Abstract
Cadmium (Cd) is considered one of the heavy metals disturbing plant biophysiological functions. The potential role of phosphorus (P) nutrition in the attenuation of Cd effects on photosynthetic efficiency, plant growth, and cadmium uptake has been investigated in hydroponically grown tomato. Two P nutrition regimes (P15: 15 mg l-1; P30: 30 mg l-1) were assessed in the presence or absence of Cd (Cd0: 0 μM; Cd25: 25 μM of CdCl2). The results showed a positive effect of P30 concentration on leaf chlorophyll content and chlorophyll a fluorescence compared to P15 treatment under Cd stress (Cd25). The disturbance of electron transfer caused by Cd at K and I-steps of OJIP transient was attenuated with sufficient P supply. P30 enhanced the performance index of photosystem II and the efficiency of electron transfer to electron acceptor at PSI acceptor side. Besides, increased P concentration improved root growth parameters and biomass accumulation in the presence of Cd. It was found that root tissues accumulated more Cd than shoots and Cd translocation was reduced with increasing P concentration. Our results reveal that Cd-P interaction induced a cascade of physiological and chemical changes in plants. An optimal P nutrition can attenuate Cd stress on plant by the promotion of nitrogen and potassium uptake, which in return improved photosynthesis efficiency, enhanced biomass accumulation and distribution, and minimized Cd accumulation and translocation in plant tissues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cadmium (Cd) is known to be one of the most stressful heavy metals to plants (Dos Santos Utmazian and Wenzel 2007; Armas et al. 2015). An excessive amount of Cd impacts the physiological functions and morphological features of most crop species (Andresen and Küpper 2013; He et al. 2017). Many studies have reported a significant decrease in photosynthetic efficiency (Dong et al. 2005), nutrients uptake (Carvalho Bertoli et al. 2012; Przedpelska-Wasowicz et al. 2012), biomass production, and crops yield in Cd-contaminated conditions (Hasan et al. 2009; Dias et al. 2013; Rusinowski et al. 2019). As well, high Cd levels affect negatively root growth and architecture, which impact nutrients uptake and mobility in plant tissues (Khan et al. 2016; Nazarian et al. 2016; Peng et al. 2017).
Phosphorus (P) is an essential nutrient needed by plants in sufficient quantity for their growth and development. P is involved in several physiologic and metabolic processes, including structural compound formation, energy transfer, cell division and elongation, carbon assimilation, and nitrogen metabolism (Malhotra et al. 2018). P can also play a protective role again environmental stress such as heavy metal accumulation and their mobility in soil and plant (Shi et al. 2015; Dai et al. 2017).
The previous studies interested in P-Cd interaction were mainly focused on the impact of P and Cd coexistence on Cd bioavailability and accumulation in plant biomass (Sajwan et al. 2002; Yu and Zhou 2009; Qiu et al. 2011). Qiu et al. (2011) have attributed the reduction of Cd uptake to the aptitude of P to fix Cd in the cell walls and forming Cd-phosphate complexes (Van Belleghem et al. 2007). Dai et al. (2017) reported that P regulated the photosynthetic pigment and proline content, and synthesis of non-protein thiols, glutathione, and phytochelatins in the leaves under Cd stress. P at appropriate content may attenuate Cd-induced stress by the enhancement of plant growth and the immobilization of Cd in the contaminated soils (Yu and Zhou 2009).
Additionally, Cd affects the photosynthesis apparatus by disturbing photosystem II (PSII) and photosystem I (PSI) activities in light-dependent photosynthesis processes. Paunov et al. (2018) reported a decrease in electron transfer rate from PSII to PSI under Cd stress conditions, which reduce the photosynthesis yield and CO2 assimilation by plants. Theoretical and technological advances in chlorophyll a fluorescence (ChlF) have been extensively contributed to better understand photosynthetic processes in plants (Kalaji et al. 2014; Tóth et al. 2020). The physiological state of PSII components, electron transport, and light-dependent biochemical reactions of the photosynthetic apparatus can be rapidly assessed by the non-invasive ChlF methods under different environment (Tuba et al. 2010; Dąbrowski et al. 2015; Kalaji et al. 2017), with interesting responses under abiotic stress conditions (Ashraf and Harris 2004; Kalaji and Loboda 2007; Da˛browski et al. 2019; Loudari et al. 2020).
Given the role of P in photosynthesis (ATP synthesis) (Cetner et al. 2020), the present study aims to elucidate how P-Cd interaction impacts photosynthesis efficiency using ChlF measurements and evaluating its effect on plant growth, nutrient uptake, and Cd accumulation.
