) was assessed using a Fluo-4 NW Calcium Assay Kit (Thermo Fisher Scientific™) using 125,000 mitochondria/50 µL following the manufacturer protocol. For both stain techniques, stained mitochondria were centrifuged at 10,670 × g/10 min, and the supernatant was discarded, p-formaldehyde at 0.1% was added and vortexed for 30 s at 1,000 rpm, and the mixture was incubated at room temperature for 30 min for preservation. Lastly, the mitochondria were centrifuged; the p-formaldehyde was discarded. The pellet was resuspended in 500 µL of DPBS (Chang et al., 2013) and stored at room temperature in darkness until the fluorescence was measured in a Sony SH800™ flow cytometer (Sony™). The mean fluorescence intensity was inversely proportional to the ΔΨm, and the Fluo-4 NW was chemically bound by electronic affinity to the intra-mitochondrial calcium. Data from ΔΨm and mCa+2 are shown as the mean fluorescence intensity (MFI).
2.9 Statistical Analysis
The Shapiro–Wilk test was applied to know the distribution of data and most passed the normality test. Hence, the biomarkers results were analysed by one-way analysis of variance (ANOVA) followed by Dunnett’s comparison test. The Kruskal–Wallis and Mann–Whitney U tests were used for the statistical analysis of mean fluorescence intensity of ΔΨm and mCa+2, using the software for descriptive statistics, “GraphPad Prism 6” (GraphPad Software, San Diego, CA, USA, www.graphpad.com). We considered statistical significance at a value of p ≤ 0.05 for all tests. With the aim to compare the effects elicited by M. aeruginosa extract with those damages induced by the positive control of PP1/PP2A inhibition (norcantharidine) on the CNS organs, we employed the “Integrated Biological Response version 2” (IBRv2) analysis developed by Sanchez et al. (2013), which is a helpful tool in the interpretation of these variables under both field and controlled conditions (Dzul-Caamal et al., 2016; Olivares-Rubio et al., 2013). Also, we applied a parametric correlation analysis (Pearson) to know the possible relationships among neurotoxicity biomarkers.
3 Results
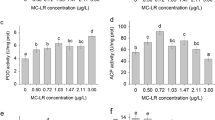
3.1 PP1/PP2A Activity
The inhibition of the enzymatic activity of PP1/PP2A in the liver of the Nile tilapia elicited by the M. aeruginosa extract (MaE) was significantly decreased, even at doses of MC-LR considered safe (Fig. 1A). Similarly, the NCTD induced an abolishment of this catalysis in a dose-dependent manner (Fig. 1B).
Activity of PP1/PP2A in the liver of the Nile tilapia (Oreochromis niloticus) treated with Microcystis aeruginosa extract (A) and with norcantharidin (B). The bars represent the mean value, and the bar errors represent the standard deviation. Biomarkers results were analysed by one-way analysis of variance (ANOVA) followed by Dunnett’s comparison test, and statistical difference was set at *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001
3.2 Activity of Enzymes Involved in Neurotransmission (AChE, CbE, and BChE)
The catalysis of AChE was irregular and did not respond consistently among the studied organs of the Nile tilapia exposed to M. aeruginosa extract (MaE) and dosed with NCTD. In the brain (Fig. 2A) and spinal cord (Fig. 2B) of specimens treated with MaE, an uneven response was found, which was only significant in the spinal cord at 5.0 µg/L. A similar patter occurred in the brain of fish dosed with NCTD (Fig. 2C); however, the low and medium doses of this substance increased the AChE activity in the spinal cord (Fig. 2D).
Activity of the enzymes involved in neurotransmission in the brain and spinal cord of the Nile tilapia (Oreochromis niloticus) treated with Microcystis aeruginosa extract (MaE) and with norcantharidine (NCTD). AChE in the brain (A) and spinal cord (B) of fish exposed to MaE. AChE in the brain (C) and spinal cord (D) of fish treated with NCTD. CbE in the brain (E) and spinal cord (F) of fish exposed to MaE. CbE in the brain (G) and spinal cord (H) of fish treated with NCTD. BChE in the brain (I) and spinal cord (J) of fish exposed to MaE. BChE in the brain (K) and spinal cord (L) of fish treated with NCTD. The bars represent the mean value, and the bar errors represent the standard deviation. Biomarkers results were analysed by one-way analysis of variance (ANOVA) followed by Dunnett’s comparison test, and statistical difference was set at *p ≤ 0.05 and **p ≤ 0.01
Interestingly, CbE activity in the brain of fish treated with MaE (Fig. 2E) or with NCTD (Fig. 2G) decreased in both cases, but it was significant only for NCTD. In contrast, in the spinal cord of O. niloticus exposed to 1.0 and 10.0 µg/L of MaE (Fig. 2F), as well as for three doses of NCTD (Fig. 2H), significant decreases were found.
As was the case for the others esterases, the BChE in the brain seems to be statistically unaltered by toxicants able to inhibit PP1/PP2A, such as the case for MaE (Fig. 2I) with the exception of the high dose of NCTD (Fig. 2K), which increased the activity of this enzyme. However, the median concentration of MaE elicited a significant increase of BChE in the spinal cord (Fig. 2J). This was not seen with NCTD, despite a similar response (Fig. 2L).
