Abstract
Groundwater from the Mocorito River aquifer in Mexico has been historically employed for both human consumption and irrigation of its overlaying agriculture fields. The aim of this research was to investigate the levels and distribution of potentially toxic elements (PTEs) in the aquifer to determine their sources and to assess their potential health risks. Groundwater samples were collected from wells at eighteen sites in two climatic seasons. In the dry season, mean dissolved concentrations (µg L−1) of As, Pb, Cd, and Cr were 3.19, 0.05, 0.02, and 0.15, respectively, and their total (unfiltered) concentrations were 4.10, 0.47, 0.05, and 0.52, respectively. While in the rainy season, their dissolved concentrations were 4.60, 0.03, 0.01, and 0.06, respectively, and their total concentrations were 5.58, 0.25, 0.01, and 0.12, respectively. On average, concentrations of the four PTEs were below national and international guidelines for drinking water. Concentrations of As exceeded the WHO (2007) guidelines (10 µg L−1) at three sites and had yielded relatively high values of both chronic daily intake and hazard quotient. Lifetime cancer risk for As indicated the probability for developing this disease of 1 in 10, 000 inhabitants. Pearson’s correlation and principal component analysis (PCA-Varimax) were carried out. According to these, all the PTE concentrations were mainly derived from natural lithogenic sources. Arsenic concentrations constitute potential human health concerns for both direct consumption and its bioaccumulation in local crops. Finally, due to high As concentrations in some sites in the aquifer, the implementation of a sustainable groundwater management plan in the MORCA, that include a monitoring of PTE levels, is recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Groundwater is an important resource for both human consumption and anthropogenic activities, including agriculture, mining, and industry (Barzegar et al., 2019; McDonough et al., 2020). Quantity and quality of this resource is, therefore, a worldwide concern because many aquifers are now being adversely impacted by over-exploitation and pollution (Afzal et al., 2014; Coyte et al., 2019; Rakib et al., 2020). That pollution includes increases in major ions (salinization) and potentially toxic elements (PTEs) such as arsenic (As), lead (Pb), cadmium (Cd), and chromium (Cr) (Bawa & Dwivedi, 2019; Erickson et al., 2019). Those increases may be due to reductions in groundwater recharge or over-exploitation of the aquifers as well as the release of these elements from natural sources (water–rock interaction, leaching, etc.) (Aullón Alcaine et al. 2020; Huq et al., 2020). PTE concentrations in aquifers may also be increased by various anthropogenic activities (e.g., mining, wastewater runoff, industry, and agriculture) that adversely affect the quality of the groundwater (Arkoc, 2014; Garau et al., 2019; Ghezzi et al., 2019), especially in shallow aquifer, which can be polluted with these and other contaminants such as nutrients (Chen et al., 2016), pesticides, hydrocarbons, and pathogen microorganisms from the anthropogenic activities carried out on the overlaying alluvial plain.
Harmful effects of PTEs on human health caused by groundwater ingestion have been reported. PTEs like As, Pb, Cd, and Cr cause pulmonary, gastrointestinal, reproductive, renal, and hematological damages, as well as short-term memory loss, disabilities in learning and coordination problems, risk of cardiovascular disease, different types of cancer, and death (Dhaliwal et al., 2020; Sobhanardakani, 2017a; Sobhanardakani et al., 2018; Wuana & Okieimen, 2011). Thus, there is an increasing need to monitor the concentrations of PTEs in aquifers, identifying their sources and assessing their potential risks (Qiao et al., 2020; Ravindra & Mor, 2019).
As a result of that need, the concentrations and the biogeochemical behavior of PTEs have been extensively investigated on a global scale (Barzegar et al., 2019; Liang et al., 2017; Rajkumar et al., 2020; Upadhyaya et al., 2014). This includes the study by Arkoc (2014) on PTEs in groundwater from the Ergene Basin, Turkey, where the average concentrations (μg L−1) of Zn (683) > Cr (16) > Fe (12) > Cu (5) > Pb (0.6) > Cd (< 0.005) were all below of the international and national guidelines. Conversely, Ayedun et al. (2015) determined the concentrations (µg L−1) of As (0.24), Cd (0.23), Cr (1.81), and Pb (7.44) in Nigerian aquifers and found that Cd and Pb concentrations exceeded the WHO (World Health Organization (WHO), 2017) guidelines. They concluded that the high Cd and Pb concentrations in those waters were caused by the discharge from waste or leaching from sewage-laden landfills, as well as industrial activities in the area, respectively. Huq et al. (2020) reported that 50 of 59 districts in Bangladesh have As levels higher than the Bangladesh (50 g L−1) and WHO (10 g L−1) standards for drinking water, and concluded that those values mainly occur because of the natural sources in the region (e.g., Himalayan orogenic deposits) and mentioned that over-exploitation of the aquifers as well as the competitive ion exchange process also contribute to the As enrichment of the groundwater.
In Mexico, high levels of As in groundwater have been associated with natural and anthropogenic sources, mainly in Zimapán-Valley, the San Antonio–El Triunfo, Santa Maria de la Paz, Los Azufres, Los Humeros and Acoculco, in Hidalgo, Southern Baja California, San Luis Potosí, and Aguascalientes states (Armienta & Segovia, 2008; Armienta et al., 2001; Razo et al., 2004; Robles-Camacho & Armienta, 2000). Armienta et al. (2001) observed that As concentrations in groundwater from Zimapan-valley ranged from nondetectable to 1000 g L−1. These high concentrations of As were associated with the chemical composition of minerals occurring in the study area such as scorodite and arsenopyrite (Krieger & Hagner, 1943). Thirty-two from sixty samples exceeded the WHO (World Health Organization (WHO), 2017) limit (10 µg L−1), and thirteen exceeded the Mexican standard of 50 µg L−1 established in the NOM-127-SSA-1994 (Secretaría de Salud (SSA), 2000). Likewise, Robles-Camacho and Armienta (2000) quantified natural Cr contamination in the groundwater from León, Valley. To this respect, the authors concluded that the Cr comes from the Ultramafic units in the Sierra de Guanajuato and hydrothermal activity. On the other hand, Rodriguez et al. (2002) mentioned that shallow aquifer from Salamanca, Guanajuato, contains high levels of Pb related with the mining activity (silver, gold, and lead ores) near to Salamanca. However, none of the above studies has conducted a risk analysis for PTE intake.
Since the behavior, bioavailability, and biogeochemical cycles of the PTEs in the aqueous ecosystems are determined by the different process in the water and particles (organic matter and sediments), such as uptake and release by particles scavenging, the levels of PTEs have been studied in both total and dissolved phases (Tang et al., 2002; Zgheib et al., 2011). PTEs from the dissolved phase are more mobile and bioavailability than those from the particulate matter (de Paiva-Magalhães et al., 2015).
There is no literature focused on quantifying levels of As and PTEs in the coastal shallow aquifers from Sinaloa, especially in the Mocorito River agriculture basin, where, similar to the mentioned by Chen et al. (2017), rural population perceives groundwater as a source of clean and safe drinking water and has used it for human consumption when regular water supplies are disrupted during catastrophic events (e.g., hurricanes and drought periods), provoking human health risks and potentially bioaccumulating into crops growing in the region.
For this reason, this research was conducted to (1) establish PTE levels (As, Pb, Cd, and Cr) in the groundwater during the dry and wet seasons, in both total and dissolved phases; (2) identify their potential sources; and (3) conduct a risk assessment for the consumption of water from this aquifer.
2 Materials and Methods
2.1 Study Area
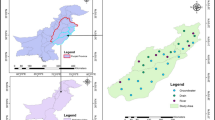
The present study focused on the Mocorito River Coastal shallow Aquifer (MORCA), located within the area called “Culiacan Valley” in northwestern Mexico (Fig. 1), where 333,000 ha of agricultural farmland are located, from which 217,000 ha are highly mechanized and technified (Karam-Quiñones, 2002). Some of the most produced crops in the region are grains and vegetables, such as corn, beans, wheat, tomato, chili, and cucumber (Instituto Nacional and de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP), 2000; García-Gutiérrez and Rodríguez-Meza, 2012; Páez-Osuna et al., 2007); these kind of crops require large amounts of agrochemicals, which can be a potentially source of metals and metalloids. The MORCA belongs to the hydrogeological area number 10 (Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), 2015), and is located between 24° 51′–25° 45′ N and 107° 38′–108° 23′ W (Comisión Nacional del Agua (CONAGUA), 2020). It has limited freshwater resources, which is being stressed by local human activities and climate change. According to Comisiόn Nacional de Agua (CONAGUA) (2020), groundwater extraction from MORCA was estimated at 89.8 × 106 m3 year−1, from which 66.0 × 106 m3 year−1 are used in agriculture, 22.8 × 106 m3 year−1 employed for public and urban used, and 1.0 × 106 m3 year−1 in domestic and other uses (e.g., human consumption during catastrophic and drought events in the region).
