Abstract
The purpose of this work was to experimentally analyze the impact of copper, chromium and a commercial pesticide with endosulfan on the escape behavior of two copepods (Notodiaptomus conifer, Argyrodiaptomus falcifer) and three cladocerans (Daphnia magna, Pseudosida variabilis and Ceriodaphnia dubia). The experimental assays were carried out using a novel hydraulic devise designed to mimic three-speed predator capture behavior. Two concentrations, one “high” and one “low”, were employed and the exposure time was 15 (±5) minutes. With two exceptions, the species exposed to heavy metals manifested higher ability to escape than controls. Both concentrations of the pesticide reduced the escape ability of cladocerans but copepods responded, in general, in a similar manner as for heavy metals. The immediate apparent advantage of low and early toxic effects is discussed and the high sensitivity of the escape behavior suggests that it could be a complementary endpoint to be used in future ecotoxicological tests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last century, chemical pollution from human activities has become one of the most important stressors of continental aquatic ecosystems. As a consequence of industrial effluents and agricultural activities, aquatic organisms are chronically exposed to sublethal concentrations of xenobiotics which can affect their fitness in different ways. One of the most relevant ecological aspects of this problem resides in the possible population and community consequences that might be due to a cascading effect (Dodson and Hanazato 1995).
Zooplankton microcrustaceans are one of the most sensitive groups to these stressors, and during the last decades, intense efforts have been directed towards assessing the impact of heavy metals (Koivisto and Ketola 1995; Zauke and Schmalenbach 2006; Bossuyt and Janssen 2005) and pesticides (Barry and Logan 1998; DeLorenzo et al. 2002) on these key groups of organisms. In general, different laboratory standard toxicity tests have been employed as well as field methods. However, due to the high effort and time of analysis they require, the need to evaluate the effects of low levels of pollutants at earlier exposure through simple and efficient methods becomes evident, in order to achieve adequate ecosystem managements and restoration practices (Rinderhagen et al. 2000).
In this context, among all biological traits, different behaviors have been documented as one of the most sensitive parameters to evaluate low level effects of pollutants on different zooplankton species (Sharp and Stearns 1997; Lopes et al. 2004). Additionally, several studies have been carried out in order to understand not only individual ethology but also its possible consequences for the population dynamics (Ohman 1988). Furthermore, it is suggested that understanding the possible modifications of the normal behavioral patterns would be of great significance for conservation and evolutionary ecology (Hanazato 1995; Hanazato and Dodson 1993).
At present, patterns of feeding (Rodgher et al. 2008; Buskey 1984), mating (Maier 1995; Lonsdale et al. 1998), swimming (Dodson et al. 1997; Lopes et al. 2004), evation (Pijanowska et al. 2006) and migration (Cohen and Fordward 2005; Dawidowicz and Loose 1992) have been well documented. However, even though several authors claim that the ability to escape from predators plays an important role along the life history of a species (Lass and Spaak 2003), no studies have been done to analyze the sensitivity of this endpoint and its potentiality in ecotoxicological studies. In addition, there is little or no information on the significance of behavioral modifications by pollutants and their relevant impact at population and community levels.
As in most prey organisms, escape behavior involves two important components: perceptual ability and the subsequent reaction to stimuli (Vitasalo et al. 1998). For those organisms with pre-contact escape strategy, perception has been described and analyzed as one of the main fundamental components of escape success. This ability involves the activation of specific sensors distributed along the body surface. The specific location of the sense organs, as well as the range of their sensitivity, have been well investigated (Strickler and Bal 1973; Gill and Crisp 1985). On the other hand, the behavioral reaction depends on an adequate synchronization between the nervous system and muscles of the antennules, thoracic and caudal appendages.
From a physiological perspective, the combination of both responses (perception and reaction) has an emergent component and requires not only an optimal individual biological stage, but also a high energetic cost which needs to be compensated with other biological traits (Stearns 1992). This aspect makes the escape ability a possible highly sensitive indicator. From an eco-toxicological point of view, it is of intrinsic interest with important implications in the diel timing of ecologically meaningful behaviors (e.g. diel vertical migration, DVM) and especially relevant in population dynamics. Therefore, despite the fact that the evaluation of both associated abilities would imply an oversimplification, it would make the results clearer to quantitative analysis and could be easily used as a complementary tool in risk assessment studies.
In this work, we designed a novel hydraulic mechanism based on earlier Szlauer (1964) experiments which mimic a predator’s feeding and swimming behaviors in order to obtain quantitative information on the escape ability of five zooplankton microcrustaceans.
The purpose of this research was to analyze the early impact of two inorganic (potassium dichromate and copper sulphate) and one organic (a commercial insecticide with the organochlorine endosulfan as active ingredient) toxics on the microcrustaceans escape behavior. Additionally, we compared the sensitivity of this behavior with other chronic endpoints frequently used in ecotoxicological assessments such as reproductive and physiological traits.
