Abstract
This study describes the capacity of different sorbents to form stable biofilms under highly hydrocarbon-polluted conditions and the degrading capacity of the microbiota present in the biofilm. With this aim, microcosms were designed in a 1 L beaker with 400 mL of culture medium or polluted wastewater and an amount equivalent of 200 mL of the selected sorbent carrier, made of cork and/or polypropylene meltblown. The culturable bacteria adhered to the sorbent carrier were quantified, and the time course of the hydrocarbon concentration was studied together with the formation of a biofilm on the carrier’s surface. The results revealed a different performance of the carriers in terms of bacterial adhesion, significantly reduction in the hydrocarbon content in water at the end of the assays, and a biofilm tolerance to high hydrocarbon concentration in the polluted water. From these results, it was concluded that the use of a sorbent, hydrophobic cork, or meltblown polypropylene, together with indigenous microbiota, constitutes a promising technology for the treatment of hydrocarbon-polluted water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Petroleum is composed of hydrocarbons and non-hydrocarbon compounds, which include basically metal porphyrins, acids, and organometallic compounds. Petroleum hydrocarbons consist mainly of a complex mixture of hydrophobic and non-aqueous compounds, such as aliphatic compounds (mainly n-alkanes), aromatic compounds, and, to a lesser extent, resins and asphaltenes (Dellagnezze et al. 2014; Grace Liu et al. 2011; Soliman et al. 2014). Water pollution by hydrocarbons is an environmental problem that affects the aquatic ecosystems, the species that inhabit these ecosystems, as well as the products that are made from them. Therefore, this contamination can be hazardous for the environment as well as for human health (Soliman et al. 2014).
With regard to aquaculture and fisheries, the presence of hydrocarbons in rivers, lakes, and seas may lead to reductions or even the loss of certain species of fish, shellfish, and other organisms unable to degrade or remove these pollutants. In the field of tourism, a spill near a tourist center is costly due to the decline in visitor numbers (Rekadwad and Khobragade 2015).
Against these problems, physicochemical methods, such as barriers and skimmers, in situ burning of crude oil in water, or the use of dispersants, have been used as conventional remediation methods. The problem with these techniques is their limitations, as they are seldom fully effective and often environmentally harmful (Al-Majed et al. 2012; Röling et al. 2002; Yoo et al. 2012). The use of sorbents is a physico-chemical technique that respects the environment and presents only few limitations. This technique uses materials that absorb the hydrocarbon compounds in their structure, but the great drawback is that a waste polluted with hydrocarbons is generated, implying a high management cost, both technically and economically (ITOPF 2014).
Today, biological methods are widely used, and they are considered the most cost-effective and environmentally sound methods. Bioremediation is considered efficient, inexpensive, and environmentally friendly for treating hydrocarbon-polluted sites (Zamani et al. 2014). The effectiveness of bioremediation for decontaminating hydrocarbon-affected waters is based on the fact that these contaminants can often be degraded by the microorganisms that inhabit these water bodies (Ward et al. 2003). In aquatic ecosystems, microorganisms used in bioremediation processes are more technically viable in coastal areas than in the open sea or vast water bodies, mainly because in the latter cases the microorganisms are diluted and washed out. One solution could be the use of a biofilm, where microorganisms are immobilized at a surface (Radwan et al. 2002). The biofilm cells have a better chance of adapting and surviving (especially during periods of stress), being protected within the matrix, which affords them a considerable advantage over planktonic microorganisms.
The efficacy of the treatment systems for water and industrial effluents using immobilized microorganisms has been demonstrated (Calderón et al. 2013; Leyva-Díaz et al. 2015; Radwan et al. 2002). Accordingly, the immobilization of the biomass can be an effective method to retain degrading microorganisms, such as hydrocarbon degraders. Microorganisms attached to a carrier have been successfully used in water treatment. In these systems, one factor to consider is the nature of support material, as the support must allow the formation of a stable biofilm and should promote the contact between the microbiota and the pollutants.