Based on the Köppen climate system classification, modified by García (1964), the area is characterized as a very warm, dry climate with an annual average temperature of 22 °C. In 2013, the annual rainfall over the MORCA region was 847 mm, with monthly averages of 0.2 mm from March to April (dry season) and 169 mm from July to September (rainy season) (Comisión Nacional del Agua (CONAGUA), 2014). MORCA has values of transmissivity that range from 1.4 × 10−3 to 7.7 × 10−3 m2 s−1, and hydraulic conductivity of 2.3 × 10−5 to 2.5 × 10−4 m s−1 with an average of 1 × 10−4 m s−1. Following the statistical level of the groundwater of MORCA, the direction of the groundwater flow has been defined in one portion of the basin as SE-NW (with statistical level between 55 and 105 masl) and in the other portion as NE-SW (statistical levels between 7 and 30 masl), parallel to the superficial flow to the Mocorito river (Comisiόn Nacional de Agua (CONAGUA), 2020). MORCA has an average recharge level of approximately 137.9 × 106 m3 year−1 (Comisiόn Nacional de Agua (CONAGUA), 2020), which correspond to (1) vertical recharge due to rainfall and river water infiltration (55.8 × 106 m3 year−1), (2) horizontal recharge because of groundwater flow from the mountains (8.7 × 106 m3 year−1), and (3) an induced recharge from water infiltration from municipal distribution net pipes and distribution channels (3.4 × 106 m3 year−1), as well as from agriculture irrigation (70 × 106 m3 year−1).
The MORCA is an unconfined shallow aquifer, primarily composed of fluvial and alluvial sediments located over a conglomerate with a low permeability, which overlying acid igneous rocks containing gersdorffite (NiAsS) and maucherite (Ni11As8) minerals (Krieger & Hagner, 1943; Universidad Nacional Autónoma de México (UNAM), 1978, which are potential sources of arsenic and other potentially toxic elements. Morales-Zepeda (2007) reported the presence of fine-grained Vertisol soil, with a high content of expansive clay minerals and enriched with organic matter because of the agriculture activities in the valley. In addition, MORCA contains predominantly calcic-sodic-bicarbonate type water (García-Beltrán, 2008; Rivera-Hernández et al., 2017).
2.2 Sampling
All the glass and high-density polyethylene materials used in this study were previously cleaned with JT Baker® trace metal grade HCl (6 N), heated at 60 °C for 24 h on a heating plate and rinsed with high purity (18.3 MΩ cm) water (MilliQ®) three times, and then cleaned with JT Baker® trace metal grade HNO3 (6 N) and rinsed with MilliQ. These protocols followed those detailed by Soto-Jimenez et al. (2006) to avoid any contamination and quantify the low-level PTE contents in the groundwater samples. All reagents used in the analyses were also trace metal grade high-purity grade.
Groundwater from eighteen wells from the MORCA were single sampled in the months of April and July of 2013, representing two weather seasons, dry (November–June) and rainy (July–October), respectively (Fig. 1). Due to environmental conditions during rainy season, the access to sample the wells SR, ELL, and EE was not possible. All sampling sites were selected based on the well network of the Comision Nacional del Agua (CONAGUA), number 10 irrigation district in Guamuchil, Sinaloa, as well as on the groundwater availability and the accessibility to each site. All the samples were collected about 1 m below the tablewater, which range from 5 to 15 m deep. The samples were collected using a bailer sampler that was rinsed two times with the groundwater, which was discarded, before to taking the samples in acid-cleaned high-density polyethylene bottles (HDPE, 60 mL). In order to avoid sample contamination, sampling procedure was carried out by two people, acting as “clean hands” and “dirty hands,” respectively (David et al., 2001; Fitzgerald, 1999; Flegal & Smith, 1995). At each sampling site, two bottles were filled with groundwater in order to assess the PTE contents in the dissolved and total phases. The bottles were individually stored in small plastic bags and grouped in larger plastic bags for transport in a cooler at 4 °C to the laboratory. During sampling, environmental parameters such as pH, temperature (°C), electrical conductivity (EC), total dissolved solids (TDS), and hardness (TH) were also collected and measured concurrently. The concentrations of major ions (Na+, Ca2+, Mg2+, Cl−, SO42−, HCO3−, and CO32−) collected at that time were published previously (Rivera-Hernández et al., 2017) and used in this study in order to assess the behavior of the PTEs and to help identify their sources.
2.3 Processing and Analysis of Samples
In the laboratory, one of the two samples from each site was filtered through white nitrocellulose Millipore filters (0.22 µm) and considered as dissolved phase (Liang et al., 2018; Wang & Liu, 2003). Filtered and unfiltered (total phase) samples were acidified with high purity analytical grade HCl (6 N) (JT Baker®), until analysis. Arsenic determination was carried out by using an atomic fluorescence spectrometer (PSA 10.055 Millennium Excalibur model) at the Institute of Marine Sciences and Limnology, Mazatlan. The samples for Pb, Cd, Cr, Fe, Al, and Mn determinations were transported to the University of California at Santa Cruz (UCSC), where they were processed in a HEPA-filtered (Class 100) trace metal clean laboratory and then analyzed with a Thermo Scientific™ Element 2™ high-resolution inductively coupled plasma mass spectrometer (HR-ICP-MS).
2.4 Quality Control
To avoid the contamination of the samples and solutions used during the analysis, all the treatment and preparation of the samples for further analysis were carried out inside a trace metal clean room. To compensate for any bias during sampling or laboratory work, field and laboratory blanks (filtered and unfiltered Milli-Q water) were processed and treated as samples. Concentrations of PTEs in filtered blanks were very similar than those in unfiltered blanks, indicating that there was no measurable PTE contamination coming from the filters. PTE concentrations obtained from these blanks, which were minimal in comparison with those from the groundwater samples, were subtracted from the concentration in samples. Concurrent measurements of a NIST reference material, Trace Elements in Natural Water (1640a), were used to determine the accuracy of the analysis by calculating the percent (%) of recovery of Pb (100.9), Cd (103.7), Cr (88.6), Fe (97.7), Al (93.5), and Mn (99.3). The precision of the analyses was derived from their coefficients of variation, which were 2.6% for Pb, 2.5% for Cd, 1.8% for Cr, 3.3% for Fe, 4.7% for Al, and 0.8% for Mn. Limits of detection, calculated as 3 times the standard deviation from concentrations of these elements in blanks, were 0.377, 0.003, 0.005, 0.002, 0.033, 0.043, and 0.002 μg L−1 for As, Pb, Cd, Cr, Fe, Al, and Mn, respectively.
2.5 Statistical Analysis and Sources Identification
All the results were analyzed with basic statistics. Shapiro–Wilk and Levene’s tests were carried out in order to know the normality and homoscedasticity of data, respectively. All the statistical analyses used the SPSS version 17.0 software package. Statistical significance was established as p < 0.05 for all the analyses.
To identify the associations and possible common sources of PTEs in the groundwater, Pearson correlation coefficients and principal components analysis (PCA-Varimax) were calculated. In order to obtain a more representative idea about the relationship among variables, PTE concentrations for both dissolved and total phases were considered for these analyses. Major ions (obtained from Rivera-Hernández et al., 2017) as well as Al, Fe, and Mn (herein analyzed) concentrations in groundwater were included in the Pearson correlation and PCA-Varimax as proxies of lithogenic sources.