Materials and methods
Selection and culture of test organisms
Two copepod (Argyrodiaptomus falcifer, Notodiaptomus conifer) and three cladocera species (Pseudosida variabilis, Ceriadaphnia dubia and Daphnia magna) were selected to evaluate their escape ability when exposed to three xenobiotics. With the exception of D. magna, all species belong to populations living in waterbodies of the alluvial plain of the Paraná River. They were collected with a planktonic net (200 μm) and cultured under constant conditions (photoperiod: 16 L–8 D; temperature: 21 ± 2°C) for several months. In the case of D. magna (a non-native species), the specimens employed in the experiments were obtained from a laboratory monoclonal stock culture belonging to the Instituto Nacional de Limnología (Santa Fe, Argentina).
During the experiments, all species were cultured in 150 ml glass containers with filtered (12 μm) and aerated pond water. Physico-chemical characteristics were measured (in mg l−1) according to the Standard Methods for the Examination of Water and Wastewater (2000): nitrates <0.1; nitrites: 0.01; ammonium: 0.29; chlorides: 3.5; sulphates: 8.3; total alkalinity: 77 CaCO3; bicarbonates: 94; sodium: 7.7; magnesium: 6.8; calcium: 12.9; potassium: 1.8; chemical demand of oxygen: 10; biological demand of oxygen: 0.08. Dissolved oxygen was 6.4 (±0.8) ppm; pH: 8.39 (±0.24); conductivity: 245.33 (±28.18) μS cm−1. The organisms were daily fed ad libitum with a Chlorella sp. concentrate (algal density: 2.8 × 105 cells ml−1).
For the escape experiments we employed adult organisms: males in the case of copepods and females in the case of anomopods (C. dubia and D. magna). Ctenopod (P.variabilis) was a special case since adults have a limited mobility, remaining almost all the time attached to a littoral substrate. Therefore, we employed planktonic juveniles which have a more constant swimming activity, in order to make an appropriate comparison of results.
Experimental design and capture assays
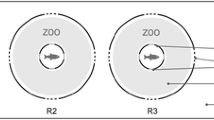
The experimental assays were carried out with a hydraulic mechanism (Fig. 1) designed to indirectly quantify the ability of the organisms to perceive and react to the approach of a possible predator (behavior hereafter referred to as “escape ability”). The escape ability was calculated through the difference between the number of exposed organisms and the number of captured ones.
The hydraulic device consisted of a transparent glass tube (“catching tube”) (long: 20 cm; diameter: 5 mm) which, moved by a piston, was vertically introduced into a bigger glass container (capacity: 18 ml) where the swimming organisms were exposed. The piston which was located in a PVC cylinder was elevated with the entry of tap water through a plastic hose. When water was taken out, a descent of the piston provoked a descent of the capture glass tube. Three capture speeds were used: 0.27, 2.87 and 15 cm s−1, which were regulated by means of a stopcock located at the extreme of the water outlet. These capture speeds were selected taking into account the range used in Szlauer’s (1964) experiments which determined a slow capture speed (SCS), a moderate capture speed (MCS) and a rapid capture speed (RCS). All experiments were conducted successively at the same temperature (21 ± 2°C) and in the daytime in a room lighted by fluorescent tubes (2242 ± 150 Lux).
In each experiment, thirty individuals per replicate were exposed and ‘catching attempts’ were repeated 100 times (replicates), except for P. variabilis where the catching experiment was repeated 50 times. In order to reduce the influence of eventual weariness or any accustoming of the animals to the movement of the tube, all catchings were carried out taking at random different groups of organisms. Nevertheless, to investigate whether these phenomena have any influence on the catches, a preliminary experiment was made. It consisted in conducting 50 identical catches that were repeated one after another in the same group of organisms of the five species utilized in this work. The results were analyzed with a Spearman correlation (X = number of successive catches; Y = number of captured individuals) and each correlations were considered significant at p < 0.05.
Tests solutions and controls
The aforementioned metals and the insecticide-acaricide with endosulfan as active ingredient used in this work were chosen because they are important and widespread pollutants in industrial areas and South American agroecosystems (Jergentz et al. 2004; Gagneten et al. 2007; Gagneten and Paggi 2009).
The stock solutions of the metallic salts (copper sulphate: CuSO4·5H20 and potassium dichromate: K2Cr2O7) were prepared by dissolving them in distilled water. The insecticide employed was Zebra Ciagro™ (Ciagro, S.A. Buenos Aires, Argentina), containing 35% of organoclhorine endosulfan as active ingredient (6, 7, 8, 9, 10, 10-hexacloro-1, 5, 5a, 6, 9, 9 a-hexahydro-6, 9-methano-2, 4, 3, benzodioxanthiopin-3-oxide). This product, as a liquid formulation, was diluted with distilled water to prepare a stock solution. The stock solution of this endosulfan formulation was prepared as in metals and for the final test solutions only the amount of the active ingredient was taken into account. The time of exposure of the organism before each experiment was 15 min (±5). This period was chosen taking into account previous works that showed the high sensitivity of the organisms to detect earlier expositions of contaminants (Lopes et al. 2004). Additionally, this range allowed to detect behavioral effects without alter or damage the individuals integrity as a consequence of a longer exposition time.