In this context, the aim of this research is to design and develop a water-treatment system based on the use of new bio-absorbents. In this context, the study seeks to determine the capacity of different sorbents to form stable biofilms in high hydrocarbon-polluted conditions. Also, an effort is made to develop and design new absorbent/carrier system with both high hydrocarbon and derivative retention capacity and high-capacity hydrocarbon-degrading microorganisms to adhere to its surface, i.e. absorbents that allow the development of biofilms with high degrading capacity. The experiments were performed at the lab scale in microcosms using hydrocarbon-polluted industrial wastewater.
2 Materials and Methods
In this study, microcosm assays were conducted to assess the bacterial adhesion capacity of carriers and the degrading capacity of the microbiota present in the biofilm. Initially, the adhesion capacity of carriers was tested using a previously isolated strain selected for its surface-adherence capacity (Rodríguez-Calvo et al. 2017). Second, surface adherence and degrading capacity of the indigenous microbiota from industrial wastewater was studied in microcosms with a selected carrier, regarding to previous studies.
2.1 Carriers
The carriers used in this study were selected based on their hydrocarbon-adsorption capacity. Selected carriers were the following:
-
CorkSorb™ 01025. Granular thermal-treated hydrophobic cork, which absorbs oil and solvents without absorbing water. This carrier is a natural biodegradable product.
-
CorkSorb™ 03025. Granular thermal-treated hydrophilic cork, which absorbs oil, solvent, and water. This carrier is a natural biodegradable product.
-
Pad Sentec™. Oil-absorbent pads composed of 100% meltblown polypropylene that absorbs only oil and not water.
-
Barrier Sentec™. Oil-absorbent barrier composed of meltblown polypropylene and cellulose pulp that absorb oil but not water.
2.2 Bacterial Strain and Culture Conditions
Pseudoalteromonas elyakovii W18 was included in this study due to its surface-adherence ability (Rodríguez-Calvo et al. 2017). The isolate was grown in Luria–Bertani (LB) (Sezonov et al. 2007) and Bushnel–Haas (BH) (Deziel et al. 1996) liquid media.
LB medium, rich in nutrients, was added of a mixture of sea salt to give the final salt concentration of 3% (w/v) (Rodriguez-Valera et al. 1981); its composition was as follows (g/L): peptone 10; yeast extract 5. BH medium used for isolating hydrocarbon-degrading microorganisms is a minimal medium with the following composition (g/L): MgSO4∙7H2O, 0.2; K2HPO4, 1; CaCl2∙2H2O, 0.02; NH4NO3∙6H2O, 1; FeCl3, 0.05; and agar, 20. The mixture of sea salt mentioned previously was added to reach the final salt concentration of 3% (w/v).
2.3 Water Samples
Industrial wastewater samples were collected from oil-storage tanks at the Compañía Logística de Hidrocarburos S.A. (CLH), located in Motril, Granada (Spain). The average concentration of Biological Oxygen Demand at 5 days (BOD5) and Chemical Oxygen Demand (COD) was 320 and 887.53 mg O2/L, respectively. These parameters were determined according to Standard Methods for the Examination of Waste and Wastewater (APHA 2005).
2.4 Bacterial Adhesion Capacity of Carriers. Microcosm Assays
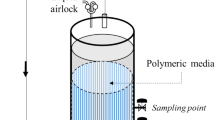
Initial microcosm assays to evaluate the bacterial adhesion capacity of carriers were prepared in a 1 L beaker with 400 mL of culture medium (LB medium and BH medium) and an amount equivalent of 200 mL of the selected sorbent in each case. These flasks were inoculated with 10 mL of an overnight culture of P. elyakovii strain W18 with shaking at 100 rpm and 28 °C for seven days. Cell growth was enumerated by dilution plating technique at days 0, 1, 2, and 7. All experiments were carried out in triplicate.
2.5 Hydrocarbon Tolerance of the Biofilm. Microcosm Assays
The second stage of microcosm assays was used to evaluate the effect of hydrocarbon content on microbial activity and biofilm formation from industrial wastewater. The 1 L beaker contained 400 mL of the wastewater without hydrocarbons, polluted with 10,000 and 20,000 ppm of diesel, respectively, as well as an amount equivalent of 200 mL of the previous carriers selected. Microcosms were incubated for 20 days at 28 °C and 100 rpm. Microbial growth was measured at 0, 1, 2, 7, 14, and 20 days. Hydrocarbon fraction was also determinate at initial and the end of the assays. All experiments were carried out in triplicate.