3.3 Levels of GABA
Despite previous findings, the levels of GABA were not consistently affected in the organs studied. The GABA levels in the brain decreased at 5 µg/L (p ≤ 0.05) and also at the high concentration of MaE (Fig. 3A); notwithstanding, in the spinal cord, (Fig. 3B) levels of this neurotransmitter decreased at 1.0 µg/L and increased at 5.0 µg/L (p ≤ 0.05). In a general way, the effects of NCTD showed a depletion of GABA in the brain (Fig. 3C), which was significant at 0.01 µg/kg (p ≤ 0.5), as was also detected for the spinal cord (Fig. 3D).
Levels of γ-aminobutyric acid (GABA) in the brain (A) and spinal cord (B) of the Nile tilapia (Oreochromis niloticus) treated with Microcystis aeruginosa extract (MaE) and with norcantharidine (NCTD) in the brain (C) and spinal cord (D). The bars represent the mean value, and the bar errors represent the standard deviation. Biomarkers results were analysed by one-way analysis of variance (ANOVA) followed by Dunnett’s comparison test, and statistical difference was set at *p ≤ 0.05
3.4 Mitochondrial Membrane Potential (ΔΨm) and Mitochondrial Ca+2 Levels (mCa+2)
In the brain, the MaE does not cause any significant change in ΔΨm; however, a decrease was noted with high concentration of this extract (Fig. 4A). Nevertheless, in the spinal cord, a significant increase in the ΔΨm (decrease of MFI of DiOC6) was found at 5.0 and 10.0 µg/L of MC-LR (Fig. 4C). Fish dosed with NCTD showed a decrease in ΔΨm in the brain, particularly when treated with a high dose (Fig. 4B). This also occurred in the spinal cord of the Nile tilapia dosed with 0.01 µg NCTD/kg (Fig. 4D).
Mitochondrial membrane potential (ΔΨm) and mitochondrial Ca+2 levels (mCa+2) in the brain and spinal cord of the Nile tilapia (Oreochromis niloticus) treated with Microcystis aeruginosa extract (left panels) and with norcantharidine (right panels). ΔΨm in the brain (A) and spinal cord (C) of fish treated with MaE. ΔΨm in the brain (B) and spinal cord (D) of fish dosed with NCTD. mCa+2 in the brain (E) and spinal cord (G) of fish treated with MaE. mCa+2 in the brain (F) and spinal cord (H) of fish dosed with NCTD. The bars represent the mean value, and the bar errors represent the standard deviation. Biomarkers results were analysed by one-way analysis of variance (ANOVA) followed by Dunnett’s comparison test, and statistical difference was set at *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001
In contrast, the level of mCa+2 was statistically abolished in the brain of Nile tilapia treated with MaE (Fig. 4E), as was also noted in the spinal cord of fish treated with the medium and high concentrations of MC-LR (Fig. 4G). However, in fish dosed with NCTD, the levels of mCa+2 increased (p ≤ 0.001) in the brain of O. niloticus exposed to 0.01 µg NCTD/kg (Fig. 4D), as well as in the spinal cord (p ≤ 0.001) in the treatments of 0.01 and 0.1 µg NCTD/kg (Fig. 4F).
3.5 Star Plot Areas Obtained from Integrated Biological Response Version 2
The integration of the biological response exhibited a clear neurotoxic effect exerted by both MaE and NCTD regarding their own controls, particularly in fish exposed to medium and high concentrations or doses. At the lower MaE concentration, the star plot area showed that the spinal cord was more affected than the brain. In fish exposed to 5.0 µg/L and 10 µg/L of MaE, the damage had a similar response between both organs. Likewise, GABA levels, ΔΨm, and mCa+2, and to some extent CbE activity, suffered the most with regard to basal values (Fig. 5 left panels). In contrast, the neurotoxicity elicited by NCTD was more consistent between organs and doses, and the response of the neurotoxicity biomarkers was similar than those elicited by MaE including to PP1/PPA2 in the liver (Fig. 5 right panels).
3.6 Correlation Between Biomarkers
In agreement with the findings of the star plot area obtained from IBRv2, the Pearson moment correlation analysis proved that MaE (Table 1) and NCTD (Table 2) caused neurotoxicity. This hypothesis is feasible to occur by increases in the number of correlations (negative and positive) between biomarkers compared with those observed in the control groups. Basal values of GABA in the brain were inversely related to AChE in the spinal cord and with CbE in the brain in both control groups. In MaE treatment control group, the results displayed a negative correlation among brain CbE with AChE in the spinal cord but positive in the brain. In the same controls, brain AChE exhibited a positive correlation with the PP1/PP2A in the liver, and the activity of BChE was positively correlated in both nervous system organs (Table 1). Interestingly, in the NCTD group, a positive correlation between ΔΨm and GABA was observed in the spinal cord, mCa+2 with AChE, and BChE with CbE in the brain of the Nile tilapia.
In treated groups, the number of relationships increased with the level of toxins (Tables 1 and 2). However, due to the higher number of correlations and their consistency with IBRv2, it is worth emphasizing the findings observed with 10.0 µg/L of MaE and 1.0 µg/kg NCTD.