Two extensive different sublethal concentrations (one high and one low) of copper, chromium and endosulfan were used in the experiments. In order to facilitate the reading, in some occasions we termed them as Cu1 and Cu2, Cr1 and Cr2, and Endosulfan1 and Endosulfan 2, respectively. Both concentrations were established taking as a reference preliminary values obtained from acute tests. Following Kenaga’s considerations (Kenaga 1982) we calculated 20% of the EC50 values in order to obtain the high level, then we divided these high value by 4 in order to obtain the low level. Each one were different depending on the sensitivity of the species and were prepared prior to each test by dissolving the stock solutions in the same medium used for culture and controls (Table 1).
Due to the asymmetric distribution of data (Table 2) and the impossibility to normalize residuals through transformations, we used a generalized linear model to analyze the effects of treatments (toxicants), capture speeds and the interaction of both factors on the capture success of each specie. To test these factors we employed the deviance as a variability mean (ANODEV) (McCullagh and Nelder 1989).
In a second analysis, and in order to reach a clearer interpretation of the effects of toxicants, the results obtained from the capture assays were grouped following an ecological-taxonomic criteria (cladocerans anomopoda, cladocerans ctenopoda and copepods). Each grouped data was statistically analyzed by employing the deviance as a variability mean (ANODEV), with the captures as dependent variable and treatments as explanatory variable. In this case, each capture speed and each toxicant concentration were analyzed separately. In all cases differences were considered significant at p < 0.05 and a Bonferroni’s sequential method was applied in order to correct the significance value (Sokal and Rohlf 1995).
Finally, based on the individual behavioral data to each toxic and to the three capture speeds, we calculated the lowest observed effect concentration (LOEC) and the non-observed effect concentration (NOEC).
Results
Preliminary experiments
As was previously indicated, in order to ascertain eventual weariness or accustoming of the animals, an initial experiment with 50 identically successive catches was performed at the moderate capture speed (MCS). The results are presented in Fig. 2. No significant correlation was found between the successiveness of catches and the number of captured copepods (N. conifer: r 2 = 0.0046, p = 0.638; A. falcifer: r 2 = 0.0192, p = 0.337) or cladocerans (D. magna r 2 = 0.014, p = 0.399; C. dubia: r 2 = 0.0042, p = 0.654; P. variabilis r 2 = <0.001, p = 0.966), indicating that during the fifty repetitions, the successiveness of catches did not influence the obtained results.
Effects of toxics
Both the magnitude and the kind of responses (higher vs. lower escape ability than controls) triggered by each specie were different depending capture speed (ANODEV: N. conifer p = 0.035; A. falcifer p < 0.01; C. dubia p < 0.01; D. magna p < 0.01; P. variabilis p < 0.01) and toxic concentration (ANODEV: N. conifer p < 0.01 to Cr, Cu and endosulfan; A. falcifer p = 0.02 to Cr, p < 0.01 to Cu and endosulfan; C. dubia p = 0.03 to Cr, p < 0.01 to Cu and endosulfan; D. magna p = 0.01 to Cr, p = 0.03 to Cu and 0.04 to endosulfan; P. variabilis p < 0.01 to Cr, Cu and endosulfan). In order to quantify each response, we calculated the difference between the arithmetic mean of captured individuals in the controls and the arithmetic mean of captured individuals in each toxic treatment. Results expressed in this way allow a clear visualization of the differences in each particular case (Fig. 3).
Differential responses to the three toxic (chromium, copper and endosulfan) at the three capture speeds (LCS, MCS and RCS). Positive values indicate that the organisms had a higher ability to escape than the respective control. On the contrary, negative values indicate that the organisms were more captured (due to a loss of ability to escape) than those of the control. The length of each bar represent the magnitude of this response. Asterisks indicate statistically significant differences from the control (ANODEV; p < 0.05)
In general, we registered fewer captures at lower than at higher concentrations of the three toxics, particularly at the LCS and MCS. This higher escape ability would be manifesting early excitation responses.
The organisms showed similar responses only in few cases: in the lower copper concentration at RCS, the higher copper concentration at MCS (in both cases manifesting early excitation responses) and in the high endosulfan concentration at the RCS. In this latter case, the interaction between endosulfan and the capture speed was statistically significant in all cases, which suggest a synergetic effect between the two stressing factors (high water turbulence and high toxicity of the compound) (ANODEV, N. conifer p = 0.024; A. falcifer p < 0.001; C. dubia p = 0.04; D. magna p = 0.04; P. variabilis p < 0.001).