2.6 Quantification of Culturable Bacteria
Growth and adhering bacteria were determinate using the dilution-plate technique. Carrier samples from each microcosm assays were taken periodically. One gram of the carrier was placed in a tube with 9 mL of sterile saline solution, and the biofilm was detached from the carrier by sonication in a bath sonicator (Ultrasonic bath, Selecta) at room temperature for 10 min. A total of 0.1 mL of serially diluted samples were plated on trypticase soy agar (TSA, Difco). The inoculated plates were incubated at 28 °C for 48 h before the colonies were counted (Silva-Castro et al. 2012).
2.7 Hydrocarbon Analysis
Total petroleum hydrocarbons (TPHs) were extracted from both water and carrier samples with a mixture of hexane:acetone 1:1 (v/v) and determined by gravimetric analysis as described elsewhere (Aguilera-Vázquez et al. 2001). For water samples, the procedure for hydrocarbon extraction was the Standard Method 5520 modified, using hexane/acetone instead of hexane/MTBE (APHA 1995). In the case of the carriers, samples were previously sonicated for 10 min. Aliphatic (alkanes C10–C20, alkanes C20–C40, branched and cyclic, phytane and pristane) and aromatic hydrocarbon fractions were analyzed on the hexane:acetone extract using a Hewlett-Packard 6890 GC system equipped with a HP-5-MS-capillary column (30 × 0.32 mm I.D.). Helium (1.6 mL min−1) was used as the carrier gas. The determinations were performed using the following temperature program: 40 °C held for 1 min isothermal, heating rate at 4 °C min−1 up to 310 °C, and final temperature held for 1.5 min. Injector and detector temperatures were 250 and 300 °C, respectively. N-alkanes and PAH were detected using a mass detector 5872 (Hewlett-Packard) and the library utilized was Wiley 275.
2.8 Scanning Electron Microscopy
Periodically, samples were taken from the carriers, fixed with glutaraldehyde (2.5% v/v) followed by three PBS washed, and dehydrated using ethanol solutions. The dry carrier samples were gold coated and examined by electron microscopy according the methodology described elsewhere (Guisado et al. 2016).
These samples were examined by scanning electron microscopy to analyze the biofilm formed on the carriers’ surface in the Centre for Scientific Instrumentation of the University of Granada (Spain), using LEO 1430VP and LEO 1430VP microscopes equipped with an INCA350 EDX system.
2.9 Statistical Analysis
The mean, variance, and standard deviation of the microbiological and chemical parameters were calculated from the values recorded from each of the triplicate samples. The data were analyzed using a one-way multifactor analysis of variance (ANOVA) and a Turkey’s test. Statistical significance limits were set at p < 0.05. All statistical analyses were carried out using SPSS (Statistical Package for the Social Sciences) software v.15.0.
3 Results
3.1 Bacterial Adhesion Capacity of Carriers. Microcosm Assays
3.1.1 Microbial Growth
The use of microorganisms adhering to a carrier forming a biofilm is one of the effective technologies for treating contaminated water. An effective system requires a suitable support as well as stability of the biofilm. In this sense, several authors have demonstrated how adhesion of bacteria to surface is strongly related to the concentration of nutrients and therefore to the density of the adhering microbial population (Borges et al. 2008; Cowell et al. 1999). Thus, assays I and II were performed to determine the effect of nutrient depletion on surface adherence for each of the carriers.
Results from the assay in which LB medium was used showed that the growth of P. elyakovii W18 adhering to both cork carriers raised consistently, reaching values close to 7 and 8 Log (UFC mL−1) in the case of CorkSorb™ 03025 and CorkSorb™ 01025, respectively. On the other hand, using “Pad Sentec™” as carrier, the mean count values of adhering microbial population slightly declined after 2 days, reaching values close to 7 Log (UFC mL−1) at the end of the experiment (7 days). In the case of “Barrier Sentec™,” similar values were found after the first day of the assay with values between 6 and 7 Log (UFC mL−1) (Fig. 1). These results, as shown in Fig. 1, indicated significant differences between the microbial growth adhering to CorkSorb™ 03025 and the rest of carriers according to a multivariate analysis based on the Tukey’s test (α = 0.05).