In the brain of fish exposed to MaE, BChE was related with AChE; also reduced number of correlations among enzymes involved in neurotransmission in the spinal cord was detected. BChE was positively related with AChE and CbE and CbE with AChE. In the brain, BChE displayed statistical correlations with AChE and CbE. GABA levels in the brain and spinal cord showed positive and significant links with AChE, CbE, and BChE in the spinal cord, as was the case for GABA in the spinal cord with the same neurotransmitter in the brain. Nonetheless, the number of relationships of mitochondrial performance with esterases and neurotransmitters increased. ΔΨm evaluated as mean fluorescence intensity in the brain was inversely related with CbE in the brain and spinal cord, BChE in the spinal cord, and GABA in the brain. In the case of ΔΨm in the spinal cord, only an inverse relationship with AChE in the brain was noted. In addition, ΔΨm in the spinal cord was related to brain mCa+2. Despite these interesting findings, the higher number of statistical correlations occurred among mCa+2 in the spinal cord with neurotoxicity biomarkers. We found positive correlations with spinal cord AChE, spinal cord CbE and BChE activities, brain CbE, and GABA in the brain and spinal cord. A negative correlation was found with ΔΨm in the brain (Table 1).
The results from fish exposed to the high doses of NCTD (1.0 µg/kg) demonstrated a positive correlation in a dose-dependent manner of the three enzymes involved in neurotransmission among the brain and spinal cord and vice versa, contrary to what was observed in the control group, where only one positive relationship was found. Remarkably, the levels of GABA in the brain and spinal cord were positively related with AChE, CbE, and BChE activities in the spinal cord. However, brain ΔΨm was negatively correlated with CbE in the brain and spinal cord, in addition to BChE and with GABA levels in the spinal cord. In contrast, ΔΨm in the spinal cord only indicated a negative relationship with AChE in the brain. However, a great number of correlations were found between mCa+2 in the spinal cord and the enzymes involved in neurotransmission and with levels of GABA in both organs studied. However, this biomarker showed a negative relationship with ΔΨm in the brain and was not linked with AChE and BChE in the brain, ΔΨm in the spinal cord, or PP1/PP2A in the liver (Table 2).
4 Discussion
In this study, it is not surprising as in other studies in fish species that some of the main effect of MCs in the liver of Oreochromis niloticus was the enzymatic inhibition of protein phosphatases such as the PP1/PP2A isoforms. However, the interesting aspect is probably its relationships with the functionality of the nervous system. The inhibition of PP1/PP2A by MCs has been widely studied in mammals and fish species since it has a high degree of specificity towards these enzymes (e.g. Carbis et al., 1997; Landsberg, 2002; Mezhoud et al., 2008; Toivola et al., 1994). Similar toxic responses have been documented for the specific inhibitor of PP1/PP2A activity, norcantharidin, using in vitro and in vivo mammal models (Deng et al., 2013; Hill et al., 2008; Wang et al., 2018). The MCs and NCDT have been recognized as inhibitors of protein phosphatase 2A through their binding with the catalytic subunit of this enzyme (Fontanillo & Köhn, 2018; Liu & Sun, 2015; Tang et al., 2016; Wang et al., 2018). Similarly, MCs and NCDT are able also to inhibit the activity of the ubiquitous Ser/Thr protein phosphatase 1 (PP1) (Fontanillo & Köhn, 2018; Liu & Sun, 2015; Tang et al., 2016; Wang et al., 2018). The PP1 regulates diverse and essential cellular processes such as cell cycle progression, protein synthesis, muscle contraction, carbohydrate metabolism, transcription, and neuronal signalling (Liang et al., 2011). On the other hand, PP2A is one of the most complex members of the PP family; it is responsible for regulating physiological processes such as neuronal stabilization by ion exchange, cardiac muscle function, and cell cycle (Lires-Deán et al., 2008). In addition, the PPs are very important in regulating Ca+2 channels in neurons through the cGMP-dependent signalling pathway (Zsombok et al., 2005). However, much less is understood about whether and how a particular type of PP contributes to regulating neuronal Ca+2 channel activities. Despite the wide involvement of PP1/PP2A in the regulation of anionic channels, our results do not contribute in a great way to solving this question, particularly in fish exposed to MaE. However, the in vitro and in vivo terrestrial models provided specific clues about the neurotoxicity, which the MCs entail. This toxic response is due to the ability of MCs to cross the blood–brain barrier and most neural cells through OATPs; therefore, the Ca+2 levels increase and could induce apoptosis (Hinojosa et al., 2019). Specifically, in SH-SY5Y cells and rat hippocampus, it has been shown that MC-LR increased the demethylation of PP2Ac, which was accompanied by associated rises in GSK-3β phosphorylation at Ser9. As a consequence, the tau hyperphosphorylation at PP2A-favorable sites occurred (Zhang et al., 2018). Based on previous reports, it has been proposed that the neurotoxicity of MCs is also related with cytoskeleton disruption in nervous system cells (Hinojosa et al., 2019). In contrast, in the brain and spinal cord of the Nile tilapia treated with the intermediate NCTD dose, statistical relationships between mCa+2 in the brain and spinal cord and PP1/PP2A activity were observed. These results would be promising to further investigate and suggest a highly specific saturable process, probably related to an increase in mCa+2 levels and also with inhibition of PP2A activity in a dose-dependent manner.