Despite the aforementioned particular differences on the responses to each toxic, some interesting behavioral patterns were exhibited by cladocerans anomopoda (D. magna and C. dubia), cladocerans ctenopoda (P.variabilis) and copepods (N. conifer and A. falcifer). Thus, in order to reach a clearer interpretation of the effects of toxics, the results obtained were grouped following an ecological-taxonomic criteria.
Figures 4 and 5 show the effects of heavy metals and endosulfan, respectively, which are representative of the behavioral responses observed in the experiments.
Number of captured a calanoid copepods (N. conifer and A. falcifer), b anomopod cladocerans (D. magna and C. dubia) and c ctenopod cladocerans (P. variabilis) at each copper and chromium concentrations. The graphics show the mean values (n = 100, except for P. variabilis where n = 50) to the three capture speeds: 0.27 cm s−1 (LCS); 2.87 cm s−1 (MCS) and 15 cm s−1 (RCS)
Number of captured a calanoid copepods (N. conifer and A. falcifer), b anomopod cladocerans (D. magna and C. dubia) and c ctenopod cladocerans (P. variabilis) at each endosulfan concentration. The graphics show the mean values (n = 100, except for P. variabilis where n = 50) to the three capture speeds: 0.27 cm s−1 (LCS); 2.87 cm s−1 (MCS) and 15 cm s−1 (RCS)
Heavy metals
In general, copepods exposed to both concentrations of chromium were not so extensively captured as controls at LCS (ANODEV, Wald χ2 = 3.3, df = 2, p = 0.19) and at MCS (ANODEV, Wald χ2 = 0.3, df = 2, p = 0.86) (Fig. 4a).
The same result was observed to copepods exposed to Cu1 at LCS (ANODEV, Wald χ2 = 3.24, df = 1, p = 0.072), however, the groups exposed to Cu2 at LCS and both concentrations of Cu at MCS were significantly less captured than the controls (ANODEV, Cu1: Wald χ2 = 9.63, df = 1, p = 0.002; Cu at MCS: Wald χ2 = 15.9, df = 2, p < 0.001, in both cases) (Fig. 4a).
The same “higher escape ability” pattern was found at RCS for the copepods exposed to the lowest concentrations of both metals (Fig. 4a), however statistical differences were found only to Cu1 (ANODEV Cr1: Wald χ2 = 0.01 df = 1:, p = 0.97; Cu1: Wald χ2 = 23.36, df = 1, p < 0.001). On the contrary, the higher concentrations of copper and chromium at RCS made the organisms more capturable than those of controls (ANODEV, Cr2: Wald χ2 = 16.18; Cu2: Wald χ2 = 3.97; df = 1, p < 0.001 in both cases) (Fig. 4a).
In case of cladocerans, the general pattern at LCS was a higher capturability of the organisms exposed to both metals. This response was particularly significant to anomopods (ANODEV, Cr: Wald χ2 = 20.42, df = 2, p < 0.001; Cu: Wald χ2 = 21.28 df = 2, p < 0.001). In case of ctenopods, differences were not statistically significant (ANODEV, Cr: Wald χ2 = 2.24, df = 2, p = 0.32; Cu: Wald χ2 = 2.8, df = 2, p = 0.24) (Fig. 4b, c).
The two higher capture speeds (MCS and RCS) registered in average, a less extensive capturability of the cladocerans exposed to both heavy metals. At MCS differences were statistically significant to anomopods exposed to both chromium concentrations (ANODEV, Wald χ2 = 17.81, df = 2, p < 0.001) and to the highest copper concentration (ANODEV, Wald χ2 = 4.86, df = 1 p = 0.027). In case of ctenopods, such diferences were not statistically significant (ANODEV, Cr: Wald χ2 = 6.04, df = 2, p = 0.05; Cu: Wald χ2 = 3.04, df = 2, p = 0.21) (Fig. 4b, c).
At RCS, the anomopods exposed to both lower concentrations were less captured than the controls (ANODEV, Cr1: Wald χ2 = 3.86 df = 1, p = 0.04; Cu1:Wald χ2 = 6.53, df = 2, p = 0.03). However, the highest concentrations of both metals did not show statistically significant differences in relation to controls (ANODEV, Cr2: Wald χ2 = 1.77, df = 1, p = 0.13; Cu2: Wald χ2 = 0.85, df = 1 p = 0.35). In case of the ctenopods submitted to RCS, both concentration of chromium and the higher concentration of copper induced a significant higher ability to escape than the controls (ANODEV, Cr: Wald χ2 = 14.03, df = 2, p = 0.001; Cu2: Wald χ2 = 4.94, df = 1, p = 0.26) (Fig. 4b, c).