Adhering of P. elyakovii W18 to the carriers CorkSorb™ 01025, CorkSorb™ 03025, Pad Sentec™, and Barrier Sentec™, in microcosms with LB medium, expressed in Log (UFC mL−1). Bacterial adhesion capacity of carrier assays. (*) Significant differences between the sorbent carriers at each sampling time. Multivariate analysis based on the Tukey’s test (α = 0.05)
Regardless, the carrier used, P. elyakovii W18, was able to grow and adhere itself in all the microcosms studied when the culture medium was LB broth (Rodríguez-Calvo et al. 2017). Previous reports have demonstrated that the concentration of nutrients and microbial-population density are important factors in bacterial surface adherence, showing a relationship between the surface adhesion rate and the lack of appropriate bacterial growth (adhering and supernatant bacteria) (Al-Juboori and Yusaf 2012; Liu et al. 2011). Therefore, a new assay in which the LB medium was substituted by BH mineral medium was planned in order to determine the response of this microorganism to nutrient depletion.
In this one, adhering bacterial growth diminished slightly with respect to LB medium. In this sense, P. elyakovii W18 grew better in microcosms with Pad Sentec™ and Barrier Sentec™ carriers (values between 6 and 7 Log UFC mL−1) than in those with Corksorb™ carriers, above all in CorkSorb™ 03025 microcosms, which resulted in no bacterial growth, as surface-adhering microbiota was lower than 4 Log UFC/mL−1 of the carrier (Fig. 2). These results, as shown in Fig. 2, indicated significant differences between the microbial growth adhering to CorkSorb™ 01025 as well as CorkSorb™ 03025, and this growth adhering to Pad Sentec™ as well as Barrier Sentec™, according to a multivariate analysis based on the Tukey’s test (α = 0.05).
Adhering of P. elyakovii W18 to the carriers CorkSorb™ 01025, CorkSorb™ 03025, Pad Sentec™, and Barrier Sentec™, in microcosms with BH medium, expressed in Log (UFC mL−1). Bacterial adhesion capacity of carrier assays. (*) Significant differences between the sorbent carriers at each sampling time. Multivariate analysis based on the Tukey’s test (α = 0.05)
3.1.2 Scanning Electron Microscopy
The adhesion capacity of carriers was confirmed by scanning electronic microscopy. This study demonstrated the presence of bacteria adhering to the carriers with a notable amount of exopolymers. Furthermore, the images reflect a larger microbial population adhering to Pad Sentec™ and Barrier Sentec™ (Figs. 3 and 4).
3.2 Hydrocarbon Tolerance of the Biofilm. Microcosm Assays
This second stage of microcosm assays was performed to evaluate the capacity of wastewater indigenous microbiota to adhere to the carriers’ surface. According to the results of previous assays in terms of microbial surface adhesion, shown previously, CorkSorb™ 01025 and Pad Sentec™ were selected as carriers for the following assays. An investigation was made of the tolerance of the microbial biofilm to different hydrocarbon concentrations and the efficacy of carriers to retain the hydrocarbon pollutants from the industrial wastewater during treatment.
3.2.1 Microbial Growth
Figures 5 and 6 show that none of the carriers proved toxic for microbial viability and the stability of adhered microorganisms was evidenced in both of them. The presence of hydrocarbons increased the microbial growth adhering to both carriers, although in the case of the carrier CorkSorb™ 01025, the biofilm was noticeably detached at the end of assays, reaching values close to 4.5 Log (UFC mL−1), while this adhering population was around 5–6.5 Log (UFC mL−1) during the assays. Furthermore, the detachment of some bacterial cells from the CorkSorb™ 01025 biofilm was detected by microscopy studies, as will be shown subsequently. As shown in Fig. 5, there are significant differences between the microbial growth in hydrocarbon absence conditions and in presence of the pollutant; on the other hand, the concentration influence is observed in significant differences between “10,000 ppm” and “20,000 ppm” mainly at the final time.