2.6 Risk Assessment
Mocorito River and the Eustaquio Buelna dam are the main water sources for human activities in the region, though a municipal net of pipes as well as irrigation channels. However, when catastrophic events (e.g., hurricanes and drought periods) occur, groundwater from MORCA is extracted from several wells for domestic and drinking purposes. For this reason, the potential health risk was assessed. The chronic daily intake (CDI) and the non-carcinogenic risk quotient (HQ) and lifetime cancer risk (LTCR) indices were calculated following the equations suggested by United States Environmental Protection Agency (USEPA) (1989), Bodrud-Doza et al. (2019), and Setia et al. (2020):
where C, DI, and BW represent the concentration of PTEs in the groundwater (μg L−1) from each site, the average daily intake rate (2 L day−1), and the average of adult body weight (72 kg), respectively. EF, DE, and AT are exposure frequency (367 day/years), exposure duration (70 years), and average time (25,550 days). According to the A to Z list of chemicals of the Integrated Risk Information System of the US Environmental Protection Agency (IRIS-USEPA; https://cfpub.epa.gov/ncea/iris_drafts/ atoz.cfm?list_type = alpha), the oral toxicity reference dose values (RfD) are 0.3, 0.5, and 1500 µg kg−1 day−1 for As, Cd, and Cr, respectively. For Pb, Health Canada (2004) suggests a value of 3.6 µg kg−1 day−1. The population is assumed to be safe when HQ < 1 (USEPA, 1999a). For LTCR calculus, the CDI was divided by 1000 in order to convert to mg kg−1 day−1 units, The oral slope factor (OSF) employed in this study was obtained from the aforementioned A to Z list of chemicals of the Integrated Risk Information System (for As, 1.5 mg−1 kg day) and Bodrud-Doza et al. (2019) (for Cd, 0.38 mg−1 kg day).
3 Results
3.1 Physicochemical Parameters
Rivera-Hernández et al. (2017) determined physicochemical parameters including pH, temperature, electric conductivity, salinity, and major ions in eleven of the sampling sites. In the present work, data from seven additional sampling sites, taken during the same sampling campaign, were included, and the maximum, minimum, and average values for pH, temperature, and electric conductivity were recalculated (Table 1). Values of pH in the MORCA groundwater varied from 7.2 to 8.6 for dry and from 6.7 to 7.7 during the rainy season. Temperature ranged from 23.8 to 29.6 °C, with an average of 26.6 ± 1.6 °C and from 27.0 to 34.0 °C, with an average of 28.6 ± 2.3 °C in the dry and rainy seasons, respectively. The values of electric conductivity ranged from 525 to 5310 μS cm−1, with an average of 2022 ± 1342 μS cm−1 in dry and from 731 to 9410 μS cm−1, with an average of 2786 ± 2460 μS cm−1 in the rainy season.
Both dissolved and total concentrations of Fe, Al, and Mn levels were quantified in the aquifer in both climatic seasons (Table 1). Significant differences (p > 0.05) were not observed between seasons for all these three elements; however, as a general trend, the highest levels of Fe and Al in the dissolved phase occurred during rainy season and varied from 5.2 to 338.4 µg L−1, with an average of 62.9 ± 86.2 µg L−1 for Fed, and from 3.8 to 269.7 µg L−1, with an average of 100.1 ± 82.8 µg L−1 for Ald. While for Fet and Alt, the highest levels were shown in dry, oscillating from not detectable (ND) to 3231.3 µg L−1, with an average of 334.5 ± 832.5 µg L−1, and from 0.02 to 11,471.5 µg L−1, with an average of 731.9 ± 2687.1 µg L−1, respectively. For Mn, both phases showed higher levels in the rainy season, ranging from ND to 436.4 µg L−1, with an average of 64.1 ± 116.2 µg L−1 for Mnd, and from 1.3 to 436.4 µg L−1, with an average of 81.0 ± 115.9 µg L−1 for Mnt.
3.2 Potentially Toxic Elements
The distribution and levels of the PTEs (As, Pb, Cd, and Cr) in both dissolved (d) and total (t) phases are shown in Figs. 2 and 3, respectively.
3.2.1 Arsenic
Asd exhibited a relatively homogeneous distribution in both climatic seasons and significant (p < 0.05) differences were not observed (t test) between them. The Asd concentration ranged from not detectable (ND) to 14.30 g L−1, with an average of 3.19 ± 3.31 g L−1 in dry season and from 0.66 to 9.98 g L−1, with an average of 4.60 ± 2.77 g L−1 in rainy season (Table 1). Slight increases in the concentration of Asd at one site (PI) in dry and at five sites (GA, GL, PI, EH, and CC) in rainy season were observed (Fig. 2a and b). For Ast, the highest values were found at the PI and EB sites, in dry, and at the PI, GL, and GA sites in rainy. The rest of the sites showed similar levels in both seasons (Fig. 3a and b). Ast levels varied from ND to 14.30 μg L−1, with an average of 4.10 ± 3.94 μg L−1, in dry season, and 2.61 to 11.96 μg L−1, with an average of 5.58 ± 2.97 μg L−1, in rainy season. Significant differences were not observed for Ast between seasons.
3.2.2 Lead
Concentrations of Pbd varied from ND to 0.17 μg L−1, with an average of 0.05 ± 0.07 μg L−1, in the dry season, and ND to 0.28 μg L−1, with an average of 0.03 ± 0.07 μg L−1 in the rainy season, with no significant differences occurring between seasons. For both seasons, Pbd levels in all sampled sites were significantly low in comparison to Pbt (Fig. 2c and d). The concentration of Pbt ranged from ND to 6.84 μg L−1, with an average of 0.47 ± 1.60 μg L−1, in dry season, and from ND to 1.08 μg L−1, with an average of 0.25 ± 0.36 μg L−1, throughout the rainy season (Table 1). The distribution of Pbt was homogeneous in both seasons (Fig. 3c and d), with an increase near Playa Colorada, during the dry season. Significant differences in Pb concentrations between seasons were not observed.
3.2.3 Cadmium
Concentrations of Cdd ranged from ND to 0.07 μg L−1 in dry and from ND to 0.05 μg L−1 in the rainy season, with an average of 0.02 ± 0.02 and 0.01 ± 0.02 μg L−1, respectively. The Cdd levels were significantly higher during the dry season (Fig. 2e) compared to those found during the rainy season (Fig. 2f). Meanwhile, Cdt showed a relatively homogeneous distribution (Fig. 3e and f), with levels ranging from ND to 0.41 μg L−1, with an average of 0.05 ± 0.09 μg L−1 in the dry season, and from ND to 0.13 μg L−1, with an average of 0.01 ± 0.03 μg L−1 during the rainy season, with no significant differences occurring between seasons.
3.2.4 Chromium
Even when no significant differences between seasons were detected for both phases, in general terms, the levels quantified for Crd and Crt tended to be higher in the dry season than in the rainy season. The sites with the highest content of Crd during the dry season were located near to Playa Colorada in the northern portion of the study area and La Reforma in the southern portion of the study area (Fig. 2g), while during the rainy season, the highest levels were observed in the north-central portion of the study area (Fig. 2h). The levels of Crd, in the dry season, varied from 0.01 to 0.63 μg L−1, with an average of 0.15 ± 0.16 μg L−1, and from ND to 0.39 μg L−1, with an average of 0.06 ± 0.12 μg L−1 during the rainy season. In contrast, Crt showed a heterogeneous distribution in both climatic seasons (Fig. 3g and h). Its levels varied from 0.01 to 5.50 μg L−1, with an average of 0.52 ± 1.26 μg L−1 during the dry season and from ND to 0.89 μg L−1, with an average of 0.12 ± 0.30 μg L−1 during the rainy season.
4 Discussion
4.1 Physicochemical Parameters
Groundwater physicochemical parameters are directly related to its water quality and serve to determine the behavior and mobility of its components, including PTEs (Barzegar et al., 2019; Rakib et al., 2020). As previously mentioned, concentrations of major ions of the MORCA were reported by Rivera-Hernández et al. (2017). They observed that the water did not present a dominant hydrochemical facie during the dry season; while the dominant hydrochemical facie was cationic (Ca2+, Mg2+, and Na+) during the rainy season. Additionally, they indicated that the pH values were inside the recommended ranges of the NOM-127-SSA-1994 (Secretaría de Salud (SSA), 2000), the World Health Organization (World Health Organization (WHO), 2017), and the United States Environmental Protection Agency (United States Environment Protection Agency (USEPA), 2001).
Concentrations of Fe, Al, and Mn in both dissolved and total phases varied between climatic seasons. For the dissolved phase, higher concentrations of these elements were observed in the rainy season, while for the total phase, the higher concentrations were observed in dry season. Iron is an essential element for the human metabolism; however, high levels of this element in drinking water can also generate adverse health effects (Salvador, 2010; Santra et al., 2018). Edet et al. (2011) and Lenntech (2015) reported that Fe values in natural groundwater range from < 0.1 to 50,000 μg L−1, which is a function of runoff, water infiltration, and the weathering and dissolution of rocks and minerals in the aquifer.