Endosulfan
In the experiment with the commercial formulation of endosulfan, copepods were, in average, less captured than the control groups (ANODEV, p < 0.001 in all capture speeds) (Fig. 5a). As a exceptional case, when the organisms were submitted to the higher concentration of endosulfan at RCS, a significantly higher capturability was registered (ANODEV, Wald χ2 = 19.7, df = 1, p < 0.001), exhibiting an important loss of ability to escape from the catching tube.
In the case of cladocerans, the general pattern was a higher capturability in all concentrations and all the capture sepeeds (Fig. 5b, c). Significant differences were found, to the anomopod group, in both endosulfan concentrations at LCS (ANODEV, Wald χ2 = 15.86, df = 2, p < 0.001) and at RCS (ANODEV, Wald χ2 = 6.78, df = 2, p = 0.034). The ctenopod group showed statistically significant differences at both endosulfan concentrations but only in the RCS (ANODEV, Wald χ2 = 12.47, df = 2, p = 0.002).
The lowest observable effect concentration (LOEC) and the no observed effect concentration (NOEC) calculated from the escape performance are shown in Table 3. Based on these data, it can be observed that cladocerans are the most sensitive organisms to the three toxics analyzed.
Discussion
Preliminary experiments showed that there were not significant correlation between the successiveness of catches and the number of captured individuals. This indicate that this stimuli did not influence their responses through time. Although some authors attributed different zooplanktonic responses as possible “learns or accustoming” (Pijanowska et al. 2006; Buskey 1984), our results are in accordance with those found by earlier Szlauer (1964) experiments, where fifty successive catches did not influence D. pulex escape behavior.
Both the magnitude and the kind of responses (higher vs. lower escape ability than controls) triggered by each specie were different depending capture speed and toxic concentration. Even though their particular differences, an interesting behavioral pattern was also recognized in cladocerans and copepods during a relatively short exposure lapse (15 min ± 5). Only with two exceptions, all the species registered an increment of the alert stage which was reflected in the higher ability to escape from the tube. These observed responses are consistent with other similar results recorded by Sullivan et al. (1983) who found that 24 h exposure to sublethal copper levels caused nauplii to be hyperactive. This pattern of escape stimulation produced by a chemical which is toxic at high concentrations is also similar to other behavioral responses documented in several toxicological bioassays (Sharp and Stearns 1997; Calabrese et al. 1999; Brown et al. 2002).
However, as many authors suggest, this immediate apparent advantage is questionable in ecotoxicology, since an increase in one physiological or behavioral process may be at the expense of another (Forbes 2000). In other terms, it would provide an important problem for risk assessors, because an increase in escape ability cannot be considered detrimental to the fitness of the individual species, but it may not be beneficial if the individual is making trade-offs with other life history aspects (e.g. feeding rate, reproduction time, refuge recognition) (Andersen et al. 2001). Furthermore, it is important to mention that in this particular case, the observed responses show the most immediate reaction of the organisms to the exposure, whereupon we can hypothesize that a higher escape ability constitutes more an involuntary behavior (reflect act) than an apparent temporary advantage. On the other hand, in addition to the long-term negative consequences of the early responses observed, the excessive alert stage may also be negative in the short term when predator–prey interactions are considered. In this line, Guerritsen and Strickler (1997) demonstrated the adverse consequences of prey hyperactivity through enhancing the encounter rate of both individuals in natural waterbodies. On the other hand, it is probable that the consequent exhaustion of available energy and the increased physiological damage cause a reduction in swimming activity (Preston et al. 1999). This reduction would reduce feeding efficiency, and hence, the energy available for general metabolism, growth and reproduction (Gliwicz 1990).
The first exception to the observed pattern occurred in the case of the escape ability in copepods exposed to the higher concentration of both metals at RCS (15 cm s−1). Their inhibition of the escape ability would be the result of the combination of the two high stress situations (the high toxicity of the metals and higher water turbulence at RCS which may cause a severe disorientation). The second exceptional case was the escape activity of the contaminated cladocerans (mainly anomopods) at LCS (0.27 cm s− 1), where vibrations are gentler. Since at 0.27 cm s−1 a high swimming activity is not required in order to escape, we hypothesize that, in spite of their phylogenetic and morphological differentiations, the high capture of these “cladocerans” may be related to an inhibition of their perceptive systems. The ecological relevant consequence of this is that the contaminated animals would be more vulnerable to some specific predators (for example ambushing ones or some invertebrates) (Vitasalo et al. 1998).
In the experimental assays with endosulfan, while both concentrations of the pesticide reduced the escape ability of cladocerans, copepods responded in a similar manner for heavy metals, mainly in the LCS and MCS (0.27 and 2.87 cm s−1). However, the higher concentration of endosulfan at 15 cm s−1 makes copepods less able to escape.