Adhering of indigenous microbiota to the carrier CorkSorb™ 01025, in microcosms with effluent wastewater that lacks hydrocarbons and polluted with 10,000 and 20,000 ppm, expressed in Log (UFC mL−1). Hydrocarbon tolerance of the biofilm assays. (*) Significant differences between “hydrocarbon absence,” and both “hydrocarbon presence (10,000 ppm)” and “hydrocarbon presence (20,000 ppm).” (•) Significant differences between “hydrocarbon absence (10,000 ppm)” and “hydrocarbon absence (20,000 ppm).” Multivariate analysis based on the Tukey’s test (α = 0.05)
Adhering of indigenous microbiota to the carrier Pad Sentec™, in microcosms with effluent wastewater that lacks hydrocarbons and polluted with 10,000 and 20,000 ppm, expressed in Log (UFC mL−1). Hydrocarbon tolerance of the biofilm assays. (*) Significant differences between “hydrocarbon absence,” and both “hydrocarbon presence (10,000 ppm)” and “hydrocarbon presence (20,000 ppm).” (†) Significant differences between “hydrocarbon absence” and “hydrocarbon presence (20,000 ppm).” (°) Significant differences between “hydrocarbon absence” and “hydrocarbon presence (10,000 ppm). (•) Significant differences between “hydrocarbon presence (10,000 ppm)” and “hydrocarbon absence (20,000 ppm).” Multivariate analysis based on the Tukey’s test (α = 0.05)
For Pad Sentec™, the microbial population adhering to the carrier proliferated throughout the assay, reaching values much higher than those in the previous assays [close to 5, 6, and 8 Log (UFC mL−1) respectively], in which hydrocarbon concentration was lower. The significant differences between the three conditions prove this behavior. This trend would indicate that hydrocarbon compounds adsorbed to the surface carrier would be used as a carbon and energy source by the adhering indigenous microorganisms, and consequently, these organic compounds can support their growth. For these reasons, comparing the two carriers, Pad Sentec™ boosted the growth of adhering microorganisms more efficiently than the CorkSorb™ 01025 carrier.
3.2.2 Hydrocarbon Analysis
GC/MS analyses were performed to evaluate the changes in hydrocarbon concentration in both wastewater and carrier samples during the incubation time. In CorkSorb™ 01025 assays at 10,000 ppm of hydrocarbon pollution, considering the mass balance of the system, the removal rates were close to 75% for all of the fractions; whereas at 20,000 ppm, the percentage of hydrocarbon removal varied depending on the hydrocarbon compounds analyzed. On the other hand, for Pad Sentec™ carrier, the majority of hydrocarbon fraction removal rates were close to 90% (Table 1).
In wastewater polluted with 10,000 ppm, in CorkSorb™ 01025 microcosms, all hydrocarbon fractions were removed by more than 66% after 20 days of treatment. Further, these assays demonstrate the capacity of CorkSorb™ 01025 to absorb the hydrocarbon compounds within its structure (Fig. 7). Otherwise, in microcosms with Pad Sentec™ carrier, hydrocarbon removal from water was more efficient (higher than 90%) and part of these pollutants remained absorbed by the carrier (Fig. 8).
In the case of wastewater polluted with 20,000 ppm, the increase in the microbial population due to higher hydrocarbon concentrations resulted in further degradation of all hydrocarbon fractions in the water, and even slightly higher in the case of Pad Sentec™ (higher than 95.5%) (Figs. 9 and 10).
3.2.3 Scanning Electron Microscopy
This microscopy analysis demonstrates the capacity of indigenous microbiota in wastewater to adhere to both surface carriers, CorkSorb ™ 01025 and Pad Sentec™, and their tolerance to hydrocarbon pollution (Fig. 11).
SEM images. a General view of CorkSorb™ 01025 structure. b General view of Pad Sentec™ structure. c Detail of bacteria adhering to CorkSorb™ 01025 structure. d Detail of bacteria adhering to Pad Sentec™ structure. e Detail of microbial detachment from CorkSorb™ 01025 structure. f Detail of bacteria adhering to Pad Sentec™ structure. Hydrocarbon tolerance of the biofilm assays
Thus, biofilm tolerance to hydrocarbons present in water to treat was demonstrated.