Based on the geochemistry chart published by the Servicio Geológico Mexicano (SGM) (2015a), there are sediment deposits with high Fe, Al, and Mn concentrations in the study area, which contribute to relatively elevated levels of these elements in the groundwater from the region. Seasonal variations in Fe, Al, and Mn concentrations in the aquifer are therefore mainly be attributed to (1) the infiltration and runoff of water towards the interior of the aquifer, which are greater during the rainy season, and (2) the increase in the residence time of water in the shallow aquifer during dry season, which causes an increase in the soil/water interactions (Sarti et al., 2020).
4.2 Potentially Toxic Elements
The average concentrations of PTEs decreased in the following order: As > Cr > Pb > Cd in both dissolved and total phases during both climatic seasons. Arsenic contamination in groundwater has often been associated with geogenic sources, mainly in countries like Bangladesh (Huq et al., 2020) and China (Sanjrani et al., 2019), in Asia, and Argentina in America (Aullón-Alcaine et al., 2020); however, several activities such as metallurgy and agriculture may also increase the concentrations of this metalloid in groundwater (Cao et al., 2019).
Twelve percent of the sites in the MORCA showed relatively high levels of As in groundwater. Sobhanardakani (2017b, 2018) reported similar levels of As in groundwater also from agricultural regions (Qaleeh Shahin, Kermanshah Province and Hamedan, Iran). However, the relatively high levels of As in MORCA for both total and dissolved phases appear to be mainly associated with the presence of Maucherita deposits (Ni11As8), which is a mineral present in the recharge zone and along the Mocorito River basin (Krieger & Hagner, 1943). Cao et al. (2019) reported that high levels of As in groundwater can be due to industrial discharges and leaching of wastewater; however, these kind of discharges were not identified in the study area.
Elevated concentrations of Pb, Cd, and Cr may also occur because of natural weathering and/or industrial inputs, including the use of PVC plastic, oil motor, cadmium batteries, and pesticides (Xiong et al., 2019). The latter source of contamination is of concern because the Mocorito River basin is one of the largest (ca. 1,250,000 ha) and most active agricultural regions in Mexico, where different grains and vegetables are cultivated (Instituto Nacional and de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP), 2000; García-Gutiérrez & Rodríguez-Meza, 2012; Páez-Osuna et al., 2007;), and these kind of crops use large quantities of chemicals (fertilizers and insecticides) (Leyva-Morales et al., 2014; Secretaria de Agricultura y Desarrollo Rural (SADER), 2019). The concentrations of PTEs analyzed were similar or lower than those quantified in different regions of Mexico and around the world (Table 1), principally in metropolis and agricultural areas, which can be affected by industrial activities and the use of agrochemicals.
As previously noted, the highest levels of Pb, Cd, and Cr in the dissolved phase were observed in dry season (Table 1). This could be due to the decrease in water renewal that leads to an increase in the soil/water interaction within the aquifer, while during the rainy season, there is an increase in the groundwater recharge into the aquifer and a dilution effect is possible to occur. That would be consistent with the determination by Buragohain et al. (2010), in their work on the seasonal variation of Pb, As, Cd, and Al in groundwater from the Dhemaji district in India, where they observed significantly higher levels of all metals in the dry season than in the rainy season. They attributed temporal variability to an increase in soil/water interaction during the dry season, which causes a concentration effect of all the constituents released from the soil.
Because groundwater extracted from the MORCA is also used for human consumption, it is As, Pb, Cd, and Cr concentrations in the aquifer were compared to the maximum permissible limits (MPL) established by both national and international official guidelines (Table 1). The parametric (United States Environment Protection Agency (USEPA), 2001) and guideline (WHO 2017) value for As (10 μg L−1) in its total phase was exceeded at two sites (PI and EB) during the dry season and two sites (PI and GL) during the rainy season (rainy), and it is important to note that two of those sites (PI and GL) are located inside private houses, where the water is used for domestic purposes. In contrast, Pb, Cd, and Cr levels were far below national and international official guidelines, with only slight increases in Pb (6.84 µg L−1) and Cr (5.50 µg L−1) concentrations at one site (EB) site during the dry season (Fig. 3).
We recommended that organizations like Comisión Nacional del Agua (CONAGUA), Junta Municipal de Agua Potable y Alcantarillado de Angostura (JUMAPAANG), Junta Municipal de Agua Potable y Alcantarillado de Mocorito (JMAPAM), and the Consejo de Cuenca de los ríos Mocorito al Quelite (Watershed Conceil for the rivers Mocorito to El Quelite) have to pay an special attention and to implement a sustainable groundwater management plan for the monitoring the levels of metals, metalloids, and potentially other pollutant in the MORCA.
4.3 Potential PTE Sources
Pearson correlation coefficients calculated for the groundwater from MORCA in dry and rainy seasons are shown in Table 2. In brief, significant and positive correlations among auxiliary parameters (TDS and TSS) and major ions (Na+, Mg2+, Ca2+, SO42−, and Cl−), as well as among the PTEs and Fe, Al and Mn were observed (r > 0.5) in both dry and rainy seasons. In addition, positive correlations between Cl−-Pbd (r = 0.62), Na+-Pbt (r = 0.52), Na+-Crt (r = 0.51), and CO32−-Cdd (r = 0.51) were also observed during the dry season (Table 2).
Those correlations were substantiated by calculating with PCA-Varimax. In the dry season, five factors accounted for 91% of total variance (Table 3). Individually, factor 1 (33% of total variance and eigenvalue 8.79) was characterized by high loads of TDS (0.99), TSS (0.98), Cl− (0.97), Ca2+ (0.96), Mg2+ (0.94), SO42− (0.91), and Na+ (0.53). Factor 2 (22% of total variance and eigenvalue 5.88) had high loads of Pbt, Alt, Crt, Cdt, and Fet (0.96, 0.96, 0.95, 0.92, and 0.89, respectively). Factor 3 (14% of total variance and eigenvalue 3.80) had high loads of Mnd (0.92), Asd (0.92), Mnt (0.88), and Ast (0.82). Factor 4 (12% of the total variance and eigenvalue 3.25) was characterized by high loads of Crd, Ald, Pbd, and Fed (0.89, 0.72, 0.72, and 0.72, respectively), and factor 5 (10% of the total variance and eigenvalue 2.81) had high loads of HCO3−, CO32−, and pH (0.87, 0.68, and 0.65, respectively). The total variation (84%) in the rainy season was also characterized by five factors (Table 3). Factor 1 (32% of the total variance and eigenvalue 8.67) was characterized by high loads of TDS (0.98), TSS (0.97), Cl− (0.98), SO42− (0.92), Mg2+ (0.86), Na+ (0.75), Pbd (0.79), and Ca2+ (0.60). Factor 2 (18% of total variance and eigenvalue 4.88) had high loads of Crt (0.97), Alt (0.94), Pbt (0.86), Fet (0.83), Crd (0.98), and HCO3− (0.58). Factor 3 (13% of the total variance and eigenvalue 3.61) was characterized by high loads of Cdt, Ald. and Cdd (0.98, 0.83, and 0.92, respectively). Factor 4 (13% of total variance and eigenvalue 3.39) had high loads of Ast (0.95), Mnt (0.91), Mnd (0.88), and Asd (0.81). Finally, factor 5 (8% of the total variance and eigenvalue 2.10) had high loads of CO32− (0.96).
According to the strong associations observed in Pearson correlations and confirmed by PCA-Varimax, two possible sources (geogenic and anthopogenic) of PTEs in the MORCA were identified for both climatic seasons. The quantification of high loads of major ions (Na+, Mg2+, and Ca2+) as well as inter-PTE interactions in almost all the factors (Table 3) suggested lithogenic origins as the main source of PTEs derived from different processes such as the ionic exchange, precipitation, and weathering of rocks and minerals as a product of the water/rock interaction (Rubio et al., 2001). This is consistent with the alluvial sedimentary origin of the MORCA, which is impacted by superficial runoff and infiltration that mobilize PTEs in the aquifer by weathering its parental material. To this respect, (Comisión Nacional del Agua (CONAGUA), (2020) reported a groundwater recharge from rainfall and river water infiltration of 55.8 × 106 m3 year−1. This infiltrated water play an important role in the enrichment of dissolved ions in groundwater (water/rock interactions).