In this study, we observed changes directly related to the activity of enzymes involved in neurotransmission, as well as the ΔΨm and mCa+2 with exposure to MCs and NCTD. The results obtained for mCa+2 levels were different for NCTD and MCs exposures; however, the star plot areas obtained from IBRv2 showed a similar tendency in the damage of the CNS of the Nile tilapia. It is well known that any changes in mCa+2 levels cause alterations in membrane potential since both variables are closely related, altering in this way the mitochondrial function or cation accumulation. The increase of mCa+2 levels can augment ATP production by altering the activity of calcium-sensitive mitochondrial matrix enzymes. Medium and high concentrations of MCs do not increase calcium levels in the mitochondria, which may be because the calcium channels are open. However, the release of calcium from the mitochondria could be related to the necro-apoptosis processes (Bai et al., 2013). On the other hand, intracellular deposits of this cation activate calcium channels, a response named store-operated calcium channels (SOCs) (Stathopulos & Ikura, 2017). The SOCs are a nearly ubiquitous Ca+2 entry pathway stimulated by numerous cell surface receptors via the reduction of Ca+2 concentration in the endoplasmic reticulum (ER). The defining feature of SOCs that distinguishes them from all other classes of Ca+2 channels is their activation by the reduction of Ca+2 concentration in the lumen of the ER. It is worth mentioning that, at the neuronal level, changes in calcium channels can promote the fusion of the synaptic vesicle membrane with the axon terminal membrane in the neurons, which causes the release of ACh to the synaptic cleft by an exocytosis mechanism (Parekh & Putney, 2005; Prakriya & Lewis, 2015; Lewis, 2011; Kawamoto et al., 2012; Finkel et al., 2015). This relationship between mCa + 2 levels and AChE levels was significantly evident in the spinal cord of the Nile tilapia treated with MaE and in the brain and spinal cord of fish dosed with NCTD. In this regard, when ACh enters to the synaptic cleft, it can bind to cholinergic receptors, whose binding occurs quickly due to the neurotransmitter being rapidly metabolized by AChE. As a consequence, the activity of AChE, and probably BChE and CbE, is affected, promoting the presence of AChE and the synthesis of BChE after exposures to MCs as noted in the current study, as well as in fish treated with NCTD, by increasing the relationships between mCa+2 with these enzymes involved in neurotransmission. Likewise, it is possible that decreases in ΔΨm, in addition to Ca+2 levels, influenced the neuronal response in addition to the flexibility of Ca+2 signalling by modifying the cytosolic Ca+2 concentrations via a regulated opening of the cellular membrane and subcellular Ca+2 sensitive channels as documented in the brain by Kawamoto et al. (2012). However, the findings of IBRv2 denote that the activity of BChE and AChE seems to be unaltered regarding the controls, in contrast to that observed with the activity of CbE. This response suggests that hydrolysis and esterification of other substrates (Perry et al., 2011) could be affected by exposure to MaE and NCDT, as well as the high substrate specificity of AChE and BChE.
Proteomic analysis showed that seventeen proteins were involved in the response to MC-LR treatment; eight of them were to be reported as being directly related with MC-LR effects: fumarylacetoacetase (EC 3.7.1.2), protein disulphide isomerase A4 and A6, 78 kDa glucose-regulated protein homolog, 40S ribosomal protein SA (EC 2.3.1.267-[ribosomal protein S5]-alanine N-acetyltransferase), prohibitin (EC 6.4.1.1-pyruvate carboxylase), cytochrome b5, and ATP synthase mitochondrial d subunit (Hubbard & McHugh, 1996; Malécot et al., 2009; Mikhailov et al., 2003). The last five are directly related to ATP generation in the mitochondria. Moreover, the concentrations of Ca+2 tend to equalize on both sides of the cell membrane producing depolarisation and causing changes in the permeability of the membrane allowing the influx of other divalent ions (Hubbard & McHugh, 1996). About the topic, we fond negative relationships between ΔΨm in the brain and mCa+2 levels in the spinal cord and positive dealings between ΔΨm in the spinal cord and mCa+2 levels in the brain of Nile tilapia treated with the higher concentration of MaE and in the higher dose of NCTD. This response suggests that the generation of a proton gradient through the mitochondrial membrane was modified, altering in this way the release of neurotransmitters evaluated through the activity of the enzymes involved in their metabolism. However, the control of this process was maintained but not the spinal cord despite the proton gradient alterations elicited by MaE and NCTD.
In addition to variations in levels of AChE, BChE, and CbE, the inhibitory neurotransmitter of the CNS, γ-aminobutyric acid (GABA) was evaluated. Approximately 20% of all neurons in the nervous system have GABA as a neurotransmitter, which prevents CNS overexcitation (Bak et al., 2006; Kalueff & Nutt, 2007). Our results showed positive relationships of GABA levels in the brain and spinal cord with mCa+2 levels in the spinal cord. In addition, GABA in the brain and spinal cord was positively related with the inhibition of AChE, BChE, and CbE on the organs in fish treated with MaE and also with the same enzymes in the spinal cord of fish dosed with NCTD. These results clearly indicate post-synaptic alterations able to induce disruption of GABA binding with its receptor, leading an overexcitation of CNS cells.
To our knowledge, previous studies about these responses are not available in fish exposed to MaE and NCTD; however, this study has opened up new opportunities to explore more consequences of eutrophication process, particularly in fish species exposed to MCs linked with blooms of some Microcystis aeruginosa strains.