2 Materials and Methods
2.1 Plant Growth Conditions
Seeds of Campbell 33 tomato cultivar were firstly germinated in growth chamber conditions (24 °C, 16/8 photoperiod, 70 % relative humidity, and 250 μmol m-2 s-1 light intensity) in commercial peat substrate and irrigated four times a week by distilled water (< 2 μs cm-1). After 23 days, seedlings were washed free of peat and transplanted in 3 liters polyethylene pot in a half-concentrated Hoagland solution (N: 242 mg l-1 as KNO3, Ca(NO3)2∙4H2O, and NH4NO3; P: 30 mg l-1 as KH2PO4; K: 232 mg l-1 as KNO3 and KH2PO4; Ca: 224 mg l-1 as Ca(NO3)2∙4H2O; Mg: 49 mg l-1 as MgSO4∙7H2O; B: 0.45 mg l-1 as H3BO3; Cu: 0.02 mg l-1 as CuSO4.5H2O; Mn: 0.5 mg l-1 as MnCl2∙4H2O; Mo: 0.0106 mg l-1 as Na2MoO4. 2H2O; Zn: 0.48 mg l-1 as ZnSO4.7H2O; and Fe: 0.5% of (NH4)5[Fe(C6H4O7)2] used at rate 1 ml l-1 of nutrient solution) (Hoagland and Arnon 1950) with three plants per pot. After this adaptive phase (1 week), the seedlings received a fully concentrated nutrient solution and exposed to two phosphorus regimes (P15:15 mg l-1 and P31: 31 mg l-1 of P) with/without cadmium (Cd0: 0 μM and Cd25: 25 μM of CdCl2) for 20 days. The experiment was arranged as a completely randomized design with three replicates and the nutrient solution was renewed once a week.
2.2 Chlorophyll Content Index
The impact of Cd and P interaction on photosynthetic efficiency was assessed by the measurement of the chlorophyll content index (CCI) in the middle of mature young leaves after 14 days of Cd and P treatment. The CCI measurements were taken by Chlorophyll meter CL-01 (Hansatech Instruments Ltd. United Kingdom).
2.3 Chlorophyll α Fluorescence Parameters
To understand the effect of Cd and P interaction on photosynthetic efficiency and electron transfer into photosystem II (PSII), the ChlF analysis was performed using Handy PEA+ fluorometer (Handy PEA+, Hansatech Instruments Ltd., UK). ChlF measurements were taken after 2 weeks of Cd and P treatments on tomato plant previously adapted to darkness for 20 min and illuminated with 650 nm light of 3000 μmol m−2 s−1 for 1 s.
The ChlF OJIP transient curve represents a polyphasic rise during the first second of illumination with four main steps (O: minimum fluorescence intensity Fo when all reaction centers are open and all QA oxidized; J and I: intermediate steps named (FJ) and (FI); P: maximum fluorescence intensity FM when all reaction centers are closed and all QA reduced (Strasser et al. 2004; Tsimilli-Michael and Strasser 2008; Oukarroum et al. 2009).
Double normalization of ChlF intensity was performed to determine the relative variable chlorophyll fluorescence (Vt) (Equation 1). The differential values (∆Vt) resulted from the subtraction of Vt (Cd0P30) from Vt of Cd exposed treatments (Cd25P15 or Cd25P30) (Tsimilli-Michael and Strasser 2013).
Other OJIP test parameters were calculated according to the following equations in Table 1 (Strasser et al. 2004; Tsimilli-Michael and Strasser 2013; Paunov et al. 2018)
2.4 Plant Growth Parameters
The harvested plants were firstly washed with distilled water and root parts were immediately speared to shoot for analyzing root architecture using LA2400 scanner and the WinRHIZO software (Regent Instruments Inc. Canada). Root morphology measurement focused mainly on total root length, average root diameter, number of tips (lateral root), forks, root surface area, and volume. Root and shoot biomasses were dried at 70 °C for 48 h and the dry weight was taken.
2.5 Cadmium and Nutrient Uptake and Translocation
The dried plant samples (root and shoot separately) were powdered and digested by nitric acid and analyzed for Cd, macro and micronutrient content using Inductively Coupled Plasma Optical Emission Spectrometry (Agilent 5110 ICP-OES, USA). The total N content in root and shoot was assessed by the Kjeldahl method (KjelMaster K-375, Netherlands). Concerning Cd mobility in the tomato plant, it was assessed by the translocation factor according to the following equation (Das and Maiti 2007):
2.6 Statistical Analysis
The studied parameters were statistically analyzed using the analysis of variance (ANOVA), considering factorial experimental design (two-way ANOVA, P*Cd) with three replicates per treatment. All data analyses were performed with the SPSS data processing software (SPSS 20.0) and mean differences between treatments were evaluated by the Student’s test at a 0.05 probability level.