Based on geochemical maps (SGM, 2015a-d) of different metals and metalloids (Fe, Al, Mn, Cd, Pb, and As) in the MORCA, there is an abundance of ferromagnesian and aluminosilicate minerals in the area. This is consistent with reports by Rosales et al. (1986), Rubio et al. (1995), and Rubio et al. (2000) on the strong, positive correlations between metals and metalloids with Fe, Al, and Mn elsewhere in Mexico. Krieger and Hagner (1943) documented the presence of maucherita (Ni11As8) in the recharge zone of the MORCA, which presumably accounts for much of the As in the aquifer.
Cadmium and Pb are two elements often associated with anthropogenic sources (Cao et al., 2019; Rehman et al., 2019). Rivera-Hernández et al. (2019) previously showed a slight enrichment of Cd and Pb in the agricultural soils overlying the aquifer. However, strong correlations between Pb-Cl− and Cd-CO32− in the aquifer attested to lithogenic origins of these elements in the MORCA. That is consistent with the determination by Peinado-Guevara et al. (2011) of evaporitic and calcareous rocks in the Sinaloa River basin, which is near MORCA recharge region and is presumably lithogenic sources of Pb and Cd in the aquifer.
Agriculture activity is the second source of dissolved ions in MORCA. Agriculture irrigation is considering a cause of ecologic damage due to either the original chemical composition of the irrigation water (Chen et al., 2020; Qian et al., 2020) or the ions leaching from the soils during irrigation. According to Comisión Nacional del Agua (CONAGUA) (2020), an average of 350 × 106 m3 of water year−1 have been used for agriculture irrigation, from which 70 × 106 m3 year−1 (20%) has been infiltrated into the subsoil.
4.4 Health Risk Assessment
Table 4 shows a decrease in the CDI values in order of As > Pb > Cr > Cd for both dry and rainy season. As previously mentioned, the presence of As in the aquifer appears to be primarily associated with the occurrence of mineral deposits of maucherita (Ni11As8), in the MORCA (Krieger & Hagner, 1943).
The results of HQ indexes for all metals studied are also shown in Table 4. The HQ indices followed a similar order of As > Pb > Cd = Cr for both seasons. HQ indices indicated, on average, that the population is not at risk of consuming water from the MORCA (i.e., HQ < 1). However, there is a potential risk for measurable As toxicity at two sites (PI (HQAs = 1.32) and EB (HQAs = 1.24)) in the dry season and another site (GL (HQAs = 1.11)) in the rainy season. Sobhanardakani (2018) also reported HQ for As < 1 in three of four provinces studied in Hamedan, Iran, considering a non-carcinogenic effect (chronic risk) for the inhabitants.
There are no values of OSF for Pb and Cr reported in the literature. According to the nomenclature suggested by United States Environmental Protection Agency (USEPA) (1999b) and the LTCR for As obtained in this study, the risk to develop cancer disease is high, with a probability of 1 in 10, 000 inhabitants (LTCR > 1E-4 and > 1E-3), except for the EE sampling site in dry season (LTCR < 1E-6). While for Cd, the LTCR are considered very low (< 1E-6), except for EB (4.28E-6) and RB (1.00E-6) sites for rainy, and RB (1.35E-6) during dry season.
5 Conclusion
Results of this study revealed variations in PTE (As, Pb, Cd, and Cr) concentrations in groundwater of Mocorito River Aquifer during the dry and rainy seasons. Statistical analyses of those concentrations and ancillary parameters (major ions, Fe, Al, and Mn) indicate that those variations are primarily due to seasonal differences in fluvial inputs, which increase during the rainy season, and the extent of solid/water interactions, which increase during the dry season when evaporation rates are also greater. The covariance of PTE concentrations with those of the ancillary parameters also attest to their predominantly lithogenic origins, as opposed to anthropogenic origins—even though the area is a center of intense agricultural activity. On the other hand, Pb, Cd, and Cr levels in the MORCA groundwater are relatively low, based on national and international environmental and human health standards. Arsenic concentrations were found to exceed the WHO (World Health Organization (WHO), 2017) guidelines for drinking water (10 μg L−1) at 12% of the sampling sites, which represented a health risk for the exposed inhabitants based on CDI, as well as HQ and LTCR indices. Although the main use of the MORCA groundwater is not for human consumption, it has been used by the population during catastrophic events when regular water supplies are disrupted (e.g., hurricanes and drought periods) and it could potentially be bioaccumulated in crops grown in the area. In addition, with our findings, government authorities will be able to make scientific-based decisions to implement groundwater management plans, such as PTE content monitoring within the aquifer, and promote the development of sustainable agriculture.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Afzal, M., Shabir, G., Iqbal, S., Mustafa, T., Khan, Q. M., & Khalid, Z. M. (2014). Assessment of heavy metal contamination in soil and groundwater at leather industrial area of Kasur, Pakistan. CLEAN - Soil, Air, Water, 42(8), 1133–1139. https://doi.org/10.1002/clen.201100715
Aullón-Alcaine, A., Schulz, C., Bundschuh, J., Jacks, G., Thunvik, R., Gustafsson, J. P., Mörth, C. M., Sracek, O., Ahmad, A., & Bhattacharya, P. (2020). Hydrogeochemical controls on the mobility of arsenic, fluoride and other geogenic co-contaminants in the shallow aquifers of northeastern La Pampa Province in Argentina. Science of the Total Environment, 715, 136671. https://doi.org/10.1016/j.scitotenv.2020.136671
Arkoc, O. (2014). Heavy metal concentrations of groundwater in the east of Ergene Basin, Turkey. Bulletin of Environmental Contamination and Toxicology, 93, 429–433. https://doi.org/10.1007/s00128-014-1347-x
Ayedun, H., Gbadebo, A. M., Idowu, O. A., & Arowolo, T. A. (2015). Toxic elements in groundwater of Lagos and Ogun States, Southwest, Nigeria and their human health risk assessment. Environmental Monitoring and Assessment, 187, 351–368. https://doi.org/10.1007/s10661-015-4319-7
Armienta, M. A., Rodríguez, R., Ceniceros, N., Cruz, O., Aguayo, A., Morales, P., & Cienfuegos, E. (2014). Groundwater quality and geothermal energy. The case of Cerro PrietoGeothermal Field. México. Renewable Energy., 63, 236–254. https://doi.org/10.1016/j.renene.2013.09.018
Armienta, M. A., & Segovia, N. (2008). Arsenic and fluoride in the groundwater of Mexico. Environmental Geochemistry and Health, 30, 345–353. https://doi.org/10.1007/s10653-008-9167-8
Armienta, M. A., Villaseñor, R., Rodriguez, R., Ongley, L. K., & Mango, H. (2001). The role of arsenic-bearing rocks in groundwater pollution at Zimapán Valley. México. Environmental Geology, 40(4–5), 571–581.