4 Discussion
This study was undertaken to select the most suitable carrier to use in bioreactors, for the treatment of hydrocarbon-polluted industrial wastewater, based on biofilm technology. The carriers under study were selected for their ability to absorb hydrocarbons from water. Thus, the first assays were performed to determine whether CorkSorb™ 03025, CorkSorb™ 01025, Barriers Sentec™, and Pad Sentec™ also allow the adherence of bacteria to their surface.
The results from this study demonstrate both microbial adherence to and hydrocarbon absorption by different carriers, manufactured mainly from cork and polypropylene, as some authors have previously reported (Al-Majed et al. 2012; Pintor et al. 2012; Schrader 1991). In addition, adhesion capacity of P. elyakovii W18 strain has been confirmed by Rodríguez-Calvo et al. (2017). Microbial surface adherence is strongly influenced by nutrient conditions (Sezonov et al. 2007). Thus, the first two assays were performed under sterile conditions by the inoculation of P. elyakovii strain W18, one of them in rich medium (LB medium) and the second under nutrient depletion (BH medium), which is closer to the nutrient conditions of industrial wastewater to be treated.
In the bacterial adhesion assays, it was established that hydrophilic cork, CorkSorb™ 03025, was not a good choice as a material for bacterial adherence and consequently biofilm formation. By contrast, sorbent materials CorkSorb™ 01025 (hydrophobic cork), Pad Sentec™, and Barrier Sentec™ (both made of meltblown polypropylene) showed good microbial adhesion, reaching values between 6 and 7 Log UFC/mL−1 of carrier, 3 logarithms higher than the first one.
These results demonstrated that biofilm formation depends largely on the surface where it develops (Donlan 2002), and therefore, carriers play a key role, as results show. It should be highlighted that, as some authors have indicated, microbial adhesion is the first step of biofilm formation and that almost any surface exposed to a hydrated environment is prone to develop this adherence (Bakker et al. 2003; Vanysacker et al. 2013). In fact, three of the four preselected carriers showed good results; other authors have reported that hydrophobicity enhances or facilitates the adherence of bacteria to the carriers (Donlan 2002; Saeki et al. 2016). In this sense, the CorkSorb™ 03025 support, hydrophilic in character, proved to be less efficient in the formation of a stable biofilm than the other substrates tested. In addition, due to its hydrophilic character, water retained inside its structure made its manipulation difficult and consequently its future application in bioreactors for treating industrial wastewater contaminated with hydrocarbons. Therefore, this material was not considered for future research.
Biofilm consists of a group of cells embedded into a polymeric matrix constituted by exopolysaccharides segregated by the biofilm’s own bacterial cells, attached to a surface. It has been demonstrated that biofilms are the most widespread microbial lifestyle (Costerton et al. 1995; Davies et al. 1998; Percival et al. 2011). From the results of the first two assays, two carriers were selected in order to study the adhesion of indigenous microbiota, CorkSorb™ 01025 and Pad Sentec™, the only one made of cork which exhibited good microbial adhesion previously and one of the two carriers made of meltblown polypropylene. In the hydrocarbon tolerance assays, differences appeared between the performances of the carriers, mainly a higher microbial population adhered to Pad Sentec™ and microbial adhesion for a longer time than in the case of CorkSorb™ 01025, in which microbial detachment occurred during the assays, as shown in Fig. 11e. Apart from this, in general, both carriers performed well, as mentioned by other authors. Albareda et al. (2008) and Ferreira and Castro (2005) pointed that the cork was a good material in terms of supporting bacterial growth; the microbial adhesion was evidenced by Bartowsky and Henschke (2008), who demonstrated that the spoilage of natural cork closures in wine bottles was due to the presence of bacteria adhering to them. Other researchers (Jurecska et al. 2013; Krivorot et al. 2011; Zhou et al. 2013) have highlighted that meltblown polypropylene was a material prone to biofilm formation in its structure.