3 Results
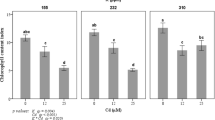
3.1 Effect of Cd-P Interaction on Chlorophyll Content
Figure 1 shows a positive effect of P concentration on tomato CCI in both cadmium concentrations (Cd0 and Cd25). In the presence of 25 μM l-1 of CdCl2 in the nutrient solution, the CCI decreased significantly by 26% in P15 compared to untreated treatment (Cd0). This reduction of CCI under Cd exposure was less accentuated with P30 treatment (20%) showing a positive effect of P-Cd interaction on chlorophyll content.
3.2 Effect of Cd-P Interaction on Chlorophyll α Fluorescence Parameters
Chlorophyll a fluorescence induction curves, as well as OJIP test parameters, have been used to assess the impact of P-Cd interaction on photosynthetic efficiency. Figure 2a shows no significant differences in initial fluorescence intensity (F0) between studied treatments. However, the maximal level (FM) was affected by Cd stress mostly under P15 conditions. Such a decline in FM under Cd stress may be related to a decrease of chlorophyll α content in tomato leaves (Paunov et al. 2018). To analyze differences between the induction curve’s form in response to P-Cd interaction, the double normalized fluorescent intensity (Vt), as well as curves of differential values related to P30Cd0 treatment (∆Vt), was performed (Fig. 2b and Fig. 2c). As a result, two positive peaks were observed at K and J steeps which describe the dissociation of the oxygen-evolving complex (OEC) and reduction of the secondary electron acceptor QB respectively (Kalaji et al. 2014). The extent of these differential curves at K steep was smaller with P30Cd25 than P15Cd25. Similarly, in J steep, Cd exposure caused a significant reduction of electron transfer rate from QA to QB at the acceptor side of PSII, in P15 treatment comparatively to P30. Also, a negative peak of ∆Vt was detected with P15 at I steep which is attributed to the reduction of electron transporters of the PSI acceptor-side (Carstensen et al. 2018), which can be explained by the high intersystem pool and PSI end electron acceptors per active PSII RC as demonstrated by Paunov et al. (2018). Other parameters and ratios were calculated to evaluate the effect of P-Cd interaction on photosynthetic function and yields. Results in Fig. 2d showed a slight increase in φPO, φEO, γRC, and PIABS and decrease of ABS/RC and δRO in P30 than P15 under Cd stress.
Effect of cadmium and phosphorus interaction on Chl a fluorescence transient curve (logarithmic time scale) (a); relative chlorophyll a fluorescence (Vt) (b); differential values (∆Vt) resulting from the subtraction of Cd0P30 treatment (0 μM l-1 CdCl2 + 30 mg l-1 P) Vt from Cd and P treatments (c); and OJIP parameters (d): φPO:Quantum yield (at t = 0) for electron transport from QA− to plastoquinone; φEO: Quantum yield of electron transport (at t = 0); ABS/RC: absorption flux (of antenna Chls) per RC; γRC: probability that a PSII Chl molecule functions as RC; PIABS:performance index (potential) for energy conservation from photons absorbed by PSII to the reduction of intersystem electron acceptors; δRO:efficiency/probability (at t = 0) with which an electron from the intersystem carriers moves to reduce end electron acceptors at the PSI acceptor side. The results are means ± SD (n = 3), and columns denoted by a different letter differ significantly at p < 0.05 according to Student’s test
3.3 P improves Plant Growth Parameters Under Cd Stress Conditions
Results in Table 2 showed that the application of 25 μM of CdCl2 in nutrient solution reduced shoot growth (leaf and steam) in both P concentrations (P15 and P30) comparatively to Cd0. In P15 treatment, Cd stress reduced shoot dry weight by 36%, though the impact of Cd on this parameter has been partially offset with P addition (P30), showing 21% of shoot dry weight improvement in comparison to P15. On the other hand, no significant effect of Cd on root dry weight was observed for both P nutrition regimes. Nevertheless, a notable positive effect of P concentration on root dry weight was detected even under Cd stress.
3.4 Effect of Cd-P Interaction on Root Growth and Morphology
Data collected, from the analysis of root morphology parameters, showed that 25 μM of CdCl2 reduced significantly total root length by 70% and 65% in P15 and P30 treatments respectively (Fig. 3b). Similarly, for root tips and forks, Cd exposure resulted in a significant reduction of lateral root number (tips) (Figs. 3d, 3e). In absence of Cd, increased P concentration in the nutrient solution (from P15 to P30) improved root tips and forks per plant, while in presence of Cd, little improvement of these two root parameters was observed in response to P (Figs. 3d, 3e). In contrast, the average root diameter was significantly improved in Cd-treated treatments compared to Cd0 in both P concentrations (Fig. 3c). However, no effect of Cd and P treatments was detected for total root volume (Fig. 3g).