5 Concluding Remarks
In summary, alterations in calcium levels are intimately intertwined with ion exchange; therefore, inhibition of phosphatases, as well as alterations in mitochondrial calcium levels, causes disruptions in neurotransmission by modifications of transporters of ATP-dependent ions by exposure to Microcystis extract. These effects related with the eutrophication process can be evaluated by the neurotoxicity biomarkers studied in the present work and highlight the need to reassess the effects of cyanotoxins produced by blooms of cyanobacteria related to human activities and climate change.
Data Availability
The authors declare that all other data supporting the findings of this study are available within the article and its supplementary information files.
References
Baganz, D., Staaks, G., & Steinberg, C. (1998). Impact of the cyanobacteria toxin, microcystin-lr on behaviour of zebrafish Danio Rerio. Water Res., 32(3), 948–952. https://doi.org/10.1016/S0043-1354(97)00207-8
Baganz, D., Staaks, G., Pflugmacher, S., & Steinberg, C. E. W. (2004). A comparative study on microcystin-LR induced behavioural changes of two fish species (Danio rerio and Leucaspius delineatus). Environmental Toxicology, 19, 564–570. https://doi.org/10.1002/tox.20063
Bai, T., Dong, D. S., & Pei, L. (2013). Resveratrol mitigates isoflurane-induced neuroapoptosis by inhibiting the activation of the Akt-regulated mitochondrial apoptotic signaling pathway. International Journal of Molecular Medicine, 32(4), 819–826. https://doi.org/10.3892/ijmm.2013.1464
Bak, L. K., Schousboe, A., & Waagepetersen, H. S. (2006). The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. Journal of Neurochemistry, 98(3), 641–653. https://doi.org/10.1111/j.1471-4159.2006.03913.x
Carbis, C. R., Rawlin, G. T., Grant, P., Mitchell, G. F., Anderson, J. W., & McCauley, I. (1997). A study of fetal carp, Cyprinus carpio L., exposed to Microcystis aeruginosa at Lake Mokoan, Australia, and possible implications for fish health. Journal of Fish Diseases, 20, 81–91. https://doi.org/10.1046/j.1365-2761.1997.d01-111.x
Carmichael, W. W., Azevedo, S. M. F. O., An, J. S., Molica, R. J. R., Jochimsen, E. M., Lau, S., Rinehart, K. L., Shaw, G. R., & Eaglesham, G. K. (2001). Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environmental Health Perspectives, 109(7), 663–668. https://doi.org/10.1289/ehp.01109663
Cazenave, J., Bistoni, M. A., Pesce, S. B., & Wunderlin, D. A. (2006). Differential detoxification and antioxidant response in diverse organs of Corydoras paleatus experimentally exposed to microcystin-RR. Aquatic Toxicology, 76(1), 1–12. https://doi.org/10.1016/j.aquatox.2005.08.011
Cazenave, J., Nores, M. L., Miceli, M., Díaz, M. P., Wunderlin, D. A., & Bistoni, M. A. (2008). Changes in the swimming activity and the glutathione S-transferase activity of Jenynsia multidentata fed with microcystin-RR. Water Research, 42(4–5), 1299–1307. https://doi.org/10.1016/j.watres.2007.09.025
Chang H, Huang H, Huang T, Yang P, Wang Y, Juan H (2013) Flow cytometric detection of mitochondrial membrane potential. Bio-protocol 3(8):e430. https://doi.org/10.21769/BioProtoc.430
Chen, J., Xie, P., Li, L., & Xu, J. (2009). First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicological Sciences, 108(1), 81–89. https://doi.org/10.1093/toxsci/kfp009
Chorus I, Bartram J (1999) Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management/edited by Ingrid Chorus and Jamie Bertram. World Health Organization. https://apps.who.int/iris/handle/10665/42827
Deng, L., Dong, J., & Wang, W. (2013). Exploiting protein phosphatase inhibitors based on cantharidin analogues for cancer drug discovery. Mini Reviews in Medicinal Chemistry., 13(8), 1166–1176. https://doi.org/10.2174/1389557511313080005
Dzul-Caamal, R., Salazar-Coria, L., Olivares-Rubio, H. F., Rocha-Gómez, M. A., Girón-Pérez, M. I., & Vega-López, A. (2016). Oxidative stress response in the skin mucus layer of Goodea gracilis (Hubbs and Turner, 1939) exposed to crude oil: A non-invasive approach. Comparative Biochemistry and Physiology Part a: Molecular & Integrative Physiology, 200, 9–20. https://doi.org/10.1016/j.cbpa.2016.05.008
Finkel, T., Menazza, S., Holmström, K. M., Parks, R. J., Liu, J., Sun, J., Liu, J., Pan, X., & Murphy, E. (2015). The ins and outs of mitochondrial calcium. Circulation Research, 116(11), 1810–1819. https://doi.org/10.1161/CIRCRESAHA.116.305484
Fontanillo, M., & Köhn, M. (2018). Microcystins: Synthesis and structure-activity relationship studies toward PP1 and PP2A. Bioorganic & Medicinal Chemistry, 26(6), 1118–1126. https://doi.org/10.1016/j.bmc.2017.08.040
Frezza, C., Cipolat, S., & Scorrano, L. (2007). Organelle isolation: Functional mitochondria from mouse liver, muscle and cultured filroblasts. Nature Protocols, 2(2), 287–295. https://doi.org/10.1038/nprot.2006.478
Gélinas, M., Juneau, P., & Gagné, F. (2012). Early biochemical effects of Microcystis aeruginosa extracts on juvenile rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology Part b: Biochemistry and Molecular Biology, 161(3), 261–267. https://doi.org/10.1016/j.cbpb.2011.12.002
Guillard, R. R. L. (1975). Culture of phytoplankton for feeding marine invertebrates. In W. L. Smith & M. H. Chanley (Eds.), Culture of Marine Invertebrate Animals (pp. 26–60). Plenum Press.