Finally, scanning electron microscopy (SEM) analyses have often been used by authors for studying and characterizing of biofilms (González-Ramírez et al. 2016; Jurecska et al. 2013; Vyas et al. 2016). In this study, SEM images showed the microbial surface adherence not only of P. elyakovii but also of indigenous microbiota from industrial wastewater studied, as shown in Figs. 3, 4, and 11.
The increase of the hydrocarbon content in the second stage of assays resulted in a larger microbial population, which proves that indigenous microbiota from the polluted water was well-adapted to the hydrocarbon presence and could have a good degrading capacity (Bao et al. 2014; Das and Chandran 2010). In addition, with this increase in hydrocarbon concentration, the microbial detachment mentioned previously occurred later than under-unpolluted conditions. With respect to the hydrocarbon content, the results from gas chromatography–mass spectrometry (CG/MS) showed a remarkable reduction in the content of hydrocarbons in water. This reduction was due to the adsorption process which takes places on the carrier surface, since hydrophobic cork and meltblown polypropylene have a high absorption capacity (Pintor et al. 2012; Wei et al. 2003) and due to a biodegradation process which occurs both in the polluted water and in the carriers. In fact, it has been demonstrated that microbiota indigenous to the contaminated area has a high degrading ability (Pandey et al. 2016). Moreover, biofilm formation enhances the degrading capacity of microorganisms, and for these reasons, it was shown that the reduction in the content of hydrocarbons in water is due to a combination of a physical process with a biological process. Furthermore, it has been demonstrated that the main advantage of these biofilm is that they improve the degrading capacity of the microorganisms that make it up, but they require a surface to adhere to (Al-Kharusi et al. 2016), as mentioned previously.
5 Conclusions
Results from this study revealed that a high hydrocarbon concentration in wastewater increased the degrading microbial population adhered to the hydrophobic cork and meltblown polypropylene and enhanced bacterial surface adhesion, delaying the biofilm detachment. Also, both carriers showed a remarkable capacity of hydrocarbon retention, achieving a rapid cleaning of the polluted water and developing a selective conditions for the colonization of hydrocarbon degrading microbiota. In this way, it can be concluded that these materials would be appropriate candidates to be used as carriers for developing hydrocarbon degrading biofilm and becoming a promising technology for the treatment of hydrocarbon-polluted water.
References
Aguilera-Vázquez, L., Soto-Cruz, N. O., Saucedo-Castañeda, G., & Gutiérrez-Rojas, M. (2001). A model system for cocomposting hydrocarbon contaminated soil by using water activity and porosity as response variables. Chemical Engineering Journal, 81(1–3), 197–202. https://doi.org/10.1016/S1385-8947(00)00218-7.
Albareda, M., Rodríguez-Navarro, D. N., Camacho, M., & Temprano, F. J. (2008). Alternatives to peat as a carrier for rhizobia inoculants: solid and liquid formulations. Soil Biology and Biochemistry, 40(11), 2771–2779. https://doi.org/10.1016/j.soilbio.2008.07.021.
Al-Juboori, R. A., & Yusaf, T. (2012). Biofouling in RO system: mechanisms, monitoring and controlling. Desalination, 302, 1–23. https://doi.org/10.1016/j.desal.2012.06.016.
Al-Kharusi, S., Abed, R. M. M., & Dobretsov, S. (2016). Changes in respiration activities and bacterial communities in a bioaugmented oil-polluted soil in response to the addition of acyl homoserine lactones. International Biodeterioration & Biodegradation, 107, 165–173. https://doi.org/10.1016/j.ibiod.2015.11.021.
Al-Majed, A. A., Adebayo, A. R., & Hossain, M. E. (2012). A sustainable approach to controlling oil spills. Journal of Environmental Management, 113, 213–227. https://doi.org/10.1016/j.jenvman.2012.07.034.
APHA (American Public Health Association) (1995) Standard methods for the examination of water and waste water. Washington, USA.
APHA (American Public Health Association) (2005) Standard methods for the examination of water and waste water. Washington, USA.
Bakker, D. P., van der Plaats, A., Verkerke, G. J., Busscher, H. J., & van der Mei, H. C. (2003). Comparison of velocity profiles for different flow chamber designs used in studies of microbial adhesion to surfaces. Applied and Environmental Microbiology, 69(10), 6280–6287. https://doi.org/10.1128/AEM.69.10.6280-6287.2003.