Changes in tomato root architecture (a) and morphological parameters in response to cadmium and phosphorus interaction; b total root length (cm); c root average diameter (mm); d root tips number; e root forks number; f root surface area (cm2); and g total root volume (cm3). The results are means ± SD (n = 3), and columns denoted by a different letter differ significantly at p < 0.05 according to Student’s test
3.5 Phosphorus Attenuates Cadmium Uptake and Translocation in Tomato
According to results in Figs. 4a and 4b, the presence of Cd in nutrient solution increased Cd accumulation in both roots and shoot biomass comparatively to Cd0. All treatments showed that root tissues were accumulated more Cd than shoots. Furthermore, the P30 treatment reduced Cd uptake and translocation factor (Fig. 4c).
Cadmium content in the root (a); shoot (b); and translocation factor (%) (c) of tomato plant grown in tow phosphorus regimes (15 and 30 mg l-1 P), in the absence and presence of cadmium (0 and 25 μM l-1 of CdCl2). The results are means ± SD (n = 3), and columns denoted by a different letter differ significantly at p < 0.05 according to Student’s test
3.6 Macronutrients Uptake in Response to P-Cd Interaction
Nutrients uptake is also affected by Cd stress; results in Table 3 showed several changes in macronutrient uptake and accumulation in tomato plant tissues. The application of Cd25 in nutrient solution increased significantly root absorption of N and K mainly in the P30 regime, showing that the positive synergy between P, N, and K is maintained even under Cd stress. Likewise, root Ca and Mg contents have been little improved under Cd stress; no P impact was noted for these two nutrients. In contrast, Cd addition reduced N and K accumulation in shoot mainly in P15 treatment and no significant effect on shoot Ca and Mg content was observed. Nevertheless, an improvement of shoot P content was noted in response to P treatment.
4 Discussion
By the present study, we demonstrate that adequate phosphorus nutrition can attenuate the impact of cadmium stress on tomato plants under hydroponic conditions. The use of P30 concentration in nutrient solution improves plant tolerance to Cd toxicity by the enhancement of chlorophyll content and photosynthetic efficiency. As mentioned in Fig. 1, Cd stress reduces leaf chlorophyll content. Puła et al. (2019) attributed this reduction to the disturbance of chlorophyll synthesis caused by the similarities and interferences of Cd with Fe and Mg in terms of chemical properties and root absorption pathways (Pagliano et al. 2006; Per et al. 2017). Similar results were reported in the previous study, indicating the inhibitory effect of Cd on chlorophyll pigment synthesis (Huang et al. 1997; Per et al. 2017; Song et al. 2019). Regarding P-Cd interaction, P30 improves the chlorophyll content index under Cd stress by 6% in contrast to P15 treatment. This result is following that of Manikandan et al. (2016) who reported that supplementation P enhanced chlorophyll content of vetiver grass grown in Cd stress conditions.
The analysis of chlorophyll a fluorescence transient shows that P plays an important role in protecting electron transfer from PSII to PSI in both Cd conditions (Cd0 and Cd25). Results in Fig. 2d indicate that increased P concentration enhances the performance index of PSII (PIABS) and the efficiency of electron transfer from the intersystems to electron acceptor at PSI acceptor side (δRO). As shown in Figs. 2a and 2c, the disturbance of electron transfer caused by Cd stress at K and I-steps of OJIP transient curves was attenuated with sufficient P supply (P30) (Cetner et al. 2020). Phosphorus is directly involved in the photosynthesis process particularly during the ATP synthesis (ADP + Pi = ATP). A disorder in P nutrition can alter this process, which causes some disturbances in light-dependent photosynthesis processes (electron transfer from PSII to PSI). As demonstrated by Carstensen et al. (2018), P deficiency reduces ATP synthase activity which caused a reduction of protons-flow from the thylakoid lumen to the chloroplast stroma, inducing lumen acidification and restriction of plastoquinones oxidation. Through these findings, we suggest that P can mitigate Cd impacts on photosynthetic apparatus by the enhancement of PSII behavior via the increase of active reaction centers (RC) number and electron transfer rate (Paunov et al. 2018). P improves also the reduction rate of the terminal electron acceptors in PSI (I-step of OJIP transient) (Kalaji et al. 2014).