Guiry in Guiry MD, Guiry GM (2020) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Available on http://www.algaebase.org/search/species/detail/?species_id=30050
Gupta, N., Pant, S. C., Vijayaraghavan, R., & Rao, P. V. L. (2003). Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology, 188, 285–296. https://doi.org/10.1016/s0300-483x(03)00112-4
Harada, K. I., & Tsuji, K. (1998). Persistence and decomposition of hepatotoxic microcystins produced by cyanobacteria in natural environment. Journal of Toxicology: Toxin Reviews, 17(3), 385–403. https://doi.org/10.3109/15569549809040400
Hestrin, N. (1949). The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. Journal of Biological Chemistry, 180(1), 249–261.
Hill, T. A., Stewart, S. G., Gordon, C. P., Ackland, S. P., Gilbert, J., Sauer, B., Sakoff, J. A., & McCluskey, A. (2008). Norcantharidin analogues: Synthesis, anticancer activity and protein phosphatase 1 and 2A inhibition. Chem Med Chem, 3(12), 1878–1892. https://doi.org/10.1002/cmdc.200800192
Hinojosa MG, Gutiérrez-Praena D, Prieto AI, Guzmán-Guillén R, Jos A, Cameán AM (2019) Neurotoxicity induced by microcystins and cylindrospermopsin: A review. Science Total Environ 10 (668):547–565. /https://doi.org/10.1016/j.scitotenv.2019.02.426
Hotta, Y., Ezaki, S., Atomi, H., & Imanaka, T. (2002). Extremely stable and versatile carboxylesterase from a hyperthermophilic archaeon. Applied and Environment Microbiology, 68(8), 3925–3931. https://doi.org/10.1128/AEM.68.8.3925-3931.2002
Hubbard, M. J., & McHugh, N. J. (1996). Mitochondrial ATP synthase F1-β-subunit is a calcium-binding protein. FEBS Letters, 391(3), 323–329. https://doi.org/10.1016/0014-5793(96)00767-3
Jonas, A., Scholz, S., Fetter, E., Sychrova, E., Novakova, K., Ortmann, J., Benisek, M., Adamovsky, O., Giesy, J. P., & Hilscherova, K. (2015). Endocrine, teratogenic and neurotoxic effects of cyanobacteria detected by cellular in vitro and zebrafish embryos assays. Chemosphere, 120, 321–327. https://doi.org/10.1016/j.chemosphere.2014.07.074
Kalueff, A. V., & Nutt, D. J. (2007). Role of GABA in anxiety and depression. Depression and Anxiety, 24(7), 495–517. https://doi.org/10.1002/da.20262
Kataoka, M., Fukura, Y., Shinohara, Y., & Baba, Y. (2005). Analysis of mitochondrial membrane potential in the cells by microchip flow cytometry. Electrophoresis, 26(15), 3025–3031. https://doi.org/10.1002/elps.200410402
Kawamoto EM, Vivar C, Camandola S (2012) Physiology and pathology of calcium signaling in the brain. Front Pharmacol 3(61). https://doi.org/10.3389/fphar.2012.00061
Kist, L. W., Rosemberg, D. B., Pereira, T. C., de Azevedo, M. B., Richetti, S. K., de Castro, L. J., Yunes, J. S., Bonan, C. D., & Bogo, M. R. (2012). Microcystin-LR acute exposure increases AChE activity via transcriptional ache activation in zebrafish (Danio rerio) brain. Comparative Biochemistry and Physiology Part c: Toxicology & Pharmacology, 155(2), 247–252. https://doi.org/10.1016/j.cbpc.2011.09.002
Khuhawar, M. Y., & Rajper, A. D. (2003). Liquid chromatographic determination of gamma-aminobutyric acid in cerebrospinal fluid using 2-hydroxynaphthaldehyde as derivatizing reagent. Journal of Chromatography. b, Analytical Technologies in the Biomedical and Life Sciences, 788(2), 413–418. https://doi.org/10.1016/s1570-0232(03)00062-x
Knedel M, Böttger R (1967) [A kinetic method for determination of the activity of pseudocholinesterase (acylcholine acyl-hydrolase 3.1.1.8.)] Klin Wochenschr 45(6):325–7. https://doi.org/10.1007/bf01747115
Komárek J, Anagnostidis K (2005) Süsswasserflora von Mitteleuropa. Cyanoprokaryota: 2. Teil/2nd Part: Oscillatoriales. Vol. 19. München: Elsevier Spektrum Akademischer Verlag.