Although we did not evaluate the mode of action of endosulfan, previous studies demonstrate that this pesticide alters the acetylcholinesterase (AchE) activity, which may hinder nerve functions and, consequently muscle responses (Bhavan and Geraldine 2001). It has been also demostrated that this kind of toxic, which alters the nervous systems, can affect metabolic and biochemical parameters (Mc Kee and Knowles 1986; Barry 1999). In addition to affecting them, many authors have documented that endosulfan affects other related behaviors such as swimming activity and hence, feeding and mating (Baudo 1987; Hanazato 2001).
In general, in this work we recognized two kinds of responses: hyperactivity with elevated alertness stage, generally at lower xenobiotic concentrations, or complete loss of escape capability. This characteristic biphasic behavior suggests the existence of specific thresholds to each xenobiotic effect. It is probable that over this specific limit of toxicity, the organisms will manifest inability to make accurate assessments of the environment through an inhibition of sensory and/or motor systems. In a broader perspective, whatever the alteration (stimulation or depression), it could leave the organisms more vulnerable to predation. The ecological significance of this finding resides in the fact that these microcrustaceans have the potential to control the bloom dynamics of some algal species through their feeding pressure (Collumb and Buskey 2004). At the same time, they could transfer the toxics to higher trophic levels generating biomagnification though the food web (Watras et al. 1998; Liu et al. 2002).
The results obtained in this work were compared with previous studies based on other known sensitive endponits such as survival, longevity, age of first reproduction and number of eggs per female (Biesinger and Christensen 1972; Winner and Farrell 1976; Gagneten and Vila 2001). Even though more information is necessary to have an adequate certainty about the real sensitivity of the escape ability in ecotoxicological tests, this study allowed the detection of lower LOEC and NOEC values than those reported by other studies. For example, Suedel et al. (1996) determined chronic values of copper with C. dubia between 3.2 and 163 μg l−1 which were higher than the LOEC values obtained in most of the species used in this study. In a similar way, the LOEC values obtained for D. magna to chromium (21 μg l−1) (Kühn et al. 1989) and endosulfan (100 μg l−1) (DeLorenzo et al. 2002) were also higher than the ones obtained for the five species analyzed in this work.
Moreover, it is important to mention that in the present study, the sublethal toxicity of copper, chromium and endosulfan occurred at lower concentrations than those proposed by national (National Hidric Resources Secretary 2003, 2005 and international (US EPA 1980) organisms for aquatic biota protection. In this sense, despite the fact that further research is needed on the effect of other xenobiotic compounds on zooplankton escape ability, our results demonstrate the sensitivity of this behavior and its potentiality as bioindicator in future ecotoxicological investigations.
From an ecological point of view, current records about the feeding ethology of zooplanktophagous fishes indicate that this behavior is a highly dynamic process lasting only 20–100 ms, involving large accelerations, and producing a rapid pulse of water through the mouth cavity (Lauder 1980). In this vein, the capture speed registered on different zooplanktophagous fishes may reach or overcome 40 cm s−1 (Webb and Skadsen 1980). Taking into account this information and in order to obtain results more consistent with the real ethology of the organisms, the consideration of the higher capture speed is suggested as the most appropriate methodology to be used in the future.
Finally, to the best of our knowledge, the present novel assay constitutes one of the first studies where the sensitivity of an ecologically important behavior such as the escape ability, is analyzed in relation with chemical stressors. Far from being a finished work, it attempts to contribute to a new research line tending to detect sensitive and early pollutant effects. At the same time it allows asking new questions to future ecotoxicological investigations. Once sufficient knowledge on the sensitivity of escape ability is achieved through similar assays with other pollutants and other species, the methodology employed in this work could be recommended as a complementary tool in ecological risk assessments as well as in ecosystem biomonitoring.