Changes in root morphology are defensive measures undertaken by plants to attenuate heavy metals stress (Shafi et al. 2010; Huang et al. 2014; Jinadasa et al. 2016). Figure 3 presents the impact of Cd on root architecture, showing that Cd decreased root length, root tips, and increased root diameter. These morphological changes (physical barrier) aim to limit Cd uptake but can disturb nutrients uptake and other bio-physiological functions as mentioned in Fig. 2 and Table 3. Our results reveal that P moderates these changes in root morphology by the enhancement of lateral root formation and root length (Fig. 3) to ensure sufficient nutrient acquisition and assimilates partitioning (root/shoot ratio, Table 2). A close relationship between P supply and phytohormones (auxin and cytokinin), involved in lateral root initiation and elongation, was documented indicating a possible protective role of P against Cd toxicity on plant roots (Bruno et al. 2017).
Our results partly agreed with other previous studies which have reported that Cd stress reduced both root and shoot dry weights and increased root/shoot ratio for many other crops (Shafi et al. 2010; Huang et al. 2014; Jinadasa et al. 2016). Besides the effect of Cd on tomato growth, the P nutrition (P30) might play an important role in limiting Cd-impact on root and shoot growth, through the improvement of photosynthetic efficiency (Fig. 2). The probable Cd immobilization in nutrient solution and/or inside roots by the formation of Cd-P complexes (CdHPO4 and Cd3(PO4)2) in vacuoles and cell wall can reduce Cd uptake as demonstrated by (Jiang et al. 2007; Qiu et al. 2011; Rizwan et al. 2017).
Our finding shows that the effect of the Cd-P interaction induces a cascade of actions linked to certain physiological processes in the plant.
Cadmic stress disrupts the absorption of macronutrients by the plant and their content in different parts of the plant, which from this study, we demonstrate that Cd-stress induce a cascade of actions of physiological processes in plants. The content of macronutrients decides about the plant photosynthetic performance, which in return influences (among other factors) biomass production and its accumulation. We suggest here that P nutrition play a key role in the attenuation of Cd-effects on biophysiological processes in plants. An optimal P-nutrition regime significantly promotes nitrogen and potassium uptake, which improves photosynthesis efficiency (including chlorophyll content and chlorophyll fluorescence parameters), enhances biomass accumulation (root and shoot) and distribution (root /shoot ratio), and minimizes Cd uptake and translocation in plant tissues.
References
Andresen, E., & Küpper, H. (2013). Cadmium toxicity in plants. Metal Ions in Life Sciences, 11, 395. https://doi.org/10.1007/978-94-007-5179-8_13.
Armas, T., Pinto, A.P., de Varennes, A., Mourato, M.P., Martins, L.L., Gonçalves, M.L.S., Mota, A.M. (2015). Comparison of cadmium-induced oxidative stress in Brassica juncea in soil and hydroponic cultures. Plant Soil, 388, 297–305. https://doi.org/10.1007/s11104-014-2330-3.
Ashraf, M., Harris, P.J.C. (2004). Potential biochemical indicators of salinity tolerance in plants. Plant Science, 166, 3–16. https://doi.org/10.1016/j.plantsci.2003.10.024.
Bruno, L., Pacenza, M., Forgione, I., Lamerton, L. R., Greco, M., Chiappetta, A., & Bitonti, M. B. (2017). In arabidopsis thaliana cadmium impact on the growth of primary root by altering SCR expression and auxin-cytokinin cross-talk. Frontiers in Plant Science, 8, 1323. https://doi.org/10.3389/fpls.2017.01323.
Carstensen, A., Herdean, A., Schmidt, S. B., Sharma, A., Spetea, C., Pribil, M., & Husted, S. (2018). The impacts of phosphorus deficiency on the photosynthetic electron transport chain1. Plant Physiology, 177(1), 271–284. https://doi.org/10.1104/PP.17.01624.
Carvalho Bertoli, A., Gabriel Cannata, M., Carvalho, R., Ribeiro Bastos, A.R., Puggina Freitas, M., dos Santos Augusto, A. (2012). Lycopersicon esculentum submitted to Cd-stressful conditions in nutrition solution: Nutrient contents and translocation. Ecotoxicology and Environmental Safety. 86, 176–181. https://doi.org/10.1016/j.ecoenv.2012.09.011.
Cetner, M. D., Kalaji, H. M., Borucki, W., & Kowalczyk, K. (2020). Phosphorus deficiency affects the i-step of chlorophyll a fluorescence induction curve of radish. Photosynthetica, 58(SI), 671–681. https://doi.org/10.32615/ps.2020.015.