Landsberg, J. H. (2002). The effects of harmful algal blooms on aquatic organisms. Fisheries Science, 10, 113–390. https://doi.org/10.1080/20026491051695
Lewis, R. S. (2011). Store-operated calcium channels: New perspectives on mechanism and function. Cold Spring Harbor Perspectives in Biology, 3, a0039701. https://doi.org/10.1101/cshperspect.a003970
Li, B., Liu, Y., Zhang, H., Liu, Y., Liu, Y., & Ping Xie, P. (2021). Research progress in the functionalization of microcystin-LR based on interdisciplinary technologies. Coordination Chemistry Reviews, 443, 214041. https://doi.org/10.1016/j.ccr.2021.214041
Liang, J., Li, T., Zhang, Y. L., Guo, Z. L., & Xu, L. H. (2011). Effect of microcystin-LR on protein phosphatase 2A and its function in human amniotic epithelial cells. Journal of Zhejiang University. Science. B, 12(12), 951–960. https://doi.org/10.1631/jzus.B1100121
Lires-Deán, M., Caramés, B., Cillero-Pastor, B., Galdo, F., López-Armada, M., & Blanco, F. J. (2008). Anti-apoptotic effect of transforming growth factor-β1 on human articular chondrocytes: Role of protein phosphatase 2A. Osteoarthritis Cartilage, 16(11), 1370–1378. https://doi.org/10.1016/j.joca.2008.04.001
Liu, J., & Sun, Y. (2015). The role of PP2A-associated proteins and signal pathways in microcystin-LR toxicity. Toxicology Letters, 236(1), 1–7. https://doi.org/10.1016/j.toxlet.2015.04.010
Malbrouck, C., & Kestemont, P. (2006). Effects of microcystins on fish. Environmental and Chemistry, 25(1), 72–86. https://doi.org/10.1897/05-029r.1
Malécot, M., Mezhoud, K., Marie, A., Praseuth, D., Puiseux-Dao, S., & Edery, M. (2009). Proteomic study of the effects of microcystin-LR on organelle and membrane proteins in Medaka fish liver. Aquatic Toxicology, 94(2), 153–161. https://doi.org/10.1016/s0009-2797(02)00075-3
Mezhoud, K., Praseuth, D., Puiseux-Dao, S., Francois, J.-C., Bernard, C., & Edery, M. (2008). Global quantitative analysis of protein expression and phosphorylation status in the liver of the medaka fish (Oryzias latipes) exposed to microcystin-LR I. Balneation Study. Aquat Toxicol, 86(2), 166–175. https://doi.org/10.1016/j.aquatox.2007.10.010
Mikhailov, A., Härmälä-Braskén, A. S., Hellman, J., Meriluoto, J., & Eriksson, J. E. (2003). Identification of ATP-synthase as a novel intracellular target for microcystin-LR. Chemico-Biological Interactions, 142(3), 223–237. https://doi.org/10.1016/s0009-2797(02)00075-3
Olivares-Rubio, H. F., Martínez-Torres, M. L., Domínguez-López, M. L., García-Latorre, E., & Vega-López, A. (2013). Pro-oxidant and antioxidant responses in the liver and kidney of wild Goodea gracilis and their relation with halomethanes bioactivation. Fish Physiology and Biochemistry, 39(6), 1603–1617. https://doi.org/10.1007/s10695-013-9812-8
Olivares-Rubio, H. F., Martínez-Torres, M. L., Nájera-Martínez, M., Dzul-Caamal, R., Domínguez-López, M. L., García-Latorre, E., & Vega-López, A. (2015). Biomarkers involved in energy metabolism and oxidative stress response in the liver of Goodea gracilis Hubbs and Turner, 1939 exposed to the microcystin-producing Microcystis aeruginosa LB85 strain. Environmental Toxicology, 30(10), 1113–1124. https://doi.org/10.1002/tox.21984
Qian, H., Liu, G., Lu, T., & Sun, L. (2018). Developmental neurotoxicity of Microcystis aeruginosa in the early life stages of zebrafish. Ecotoxicology and Environmental Safety, 151, 35–41. https://doi.org/10.1016/j.ecoenv.2017.12.059
Parekh, A. B., & Putney, J. W. (2005). Store-operated calcium chan-nels. Physiological Reviews, 85, 757–810. https://doi.org/10.1152/physrev.00057.2003
Perry, S. W., Norman, J. P., Barbieri, J., Brown, E. B., & Gelbard, H. A. (2011). Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. BioTechniques, 50(2), 98–115. https://doi.org/10.2144/000113610
Prakriya, M., & Lewis, R. S. (2015). Store-operated calcium channels. Physiological Reviews, 95(4), 1383–1436. https://doi.org/10.1152/physrev.00020.2014
Sanchez, W., Burgeot, T., & Porcher, J. (2013). A novel “Integrated biomarker response” calculation based on reference deviation concept. Environmental Science and Pollution Research, 20, 2721–2725. https://doi.org/10.1007/s11356-012-1359-1
Silberman, S. R., Speth, M., Nemani, R., Ganapathi, M. K., Dombradi, V., Paris, H., & Lee, E. Y. (1984). Isolation and characterization of rabbit skeletal muscle protein phosphatases C-I and C-II. Journal of Biological Chemistry, 259(5), 2913–2922.