References
Andersen HR, Wollenberger L, Halling-Sorensen B, Ole Kusk K (2001) Development of copepod nauplii to copepodites—a parameter for chronic toxicity including endocrine disruption. Environ Toxicol Chem 20:2821–2829
Barry MJ (1999) The effects of a pesticide on inducible phenotipic plasticity in Daphnia. Environ Pollut 104:217–224
Barry MJ, Logan DC (1998) The use of temporary pond microcosms for aquatic toxicity testing: direct and indirect effects of endosulfan on community structure. Aquat Toxicol 41:101–124
Baudo R (1987) Ecotoxicology testing with Daphnia. In: Peters RH, De Bernardi R (eds) Daphnia, a special edition of Memorie dell’Istituto Italiano di Idrobiologia. Pallanza, Italy, pp 461–482
Bhavan SP, Geraldine P (2001) Biochemical stress responses in tissues of the prawn Macrobrachium malcolmsonii on exposure to Endosulfan. Pest Biochem Physiol 70:27–41
Biesinger KE, Christensen GM (1972) Effects of various metals on survival, growth, reproduction, and metabolism of Daphnia magna. J Fish Res Board Can 29:1691–1700
Bossuyt BTA, Janssen CR (2005) Copper toxicity to different field-collected cladoceran species: intra- and inter-species sensitivity. Environ Pollut 136:145–154
Brown RJ, Rundle SD, Hutchinson TH, Williams TD, Jones MB (2002) A copepod life-cycle test and growth model for interpreting the effects of lindane. Aquat Toxicol 63:1–11
Buskey EJ (1984) Swimming pattern as an indicator of the roles of copepod sensory systems in the recognition of food. Mar Biol 79:165–175
Calabrese EJ, Baldwin LA, Holland CD (1999) Hormesis: a highly generalizable and reproducible phenomenon with important implications for risk assessment. Risk Anal 19:261–281
Cohen JH, Fordward RB (2005) Diel vertical migration of the marine copepod Calanopia americana. I. Twilight DVM and its relationship to the diel light cycle. Mar Biol 147:387–398
Collumb CJ, Buskey EJ (2004) Effects of the toxic red tide dinoflagellate (Karenia brevis) on survival, fecal pellet production and fecundity of the copepod Acartia tonsa. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA (eds) Harmful algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO
Dawidowicz P, Loose CJ (1992) Metabolic costs during predator-induced diel vertical migration of Daphnia. Limnol Oceanogr 37:1589–1595
DeLorenzo ME, Taylor LA, Lund SA, Pennington PL, Strozier ED, Fulton MH (2002) Toxicity and bioconcentration potential of the agricultural pesticide endosulfan in phytoplankton and zooplankton. Arch Environ Contam Toxicol 42:173–181
Dodson S, Hanazato T (1995) Commentary on effects of anthropogenic and natural organic chemicals on development, swimming behavior, and reproduction of Daphnia, a key member of aquatic ecosystems. Environ Health Persp 103:7–11
Dodson SI, Ryan S, Tollrian R, Lamper W (1997) Individual swimming behavior of Daphnia: effects of food, light and container size in four clones. J Plankton Res 19:1537–1552
Forbes VE (2000) Is hormesis an evolutionary expectation? Funct Ecol 14:12–24
Gagneten AM, Paggi JC (2009) Effects of heavy metal contamination (Cr, Cu, Pb, Cd) and eutrophication on zooplankton in the lower basin of the Salado river (Argentina). Water Air Soil Poll 198:317–334
Gagneten AM, Vila I (2001) Effects of Cu+2 and pH on the fitness of Ceriodaphnia dubia (Richard 1894) (Crustacea, Cladocera) in microcosm experiments. Environ Toxicol 16:428–438
Gagneten AM, Gervasio S, Paggi JC (2007) Heavy metal pollution and eutrophication in the lower Salado river basin (Argentina). Water Air Soil Poll 178:335–349
Gill CW, Crisp DJ (1985) Sensitivity of intact and antennule amputated copepods to water disturbance. Mar Ecol Prog Ser 21:221–227
Gliwicz ZM (1990) Why do cladocerans fail to control algal blooms? Hydrobiologia 200–201:83–97
Guerritsen J, Strickler JR (1997) Encounter probabilities and community structure in zooplankton: a mathematical model. Can J Fish Res Board 34:73–82
Hanazato T (1995) Combined effect of the insecticide carbaryl and the Chaoborus kairomone on helmet development in Daphnia ambigua. Hydrobiologia 310:95–100
Hanazato T (2001) Pesticide effects on freshwater zooplankton: an ecological perspective. Environ Poll 112:1–10
Hanazato T, Dodson SI (1993) Morphological responses of four species of cyclomorphic Daphnia to a short-term exposure to the insecticide carbaryl. J Plankton Res 14:1743–1755
Jergentz S, Pessacq P, Mugni H, Bonetto C, Schulz R (2004) Linking in situ bioassays and dinamics of macroinvertebrates to assess agricultural contamination in streams of the Argentine pampa. Ecotox Environ Safe 59:133–141
Kenaga E (1982) Predictability of chronic toxicity from acute toxicity of chemicals in fish and aquatic invertebrates. Environ Toxicol Chem 1:347–358
Koivisto S, Ketola M (1995) Effects of copper on life-history traits of Daphnia pulex and Bosmina longirostris. Aquat Toxicol 32:255–269