Da˛Browski, P., Baczewska-Dąbrowska, A. H., Kalaji, H. M., Goltsev, V., Paunov, M., Rapacz, M., et al. (2019). Exploration of chlorophyll a fluorescence and plant gas exchange parameters as indicators of drought tolerance in perennial ryegrass. Sensors (Switzerland), 19(12), 2736. https://doi.org/10.3390/s19122736.
Dąbrowski, P., Pawluśkiewicz, B., Baczewska, A. H., Oglęcki, P., & Kalaji, H. (2015). Chlorophyll a fluorescence of perennial ryegrass (Lolium perenne L.) varieties under long term exposure to shade. Zemdirbyste-Agriculture, 102, 305. https://doi.org/10.13080/z-a.2015.102.039.
Dai, M., Lu, H., Liu, W., Jia, H., Hong, H., Liu, J., Yan, C. (2017). Phosphorus mediation of cadmium stress in two mangrove seedlings Avicennia marina and Kandelia obovata differing in cadmium accumulation. Ecotoxicology and Environmental Safety, 139, 272–279. https://doi.org/10.1016/j.ecoenv.2017.01.017.
Das, M., & Maiti, S. K. (2007). Metal accumulation in A. baccifera growing naturally on abandoned copper tailings pond. Environmental Monitoring and Assessment, 127(1–3), 119–125. https://doi.org/10.1007/s10661-006-9265-y.
Dias, M.C., Monteiro, C., Moutinho-Pereira, J., Correia, C., Gonçalves, B., Santos, C. (2013). Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiologiae Plantarum, 35, 1281–1289. https://doi.org/10.1007/s11738-012-1167-8.
Dong, J., Wu, F.B., Zhang, G.P. (2005). Effect of cadmium on growth and photosynthesis of tomato seedlings. Journal of Zhejiang University. Science, 6, 974–980. https://doi.org/10.1631/jzus.2005.B0974.
Dos Santos Utmazian, M. N., & Wenzel, W. W. (2007). Cadmium and zinc accumulation in willow and poplar species grown on polluted soils. Journal of Plant Nutrition and Soil Science, 170(2), 265–272. https://doi.org/10.1002/jpln.200622073.
Hasan, S. A., Fariduddin, Q., Ali, B., Hayat, S., & Ahmad, A. (2009). Cadmium: toxicity and tolerance in plants. Journal of Environmental Biology, 30(2), 165–174.https://doi.org/10.1016/c2017-0-02050-5.
He, S., Yang, X., He, Z., & Vc, B. (2017). Morphological and physiological responses of plants to cadmium toxicity: a review. Pedosphere, 3, 53–60. https://doi.org/10.1016/S1002-0160(17)60339-4.
Hoagland, D R; Arnon, D. I. (1938). Agricultural Experiment Station the Water-Culture Method for Growing Plants Without Soil. California Experiment Station, C347, 1–39.
Huang, X. D., Mcconkey, B. J., Babu, T. S., & Greenberg, B. M. (1997). Mechanisms of photoinduced toxicity of photomodified anthracene to plants: Inhibition of photosynthesis in the aquatic higher plant Lemna gibba (duckweed). Environmental Toxicology and Chemistry, 16(8), 1707–1715. https://doi.org/10.1002/etc.5620160819.
Huang, B., Xin, J., Dai, H., Liu, A., Zhou, W., Yi, Y., & Liao, K. (2014). Root morphological responses of three hot pepper cultivars to Cd exposure and their correlations with Cd accumulation. Environmental Science and Pollution Research, 22(2), 1151–1159. https://doi.org/10.1007/s11356-014-3405-7.
Jiang, H. M., Yang, J. C., & Zhang, J. F. (2007). Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environmental Pollution, 147(3), 750–756. https://doi.org/10.1016/j.envpol.2006.09.006.
Jinadasa, N., Collins, D., Holford, P., Milham, P. J., & Conroy, J. P. (2016). Reactions to cadmium stress in a cadmium-tolerant variety of cabbage (Brassica oleracea L.): is cadmium tolerance necessarily desirable in food crops? Environmental Science and Pollution Research, 23(6), 5296–5306. https://doi.org/10.1007/s11356-015-5779-6.
Kalaji, H. M., & Loboda, T. (2007). Photosystem II of Barley seedlings under cadmium and lead stress. Plant, Soil and Environment, 53(12), 511–516. https://doi.org/10.17221/2191-pse.
Kalaji, H.M., Jajoo, A., Oukarroum, A., Brestic, M., Zivcak, M., Samborska, I.A., Cetner, M.D., Łukasik, I., Goltsev, V., Ladle, R.J., et al. (2014). The use of chlorophyll fluorescence kinetics analysis to study the performance of photosynthetic machinery in plants. In Emerging Technologies Managing Crop Stress Tolererance, 2, 347–384. https://doi.org/10.1016/B978-0-12-800875-1.00015-6.