Barzegar, R., Moghaddam, A. A., Soltani, S., Fijani, E., Tziritis, E., & Kazemian, N. (2019). Heavy metal(loid)s in the groundwater of Shabestar Area (NW Iran): Source identification and health risk assessment. Exposure and Health, 11, 251–265. https://doi.org/10.1007/s12403-017-0267-5
Bawa, R., & Dwivedi, P. (2019). Impact of land cover on groundwater quality in the Upper Floridan Aquifer in Florida. United States. Environmental Pollution, 252, 1828–1840. https://doi.org/10.1016/j.envpol.2019.06.054
Bodrud-Doza, M., Islam, S. M. D., Hasan, M. T., Alam, F., Haque, M. M., Rakib, M. A., Asad, M. A., & Rahman, M. A. (2019). Groundwater pollution by trace metals and human health risk assessment in central west part of Bangladesh. Groundwater for Sustainable Development, 9, 100219. https://doi.org/10.1016/j.gsd.2019.100219
Buragohain, M., Bhuyan, B., & Sarma, H. P. (2010). Seasonal variations of lead, arsenic, cadmium and aluminium contamination of groundwater in Dhemaji district, Assam. India. Environmental Monitoring and Assessment, 170, 345–351. https://doi.org/10.1007/s10661-009-1237-6
Cao, X., Lu, Y., Wang, Ch., Zhang, M., Yuan, J., Zhang, A., Song, Sh., Baninla, Y., Khan, K., & Wang, Y. (2019). Hydrogeochemistry and quality of surface water and groundwater in the drinking water source area of an urbanizing region. Ecotoxicology and Environmental Safety, 186, 109628. https://doi.org/10.1016/j.ecoenv.2019.109628
Chen, J., Qian, H., Gao, Y., Wang, H., & Zhang, M. (2020). Insights into hydrological and hydrochemical processes in response to water replenishment for lakes in arid regions. Journal of Hydrology, 581, 124386. https://doi.org/10.1016/j.jhydrol.2019.124386
Chen, J., Wu, H., Qian, H., & Gao, Y. (2017). Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of Northwest China. Exposure and Health, 9(3), 183–195. https://doi.org/10.1007/s12403-016-0231-9
Chen, J., Wu, H., & Qian, H. (2016). Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, northwest China. Exposure and Health, 8(3), 349–359. https://doi.org/10.1007/s12403-016-0208-8
Comisión Nacional de Agua (CONAGUA). (2020). Actualización de la disponibilidad medial anual de agua en el acuífero Río Mocorito (2503), Estado de Sinaloa. Diario Oficial de la Federación, 04 de enero de 2018, Gobierno Federal de los Estados Unidos Mexicanos, pp. 2–30. https://sigagis.conagua.gob.mx/gas1/Edos_Acuiferos_18/sinaloa/DR_2503.pdf (accessed 25 November 2020)
Comisión Nacional del Agua (CONAGUA). (2014). Información histórica, temporada de ciclones 2014. Servicio Meteorologico Nacional. https://smn.conagua.gob.mx/tools/DATA/Climatolog%C3%ADa/Pron%C3%B3stico%20clim%C3%A1tico/Temperatura%20y%20Lluvia/PREC/2013.pdf. Accessed 24 June 2021
Coyte, R. M., Singh, A., Furst, K. E., Mitch, W. A., & Vengosh, A. (2019). Co-occurrence of geogenic and anthropogenic contaminants in groundwater from Rajasthan Indian. Science of the Total Environment, 688(20), 1216–1227. https://doi.org/10.1016/j.scitotenv.2019.06.334
David, N., Bell, D., Gold, J. (2001). Field sampling manual for the regional monitoring program for trace substances. https://www.sfei.org/sites/default/files/biblio_files/FOM2001_0.pdf (accessed: 30 august 2020).
De Paiva-Magalhães, D., da Costa-Marques, M. R., Fernandes-Baptista, D., & Forsin-Buss, D. (2015). Metal bioavailability and toxicity in freshwaters. Environmental Chemistry Letters, 13, 69–87. https://doi.org/10.1007/s10311-015-0491-9
Dhaliwal, S. S., Singh, J., Taneja, P. K., & Mandal, A. (2020). Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environmental Science and Pollution Research, 27, 1319–1333. https://doi.org/10.1007/s11356-019-06967-1
Edet, A., Nganje, T. N., Ukpong, A. J., & Ekwere, A. S. (2011). Groundwater chemistry and quality of Nigeria: A status review. African Journal of Environmental Science and Technology, 5(13), 1152–1169. https://doi.org/10.5897/AJESTX11.011
Erickson, M. L., Yager, R. M., Kauffman, L. J., & Wilson, J. T. (2019). Drinking water quality in the glacial aquifer system, northern USA. Science of the Total Environment, 694(1), 133735. https://doi.org/10.1016/j.scitotenv.2019.133735
Fitzgerald, W.F. (1999). Clean hands, dirty hands: Clair Patterson and the aquatic biogeochemistry of mercury. In: Davidson, C.I. (Ed.), Clean Hands, Clair Patterson’s Crusade against Environmental Lead Contamination. Nova Science, Commack, NY, pp. 119–137.
Flegal, A. R., & Smith, D. R. (1995). Measurements of environmental lead contamination and human exposure. Reviews of Environmental Contamination and Toxicology, 143, 1–45.
Garau, M., Garau, G., Diquattro, S., Roggero, P. P., & Castaldi, P. (2019). Mobility, bioaccessibility and toxicity of potentially toxic elements in a contaminated soil treated with municipal solid waste compost. Ecotoxicology and Environmental Safety, 186, 1–10. https://doi.org/10.1016/j.ecoenv.2019.109766
García-Beltrán, A.N. (2008). Metodología para la generación y evaluación de políticas de operación en sistemas de recursos hídricos. Aplicación a un sistema en México. PhD Thesis. Universitat Politècnica de València. https://doi.org/10.4995/Thesis/10251/1970. (accessed, 17 February 2020).
García, E. (1964). Modificaciones al sistema de clasificación climática de Koppen. Offset Larios, Mexico City.
García-Gutiérrez, C., & Rodríguez-Meza, G. D. (2012). Problemática y riesgo ambiental por el uso de plaguicidas en Sinaloa. Ra Ximhai Revista De Sociedad Cultura y Desarrollo Sustentable, 8(3), 1–10.
Ghezzi, L., D’Orazio, M., Doveri, M., Lelli, M., Petrini, R., & Giannecchini, R. (2019). Groundwater and potentially toxic elements in a dismissed mining area: Thallium contamination of drinking spring water in the Apuan Alps (Tuscany, Italy). Journal of Geochemical Exploration, 197, 84–92. https://doi.org/10.1016/j.gexplo.2018.11.009
Ghobadi, A., Cheraghi, M., Sobhanardakani, S., Lorestani, B., & Merrikhpour, H. (2020). Hydrogeochemical characteristics, temporal, and spatial variations for evaluation of groundwater quality of Hamedan-Bahar Plain as a major agricultural region. West of Iran. Environmental Earth Sciences, 79, 428. https://doi.org/10.1007/s12665-020-09177-y
Health Canada. (2004). Federal contaminated site risk assessment in Canada. Part II: Health Canada toxicological reference values (TRVs). Health Canada, Ottawa.
Huq, M. E., Fahad, S., Shao, Z., Sarven, M. S., Khan, I. A., Alam, M., Saeed, M., Ullah, H., Adnan, M., Saud, S., Cheng, Q., Ali, S., Wahid, F., Zamin, M., Raza, M. A., Saeed, B., Riaz, M., & Khan, W. U. (2020). Arsenic in a groundwater environment in Bangladesh: Occurrence and mobilization. Journal of Environmental Management, 262, 110318. https://doi.org/10.1016/j.jenvman.2020.110318
Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). (2000). Guía para la asistencia técnica agrícola. Área de influencia del campo experimental. Fundación Produce Sinaloa. Consejo consultivo zona norte, pp 284.
Karam-Quiñones, C. (2002). Los agroquímicos: una perspectiva jurídica-ambiental. Análisis del caso Sinaloa. Colegio de Sinaloa. Culiacán, Sinaloa, México. P.p. 404.
Krieger, P., & Hagner, A. F. (1943). Gold-nickel mineralization at Alistos Sinaloa, Mexico. American Mineralogist: Journal of Earth and Planetary Materials, 28(4), 257–271.
Lenntech. (2015). Iron in groundwater. Lenntech water treatment and purification holding B.V, Rotterdamseweg, Netherlands. https://www.lenntech.com/groundwater/iron.htm (accessed: 9 March 2020)
Leyva-Morales, J. B., García de la Parra, L. M., Bastidas Bastidas, P. J., Astorga Rodríguez, J. E., Bejarano Trujillo, J., Cruz Hernández, A., et al. (2014). Pesticide use in a technified agricultural valley in Northwest Mexico [Uso de plaguicidas en un valle agrícola tecnificado en el Noroeste de México]. Revista Internacional De Contaminación Ambiental, 30(3), 247–261.
Liang, B., Han, G., Liu, M., Yang, K., Li, X., & Liu, J. (2018). Distribution, sources, and water quality assessment of dissolved heavy metals in the Jiulongjiang river water, Southeast China. International Journal of Environmental Research and Public Health, 15, 2752. https://doi.org/10.3390/ijerph15122752
Liang, Y., Yi, X., Dang, Z., Wang, Q., Luo, H., & Tang, J. (2017). Heavy metal contamination and health risk assessment in the vicinity of a tailing pond in Guangdong, China. International Journal of Environmental Research and Public Health, 14, 1557. https://doi.org/10.3390/ijerph14121557
McDonough, L. K., O’Carrolla, D. M., Meredith, K., Andersen, M. S., Brügger, C., Huang, H., Rutlidge, H., Behnke, M. I., Spencer, R. G. M., McKenna, A., Marjo, C. E., Oudone, P., & Baker, A. (2020). Changes in groundwater dissolved organic matter character in a coastal sand aquifer due to rainfall recharge. Water Research, 169, 115201. https://doi.org/10.1016/j.watres.2019.115201
Mora, A., Mahlknecht, J., Rosales-Lagarde, L., & Hernández-Antonio, A. (2017). Assessment of major ions and trace elements in groundwater supplied to the Monterrey metropolitan area, Nuevo León. Mexico. Environmental Monitoring and Assessment, 189, 394. https://doi.org/10.1007/s10661-017-6096-y
Morales-Zepeda, F. (2007). El impacto de la biotecnología en la formación de redes institucionales en el sector hortofrutícola de Sinaloa, México. PhD Thesis, Departamento de geografía física y análisis geográfico regional, Universidad de Barcelona. España, pp 441.