Kühn R, Pattard M, Pernak K, Winter A (1989) Results of the harmful effects of water pollutants to Daphnia magna in the 21 day reproduction test. Water Res 23:501–510
Lass S, Spaak P (2003) Chemically induced anti- predator defences in plankton: a review. Hydrobiologia 491:221–239
Lauder GV (1980) Hydrodinamic of prey capture by teleost fishes. Proceeding of the 2nd conference on biofluid mechanics, vol 2. Plenum Press, New York, pp 161–181
Liu XJ, Ni IH, Wang WX (2002) Trophic transfer of heavy metals from freshwater zooplankton Daphnia magna to zebrafish Danio reiro. Water Res 36:4563–4569
Lonsdale DJ, Frey MA, Snell TW (1998) The role of chemical signals in copepod reproduction. J Marine Syst 15:1–12
Lopes I, Donald JB, Riveiro R (2004) Avoidance of copper contamination by field population of Daphnia longispina. Environ Toxicol Chem 7:1702–1708
Maier G (1995) Mating frequency and interspecific matings in some freshwater cyclopoid copepods. Oecologia 101:245–250
Mc Kee MJ, Knowles CO (1986) Effects of fenvalerate on biochemical parameters, survival, and reproduction of Daphnia magna. Ecotox Environ Safe 12:70–84
McCullagh P, Nelder JA (1989) Generalized linear models, 2nd edn. New York
National Hidric Resources Secretary (2003) Desarrollo de niveles guía nacionales de calidad de agua ambiente correspondiente a cromo. Argentina
National Hidric Resources Secretary (2005) Desarrollo de niveles guía nacionales de calidad de agua ambiente correspondiente a cobre. Argentina
Ohman MD (1988) Behavioral responses of zooplankton to predation. B Mar Sci 43:530–550
Pijanowska J, Dawidowicz P, Weider LJ (2006) Predator-induced escape response in Daphnia. Arch Hydrobiol 167:77–87
Preston BJ, Cecchine G, Snell TW (1999) Effects of pentachlorophenol on predator avoidance behavior of the rotifer Brachionus calyciflorus. Aquat Toxicol 44:201–212
Rinderhagen M, Ritterhoff J, Zauke GP (2000) Biomonitoring of polluted water—reviews on actual topics, vol. 9. Environmental research forum. Trans Tech Publications, Uetikon-Zuerich, pp 161–194
Rodgher S, Lombardi AT, Gama Mela MG, Tonietto AE (2008) Change in life cycle parameters and feeding rate of Ceriodaphnia silvestrii Daday (Crustacea, Cladocera) exposure to dietary copper. Ecotoxicology 17:826–833
Sharp AA, Stearns DE (1997) Sublethal effects of cupric ion activity on the grazing behaviour of three calanoid copepods. Mar Poll Bull 34:1041–1048
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. WH Freeman and Co., New York
Standard Methods (2000) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington, DC
Stearns SC (1992) The evolution of life histories. Oxford Univerisity Press, Oxford
Strickler RI, Bal A (1973) Setae of the first antennae of the copepod Cyclops scutifer (Sars): Their structure and importance. Proc Natl Acad Sci USA 70:2656–2659
Suedel BC, Deaver E, Rodgers JH (1996) Experimental factors that may affect toxicity of aqueous and sediment-bound copper to freshwater organisms. Arch Environ Contam Toxicol 30:40–46
Sullivan BK, Buskey E, Miller DC, Ritacco PJ (1983) Effects of copper and cadmium on growth, swimming and predator avoidance in Eurytemora affinis (Copepoda). Mar Biol 77:299–306
Szlauer L (1964) Reaction of Daphnia pulex de Geer to the approach of different objects. Polskie Arch Hydrobiologii 12:5–16
United States Environmental Protection Agency (1980) Ambient water quality criteria for endosulfan. EPA 440/5-80-046. Office of Water Regulations and Standards, Washington, DC
Vitasalo M, Kioerboe T, Flinkman J, Pedersen LW, Visser AW (1998) Predation vulnerability of planktonic copepods: consequences of predator foraging strategies and prey sensory abilities. Mar Ecol Prog Ser 175:129–142
Watras CJ, Back RC, Halvorsen S, Hudson RJM, Morrison KA, Wente SP (1998) Bioaccumulation of mercury in pelagic freshwater food webs. Sci Total Environ 219:183–208
Webb PW, Skadsen JM (1980) Strike tactics of Esox. Can J Zool 58:1462–1469
Winner RW, Farrell MP (1976) Acute and chronic toxicity of copper to four species of Daphnia. J Fish Res Board Can 33:1685–1691
Zauke GP, Schmalenbach I (2006) Heavy metals in zooplankton and decapod crustaceans from the Barents Sea. Sci Total Environ 359:283–294
Acknowledgments
We thank the anonymous reviewers for their constructive comments and important contributions. We are also grateful to Leonardo Paggi and Oscar Mendoza for their technical assistance. This research was supported with grants from the Universidad Nacional del Litoral, Santa Fe, Argentina (Project CAI+D 2009 No. PI 69-351).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutierrez, M.F., Paggi, J.C. & Gagneten, A.M. Microcrustaceans escape behavior as an early bioindicator of copper, chromium and endosulfan toxicity. Ecotoxicology 21, 428–438 (2012). https://doi.org/10.1007/s10646-011-0803-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0803-1