Kalaji, H. M., Dąbrowski, P., Cetner, M. D., Samborska, I. A., Łukasik, I., Brestic, M., et al. (2017). A comparison between different chlorophyll content meters under nutrient deficiency conditions. Journal of Plant Nutrition, 40(7), 1024–1034. https://doi.org/10.1080/01904167.2016.1263323.
Khan, A., Khan, S., Alam, M., Khan, M. A., Aamir, M., Qamar, Z., Rehman, Z. U., & Perveen, S. (2016). Toxic metal interactions affect the bioaccumulation and dietary intake of macro- and micro-nutrients. Chemosphere, 146, 121–128.https://doi.org/10.1016/j.chemosphere.2015.12.014.
Loudari, A., Benadis, C., Naciri, R., Soulaimani, A., Zeroual, Y., El Gharous, M., et al. (2020). Salt stress affects mineral nutrition in shoots and roots and chlorophyll a fluorescence of tomato plants grown in hydroponic culture. Journal of Plant Interactions, 15(1), 398–405. https://doi.org/10.1080/17429145.2020.1841842.
Malhotra, H., Vandana Sharma, S., Pandey, R. (2018). Phosphorus nutrition: plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance, 171–190. https://doi.org/10.1007/978-981-10-9044-8_7.
Manikandan, R., Ezhili, N., & Venkatachalam, P. (2016). Phosphorus Supplementation Alleviation of the Cadmium-Induced Toxicity by Modulating Oxidative Stress Mechanisms in Vetiver Grass [ Chrysopogon zizanioides (L.) Roberty]. Journal of Environmental Engineering, 142(9). https://doi.org/10.1061/(asce)ee.1943-7870.0001112.
Nazarian, H., Amouzgar, D., Sedghianzadeh, H. (2016). Effects of different concentrations of cadmium on growth and morphological changes in basil (Ocimum Basilicum L.). Pakistan Journal of Botany, 48(3), 945–952.
Oukarroum, A., Schansker, G., & Strasser, R. J. (2009). Drought stress effects on photosystem i content and photosystem II thermotolerance analyzed using Chl a fluorescence kinetics in barley varieties differing in their drought tolerance. Physiologia Plantarum, 137(2), 188–199. https://doi.org/10.1111/j.1399-3054.2009.01273.x.
Pagliano, C., Raviolo, M., Dalla Vecchia, F., Gabbrielli, R., Gonnelli, C., Rascio, N., et al. (2006). Evidence for PSII donor-side damage and photoinhibition induced by cadmium treatment on rice (Oryza sativa L.). Journal of Photochemistry and Photobiology B: Biology, 84(1), 70–78. https://doi.org/10.1016/j.jphotobiol.2006.01.012.
Paunov, M., Koleva, L., Vassilev, A., Vangronsveld, J., & Goltsev, V. (2018). Effects of different metals on photosynthesis: cadmium and zinc affect chlorophyll fluorescence in durum wheat. International Journal of Molecular Sciences, 19(3), 787. https://doi.org/10.3390/ijms19030787.
Peng, Q., Chen, W., Wu, L., Bai, L. (2017). The uptake, accumulation, and toxic effects of cadmium in barnyardgrass (Echinochloa crus-galli). Polish Journal of Environmental Studies, 26, 779–784. https://doi.org/10.15244/pjoes/65780.
Per, T. S., Masood, A., & Khan, N. A. (2017). Nitric oxide improves S-assimilation and GSH production to prevent inhibitory effects of cadmium stress on photosynthesis in mustard (Brassica juncea L.). Nitric Oxide - Biology and Chemistry, 68, 111–124. https://doi.org/10.1016/j.niox.2016.12.012.
Przedpelska-Wasowicz, E., Polatajko, A., & Wierzbicka, M. (2012). The influence of cadmium stress on the content of mineral nutrients and metal-binding proteins in arabidopsis halleri. Water, Air, and Soil Pollution, 223, 5445–5458. https://doi.org/10.1007/s11270-012-1292-4.
Puła, J., Barabasz-Krasny, B., Lepiarczyk, A., Zandi, P., & Mozdzeń, K. (2019). Activity of the photosynthetic apparatus in Phaseolus vulgaris L. leaves under the cadmium stress. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 47(2), 405–411. https://doi.org/10.15835/nbha47111328.
Qiu, Q., Wang, Y., Yang, Z., & Yuan, J. (2011). Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chemical Toxicology, 49, 2260-2267. https://doi.org/10.1016/j.fct.2011.06.024.