Páez-Osuna, F., Ramírez, G., Ruíz-Fernández, A.C., Soto-Jiménez, M.F. (2007). La contaminación por nitrógeno y fosforo en Sinaloa: flujos, fuentes, efectos y opciones de manejo. Serie lagunas costeras de Sinaloa. Primera edición. Universidad Nacional Autónoma de México. Instituto de Ciencias del Mar y Limnología, Unidad Académica Mazatlán.
Peinado-Guevara, H. J., Green-Ruiz, C. R., Herrera-Barrientos, J., Escolero-Fuentes, O. A., Delgado-Rodríguez, O., Belmonte-Jiménez, S. I., & Ladrón de Guevara, M. Á. (2011). Calidad y aptitud de uso agrícola y doméstico del agua del acuífero del río Sinaloa, porción costera. Hidrobiologica, 21(1), 63–76.
Qian, H., Chen, J., & Howard, K. W. F. (2020). Assessing groundwater pollution and potential remediation processes in a multi-layer aquifer system. Environmental Pollution, 263, 114669. https://doi.org/10.1016/j.envpol.2020.114669
Qiao, D., Wang, G., Li, X., Wang, S., & Zhao, Y. (2020). Pollution, sources and environmental risk assessment of heavy metals in the surface AMD water, sediments and surface soils around unexploited Rona Cu deposit, Tibet. China. Chemosphere, 248, 125988. https://doi.org/10.1016/j.chemosphere.2020.125988
Rajkumar, H., Naik, P. K., & Rishi, M. S. (2020). A new indexing approach for evaluating heavy metal contamination in groundwater. Chemosphere, 245, 125598. https://doi.org/10.1016/j.chemosphere.2019.125598
Rakib, M. A., Sasaki, J., Matsuda, H., Quraishi, S. B., Mahmud, M. J., Bodrud-Doza, M., Ullah, A. K. M. A., Fatema, K. J., Newaz, M. A., & Bhuiyan, M. A. H. (2020). Groundwater salinization and associated co-contamination risk increase severe drinking water vulnerabilities in the southwestern coast of Bangladesh. Chemosphere, 246, 125646. https://doi.org/10.1016/j.chemosphere.2019.125646
Ravindra, K., & Mor, S. (2019). Distribution and health risk assessment of arsenic and selected heavy metals in Groundwater of Chandigarh. India. Environmental Pollution, 250, 820–830. https://doi.org/10.1016/j.envpol.2019.03.080
Razo, I., Carrizales, L., Castro, J., Díaz-Barriga, F., & Monroy, M. (2004). Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water, Air and Soil Pollution, 152, 129–152.
Rehman, A., Bukhari, S. M., Andleeb, Sh., Mahmood, A., Erinle, K. O., Naeem, M. M., & Imran, Q. (2019). Ecological risk assessment of heavy metals in vegetables irrigated with groundwater and wastewater: The particular case of Sahiwal district in Pakistan. Agricultural Water Management, 226, 105816. https://doi.org/10.1016/j.agwat.2019.105816
Rivera-Hernández, J. R., Alvarado-Zambrano, D., Gonzales, L. A., & Green-Ruiz, C. (2019). Subtotal content and geochemical fractionation of potential toxic elements in agricultural soils from Mocorito River basin in NW Mexico: Environmental and health implications. International Journal of Environmental Health Research, 1, 1–17. https://doi.org/10.1080/09603123.2019.1700939
Rivera-Hernández, J. R., Green-Ruiz, C., Pelling-Salazar, L. E., & Trejo-Alduenda, A. (2017). Hidroquímica del acuífero costero del Río Mocorito, Sinaloa, México: Evaluación de la calidad del agua para consumo humano y agricultura. Hidrobiologica, 27(1), 103–113.
Robles-Camacho, J., & Armienta, M. A. (2000). Natural chromium contamination of groundwater at León Valley México. Journal of Geochemical Exploration, 68, 167–181.
Rodriguez, R., Armienta, M. A., Berlin, J., & Mejia, J. A. (2002). Arsenic and lead pollution of the Salamanca aquifer, Mexico: Origin, mobilization and restoration alternatives. Groundwater Quality: Natural and Enhanced Restoration of Groundwater Pollution, 275, 561–565.
Rosales, L., Carranza, A., Álvarez, U. (1986). Sedimentological and chemical studies in sediments from Alvarado lagoon system, Veracruz, Mexico. Universidad Nacional Autónoma de México. Anuario Instituto de Ciencias del Mar y Limnología 13(3), 19–28.
Rubio, B., Nombela, M., & Vilas, F. (2000). Geochemistry of major and trace elements in sediments of the Ría Vigo: Assessment of metal pollution. Marine Pollution Bulletin, 40(11), 968–980.
Rubio, B., Nombela, M., Vilas, F., Alejo, I., García-Gil, S., García-Gil, E., & Pazos, O. (1995). Distribución y enriquecimiento de metales pesados en sedimentos actuales de la parte interna de la Ría de Pontevedra. Thalassas, 11(3), 968–980.
Rubio, B., Pye, K., Rae, J., & Rey, D. (2001). Sedimentological characteristics, heavy metal distribution and magnetic properties in subtidal sediments, Ría de Pontevedra. Sedimentology, 48, 1–20.
Salvador, G. A. (2010). Iron in neuronal function and dysfunction. BioFactors, 36(2), 103–110. https://doi.org/10.1002/biof.80
Sanjrani, M. A., Zhou, B., Zhao, H., Bhutto, S. A., Muneer, A. S., & Xia, S. B. (2019). Arsenic contaminated groundwater in China and its treatment options, a review. Applied Ecology and Environmental Research, 17(2), 1655–1683. https://doi.org/10.15666/aeer/1702_16551683
Santra, D., Mandal, S., Santra, A., & Ghorai, U. K. (2018). Cost-effective, wireless, portable device for estimation of hexavalent chromium, fluoride, and iron in drinking water. Analytical Chemistry, 90, 12815–12823. https://doi.org/10.1021/acs.analchem.8b03337
Sarti, G., Sammartino, I., & Amorosi, A. (2020). Geochemical anomalies of potentially hazardous elements reflect catchment geology: An example from the Tyrrhenian coast of Italy. Science of the Total Environment, 714, 136870. https://doi.org/10.1016/j.scitotenv.2020.136870
Secretaria de Agricultura y Desarrollo Rural (SADER) and Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA). (2019). Manual para el buen uso y manejo de plaguicidas en campo, first ed. SENASICA, Mexico City. https://www.gob.mx/cms/uploads/attachment/file/452645/MANUAL_PARA_EL_BUEN_USO_Y_MANEJO_DE_PLAGUICIDAS_EN_CAMPO.pdf. (accessed: 17 February 2020).
Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). (2015). Acuerdo por el que se da a conocer el resultado de los estudios técnicos de aguas nacionales subterráneas del Acuífero Río Mocorito, Clave 2503, en el Estado de Sinaloa, Región Hidrológico-Administrativa Pacífico Norte. Diario Oficial de la Federación, 26 de agosto de 2015, Gobierno Federal de los Estados Unidos Mexicanos, Primera sección. http://www.dof.gob.mx/nota_detalle.php?codigo=5405188&fecha=26/08/2015 (accessed: 6 February 2020).
Secretaría de Salud (SSA). (2000). Modificación a la Norma Oficial Mexicana NOM-127-SSA1–1994, Salud ambiental. Agua para uso y consumo humano. Límites permisibles de calidad y tratamientos a que debe someterse el agua para su potabilización. Diario Oficial de la Federación, 22 de noviembre de 2000, Gobierno Federal de los Estados Unidos Mexicanos, Primera sección, 248–55. http://www.dof.gob.mx/nota_detalle.php?codigo=2063863&fecha=22/11/2000 (accessed: 17 February 2020).