Stathopulos, P. B., & Ikura, M. (2017). Store operated calcium entry: From concept to structural mechanisms. Cell Calcium, 63, 3–7. https://doi.org/10.1016/j.ceca.2016.11.005
Tang, Z. B., Chen, Y. Z., Zhao, J., Guan, X. W., Bo, Y. X., Chen, S. W., & Hui, L. (2016). Conjugates of podophyllotoxin and norcantharidin as dual inhibitors of topoisomerase II and protein phosphatase 2A. European Journal of Medicinal Chemistry, 123, 568–576. https://doi.org/10.1016/j.ejmech.2016.07.031
Toivola, D. M., Erikssonb, J. E., & Brautigan, D. L. (1994). Identification of protein phosphatase 2A as the primary target for microcystin-LR in rat liver homogenates. FEBS Letters, 344, 175–180. https://doi.org/10.1016/0014-5793(94)00382-3
Vega-López, A., Pagadala, N. S., López-Tapia, B. P., Madera-Sandoval, R. L., Rosales-Cruz, E., Nájera-Martínez, M., & Reyes-Maldonado, E. (2019). Is related the hematopoietic stem cells differentiation in the Nile tilapia with GABA exposure? Fish & Shellfish Immunology, 93, 801–814. https://doi.org/10.1016/j.fsi.2019.08.032
Wang, G., Dong, J., & Deng, L. (2018). Overview of cantharidin and its analogues. Current Medicinal Chemistry, 25(17), 2034–2044. https://doi.org/10.2174/0929867324666170414165253
Wang H, Xu C, Liu Y, Jeppesen E, Svenning JC, Wu J, Zhang W, Zhou T, Wang P, Nangombe S, Ma J, Duan H, Fang J, Xie P. (2021) From unusual suspect to serial killer: Cyanotoxins boosted by climate change may jeopardize megafauna. Innovation (N Y). 2(2):100092. https://doi.org/10.1016/j.xinn.2021.100092
Wiegand, C., & Pflugmacher, S. (2005). Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicology and Applied Pharmacology, 203(3), 201–218. https://doi.org/10.1016/j.taap.2004.11.002
Wu, Q., Yan, W., Liu, C., Li, L., Yu, L., Zhao, S., & Li, G. (2016). Microcystin-LR exposure induces developmental neurotoxicity in zebrafish embryo. Environmental Pollution, 213, 793–800. https://doi.org/10.1016/j.envpol.2016.03.048
Wu, Q., Yan, W., Cheng, H., Liu, C., Hung, T. C., Guo, X., & Li, G. (2017). Parental transfer of microcystin-LR induced transgenerational effects of developmental neurotoxicity in zebrafish offspring. Environmental Pollution, 231(Pt 1), 471–478. https://doi.org/10.1016/j.envpol.2017.08.038
Zhang, Y., Zhang, J., Wang, E., Qian, W., Fan, Y., Feng, Y., Yin, H., Li, Y., Wang, Y., & Yuan, T. (2018). Microcystin-leucine-arginine induces tau pathology through Bα degradation via protein phosphatase 2A demethylation and associated glycogen synthase kinase-3β phosphorylation. Toxicological Sciences, 162(2), 475–487. https://doi.org/10.1093/toxsci/kfx271
Zhang, H., Li, B., Liu, Y., Chuan, H., Liu, Y., & Xie, P. (2022). Immunoassay technology: Research progress in microcystin-LR detection in water samples. Journal of Hazardous Materials, 424(Pt B), 127406. https://doi.org/10.1016/j.jhazmat.2021.127406
Zsombok, A., Schrofner, S., Hermann, A., & Kerschbaum, H. H. (2005). A cGMP-dependent cascade enhances an L-type-like Ca2+ current in identified snail neurons. Brain Research, 1032(1–2), 70–76. https://doi.org/10.1016/j.brainres.2004.11.003
Funding
This study was supported by Instituto Politécnico Nacional SIP code 20180917 and SIP code 20201044. M. Najera-Martínez is a graduate DSc in Chemobiological Sciences which received financial support by a postdoctoral fellowship granted by CONACyT, México. G.G. Landon-Hernández is a graduate BSc student. J.P. Romero-López is a graduate DSc in Chemobiological Sciences who received scholarship from CONACyT and BEIFI-IPN. M.L. Domínguez-López and A. Vega-López are fellow of Estímulos al Desempeño en Investigación and Comisión y Fomento de Actividades Académicas (Instituto Politécnico Nacional) and Sistema Nacional de Investigadores (SNI, CONACyT, México).
Author information
Authors and Affiliations
Contributions
MNM, GGLH, and JPRL conducted the experiments; MNM, MLDL, and AVL designed the experiments and interpreted the results; and AVL wrote the manuscript.
Corresponding author
Ethics declarations
Ethics Standards
The study was performed in agreement with Article 38 and Chapter V of the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 for the protection of animals used for scientific purposes (https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nájera-Martínez, M., Landon-Hernández, G.G., Romero-López, J.P. et al. Disruption of Neurotransmission, Membrane Potential, and Mitochondrial Calcium in the Brain and Spinal Cord of Nile Tilapia Elicited by Microcystis aeruginosa Extract: an Uncommon Consequence of the Eutrophication Process. Water Air Soil Pollut 233, 6 (2022). https://doi.org/10.1007/s11270-021-05480-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05480-x