Rizwan, M., Ali, S., Adrees, M., Ibrahim, M., Tsang, D. C. W., Zia-ur-Rehman, M., et al. (2017). A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere, 182, 90–105. https://doi.org/10.1016/j.chemosphere.2017.05.013.
Rusinowski, S., Krzyżak, J., Sitko, K., Kalaji, H. M., Jensen, E., & Pogrzeba, M. (2019). Cultivation of C4 perennial energy grasses on heavy metal contaminated arable land: Impact on soil, biomass, and photosynthetic traits. Environmental Pollution, 250, 300–311. https://doi.org/10.1016/j.envpol.2019.04.048.
Sajwan, K. S., Paramasivam, S., Richardson, J. P., & Alva, A. K. (2002). Phosphorus alleviation of cadmium phytotoxicity. Journal of Plant Nutrition, 25, 2027–2034. https://doi.org/10.1081/PLN-120013292.
Shafi, M., Guoping, Z., Bakht, J., Khan, M. A., Ejaz-Ul-Islam, Khan, M. D., & Raziuddin. (2010). Effect of cadmium and salinity stresses on root morphology of wheat. Pakistan Journal of Botany, 42(4), 2747–2754.
Shi, G. L., Zhu, S., Bai, S. N., Xia, Y., Lou, L. Q., & Cai, Q. S. (2015). The transportation and accumulation of arsenic, cadmium, and phosphorus in 12 wheat cultivars and their relationships with each other. Journal of Hazardous Materials, 299, 94–102. https://doi.org/10.1016/j.jhazmat.2015.06.009.
Song, X., Yue, X., Chen, W., Jiang, H., Han, Y., & Li, X. (2019). Detection of cadmium risk to the photosynthetic performance of Hybrid pennisetum. Frontiers in Plant Science, 10. https://doi.org/10.3389/fpls.2019.00798.
Strasser, R. J., Tsimilli-Michael, M., & Srivastava, A. (2004). Analysis of the Chlorophyll a Fluorescence Transient. In Advances in Photosynthesis and Respiration: Chlorophyll Fluorescence, a Signature of Photosynthesis 321–362. https://doi.org/10.1007/978-1-4020-3218-9_12.
Tóth, S.Z., Oukarroum, A., Schansker, G. (2020). Probing the photosynthetic apparatus noninvasively in the laboratory of Reto Strasser in the countryside of Geneva between 2001 and 2009. Photosynthetica, 58(SI), 560–572. https://doi.org/10.32615/ps.2020.003.
Tsimilli-Michael, M., & Strasser, R. J. (2008). Experimental Resolution and Theoretical Complexity Determine the Amount of Information Extractable from the Chlorophyll Fluorescence Transient OJIP. In Photosynthesis. Energy from the Sun, 697–701. https://doi.org/10.1007/978-1-4020-6709-9_156.
Tsimilli-Michael M, Strasser RJ (2013) The energy flux theory 35 years later: Formulations and applications. Photosynthesis Research, 117, 289–320. https://doi.org/10.1007/s11120-013-9895-1.
Tuba, Z., Saxena, D.K., Srivastava, K., Singh, S., Czobel, S., Kalaji, H.M. (2010). Chlorophyll a fluorescence measurements for validating the tolerant bryophytes for heavy metal (Pb) biomapping. Current Science, 98(11), 1505–1508. Retrieved 16 Jan 2020 from http://www.jstor.org/stable/24108223.
Van Belleghem, F., Cuypers, A., Semane, B., Smeets, K., Vangronsveld, J., D’Haen, J., Valcke, R. (2007). Subcellular localization of cadmium in roots and leaves of Arabidopsis thaliana. New Phytologist, 173, 495–508. https://doi.org/10.1111/j.1469-8137.2006.01787.x.
Yu, Z., Zhou, Q. (2009). Growth responses and cadmium accumulation of Mirabilis jalapa L. under interaction between cadmium and phosphorus. Journal of Hazardous Materials, 167, 38–43. https://doi.org/10.1016/j.jhazmat.2008.12.082.
Acknowledgements
The authors would like to thank Mohammed VI Polytechnic University (UM6P), especially Plant Stress Physiology Laboratory and Agricultural Innovation and Technology Transfer Center for their valuable support.
Author information
Authors and Affiliations
Contributions
All authors contributed to this article, revised the text and results at different stages of the writing process, and read and approved the current manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chtouki, M., Naciri, R., Soulaimani, A. et al. Effect of Cadmium and Phosphorus Interaction on Tomato: Chlorophyll a Fluorescence, Plant Growth, and Cadmium Translocation. Water Air Soil Pollut 232, 84 (2021). https://doi.org/10.1007/s11270-021-05038-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05038-x