Servicio Geológico Mexicano (SGM). (2015a). Carta geoquímica por Fe, escala: 1: 250,000, Pericos, Sinaloa, México. https://mapserver.sgm.gob.mx/Cartas_Online/geoquimica/39_G13-7_fe.pdf (accessed: 17 February 2020).
Servicio Geológico Mexicano (SGM). (2015b). Carta geoquímica por As, escala: 1: 250,000, Pericos, Sinaloa, México. https://mapserver.sgm.gob.mx/Cartas_Online/geoquimica/39_G13-7_as.pdf (accessed: 17 February 2020).
Servicio Geológico Mexicano (SGM). (2015c). Carta geoquímica por Pb, escala: 1: 250,000, Pericos, Sinaloa, México. https://mapserver.sgm.gob.mx/Cartas_Online/geoquimica/39_G13-7_pb.pdf (accessed: 17 February 2020).
Servicio Geológico Mexicano (SGM). (2015d). Carta geoquímica por Cd, escala: 1: 250,000, Pericos, Sinaloa, México. https://mapserver.sgm.gob.mx/Cartas_Online/geoquimica/39_G13-7_cd.pdf (accessed: 17 February 2020).
Setia, R., Dhaliwal, S.S., Kumar, V., Singh, R., Kukal, S.S., Pateriya, B. (2020). Impact assessment of metal contamination in surface water of Sutlej River (India) on human health risks. Environmental Pollution. 114907https://doi.org/10.1016/j.envpol.2020.114907
Sobhanardakani, S. (2017a). Potential health risk assessment of heavy metals via consumption of caviar of Persian sturgeon. Marine Pollution Bulletin, 123, 34–38.
Sobhanardakani, S. (2017b). arsenic health risk assessment through groundwater drinking (case study: Qaleeh Shahin Agricultural Region, Kermanshah Province, Iran). Pollution, 4(1), 77–82. https://doi.org/10.22059/poll.2017.236875.291
Sobhanardakani, S. (2018). Health risk assessment of inorganic arsenic through groundwater drinking pathway in some agricultural districts of Hamedan, West of Iran. Avicenna Journal of Environmental Health Engineering, 5(2), 73–77. https://doi.org/10.15171/ajehe.2018.10
Sobhanardakani, S., Tayebi, L., & Hosseini, S. V. (2018). Health risk assessment of arsenic and heavy metals (Cd, Cu Co, Pb, and Sn) through consumption of caviar of Acipenser persicus from Southern Caspian Sea. Environmental Science and Pollution Research, 25, 2664–2671. https://doi.org/10.1007/s11356-017-0705-8
Soto-Jimenez, M. F., Hibdon, S. A., Rankin, Ch. W., Aggarawl, J., Ruiz-Fernandez, A. C., Paez-Osuna, F., & Flegal, A. R. (2006). Chronicling a century of lead pollution in Mexico: Stable lead isotopic composition analyses of dated sediment cores. Environmental Science and Technology, 40(3), 764–770. https://doi.org/10.1021/es048478g
Tang, D., Warnken, K. W., & Santschi, H. (2002). Distribution and partitioning of trace metals (Cd, Cu, Ni, Pb, Zn) in Galveston Bay waters. Marine Chemistry, 78, 29–45.
Thuyet, D. Q., Saito, H., Saito, T., Moritani, S., Kohgo, Y., & Komatsu, T. (2016). Multivariate analysis of trace elements in shallow groundwater in Fuchu in western Tokyo metropolis. Japan. Environmental Earth Sciences, 75, 559. https://doi.org/10.1007/s12665-015-5170-4
United States Environmental Protection Agency (USEPA). (1989). Risk assessment guidance for superfund. Human health evaluation manual, (part a) (Vol. 1). Washington, D.C.: Office of Emergency and Remedial Response.
United States Environmental Protection Agency (USEPA). (1999a). Guidance for performing aggregate exposure and risk assessments, Office of Pesticide Programs, Washington, DC. https://archive.epa.gov/scipoly/sap/meetings/web/pdf/guidance.pdf (accessed: 9 March 2020)
United States Environmental Protection Agency (USEPA). (1999b). A risk assessment–multi way exposure spread sheet calculation tool. United States Environmental Protection Agency, Washington, DC
United States Environmental Protection Agency (USEPA). (2001). Parameters of water quality: Interpretation and standards. Ireland. 133 pp. https://www.epa.ie/pubs/advice/water/quality/Water_Quality.pdf (accessed: 2 March 2020).
Upadhyaya, D., Survaija, M. D., Basha, S., Mandal, S. K., Thorat, R. B., Haldar, S., Goel, S., Dave, H., Baxi, K., Trivedi, R. H., & Mody, K. H. (2014). Occurrence and distribution of selected heavy metals and boron in groundwater of the Gulf of Khambhat region, Gujarat. India. Environmental Science and Pollution Research, 21, 3880–3890. https://doi.org/10.1007/s11356-013-2376-4
Universidad Nacional Autónoma de México (UNAM). (1978). Atlas geológico y evaluación geológica minera del Estado de Sinaloa. Rodríguez R., Cordoba D. (eds). Instituto de Geología. Universidad Nacional Autónoma de México. Hojas I ‘‘Mocorito’’; II ‘‘Culiacán’’; III ‘‘Tamazula’’ y IV ‘‘La Peña’’, México.
Wang, Z.-L., & Liu, C.-Q. (2003). Distribution and partition behavior of heavy metals between dissolved and acid-soluble fractions along a salinity gradient in the Changjiang Estuary, eastern China. Chemical Geology, 202, 383–396. https://doi.org/10.1016/j.chemgeo.2002.05.001
Wuana, R., & Okieimen, F. (2011). Heavy metals in contaminated soils: A review of sources, chemistry, risks and best strategies for remediation. International Scholarly Research Network Ecology, 402647, 1–20. https://doi.org/10.5402/2011/402647
World Health Organization (WHO). (2017). Guidelines for drinking-water quality: Fourth edition incorporating the first addendum. Geneva, 541 pp. https://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf?sequence=1 (accessed 2 March 2020).
Xiong, X., Liu, X., Yu, I. K. M., Wang, L., Zhou, J., Sun, X., Rinklebe, J., Shaheen, S. M., Ok, Y. S., Lin, Z., & Tsang, D. C. W. (2019). Potentially toxic elements in solid waste streams: Fate and management approaches. Environmental Pollution, 253, 680–707. https://doi.org/10.1016/j.envpol.2019.07.012
Zgheib, S., Moilleron, R., Saad, M., & Chebbo, G. (2011). Partition of pollution between dissolved and particulate phases: What about emerging substances in urban stormwater catchments? Water Research, 45, 913–925. https://doi.org/10.1016/j.watres.2010.09.032
Zhou, M., Liao, B., Shu, W., Yang, B., & Lan, C. (2015). Pollution assessment and potential sources of heavy metals in agricultural soils around four Pb/Zn mines of Shaoguan City, China. Soil and Sediment Contamination: An International Journal, 24(1), 76–89. https://doi.org/10.1080/15320383.2014.914152.
Acknowledgements
The authors thank Sharon Hibdon† and Rob Frank of the University of California at Santa Cruz, for their support in the treatment, processing, and chemical analysis in the ICP-MS. In addition, they extend their appreciation to Francisco Montes of the National Water Commission (CONAGUA) in Guamuchil, Sinaloa, and to Alejandra Trejo Alduenda, Nuria Alonso, and Hernán Quiroga for their invaluable support during the fieldwork.
Funding
This research was supported by the Programa de Apoyo para la Superación del Personal Académico (PASPA) and Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPPIT-IN107813) of the Universidad Nacional Autónoma de México. J. R. Rivera-Hernández and L. Pelling-Salazar had doctorate and master scholarship, respectively, from Consejo Nacional de Ciencia y Tecnología.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rivera-Hernández, J.R., Green-Ruiz, C.R., Pelling-Salazar, L.E. et al. Monitoring of As, Cd, Cr, and Pb in Groundwater of Mexico’s Agriculture Mocorito River Aquifer: Implications for Risks to Human Health. Water Air Soil Pollut 232, 291 (2021). https://doi.org/10.1007/s11270-021-05238-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05238-5