Bao, M., Sun, P., Yang, X., Wang, X., Wang, L., Cao, L., & Li, F. (2014). Biodegradation of marine surface floating crude oil in a large-scale field simulated experiment. Environmental Science: Processes & Impacts, 16(8), 1948–1956. https://doi.org/10.1039/C4EM00166D.
Bartowsky, E. J., & Henschke, P. A. (2008). Acetic acid bacteria spoilage of bottled red wine—a review. International Journal of Food Microbiology, 125(1), 60–70. https://doi.org/10.1016/j.ijfoodmicro.2007.10.016.
Borges, M. T., Nascimento, A. G., Rocha, U. N., & Tótola, M. R. (2008). Nitrogen starvation affects bacterial adhesion to soil. Brazilian Journal of Microbiology, 39(3), 457–463. https://doi.org/10.1590/S1517-83822008000300009.
Calderón, K., González-Martínez, A., Gómez-Silván, C., Osorio, F., Rodelas, B., & González-López, J. (2013). Archaeal diversity in biofilm technologies applied to treat urban and industrial wastewater: recent advances and future prospects. International Journal of Molecular Sciences, 14(9), 18572–18598. https://doi.org/10.3390/ijms140918572.
Costerton, J. W., Lewandowski, Z., Caldwell, D. E., Korber, D. R., & Lappin-Scott, H. M. (1995). Microbial biofilms. Annual Review of Microbiology, 49, 711–745. https://doi.org/10.1146/annurev.mi.49.100195.003431.
Cowell, B. A., Willcox, P., Herbert, B., & Schneider, R. P. (1999). Effect of nutrient limitation on adhesion characteristics of Pseudomonas aeruginosa. Journal of Applied Microbiology, 86(6), 944–954. https://doi.org/10.1046/j.1365-2672.1999.00773.x.
Das, N., & Chandran, P. (2010). Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnology Research International, 2011, e941810. https://doi.org/10.4061/2011/941810.
Davies, D. G., Parsek, M. R., Pearson, J. P., Iglewski, B. H., Costerton, J. W., & Greenberg, E. P. (1998). The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science (New York, N.Y.), 280(5361), 295–298.
Dellagnezze, B. M., de Sousa, G. V., Martins, L. L., Domingos, D. F., Limache, E. E. G., de Vasconcellos, S. P., et al. (2014). Bioremediation potential of microorganisms derived from petroleum reservoirs. Marine Pollution Bulletin, 89(1–2), 191–200. https://doi.org/10.1016/j.marpolbul.2014.10.003.
Deziel, E., Paquette, G., Villemur, R., Lepine, F., & Bisaillon, J. (1996). Biosurfactant production by a soil Pseudomonas strain growing on polycyclic aromatic hydrocarbons. Applied and Environmental Microbiology, 62(6), 1908–1912.
Donlan, R. M. (2002). Biofilms: microbial life on surfaces. Emerging Infectious Diseases, 8(9), 881–890. https://doi.org/10.3201/eid0809.020063.
Ferreira, E. M., & Castro, I. V. e. (2005). Residues of the cork industry as carriers for the production of legume inoculants. Silva Lusitana, 13(2), 159–167.
González-Ramírez, A. I., Ramírez-Granillo, A., Medina-Canales, M. G., Rodríguez-Tovar, A. V., & Martínez-Rivera, M. A. (2016). Analysis and description of the stages of Aspergillus fumigatus biofilm formation using scanning electron microscopy. BMC Microbiology, 16(1), 243. https://doi.org/10.1186/s12866-016-0859-4.
Grace Liu, P.-W., Chang, T. C., Whang, L.-M., Kao, C.-H., Pan, P.-T., & Cheng, S.-S. (2011). Bioremediation of petroleum hydrocarbon contaminated soil: effects of strategies and microbial community shift. International Biodeterioration & Biodegradation, 65(8), 1119–1127. https://doi.org/10.1016/j.ibiod.2011.09.002.
Guisado, I. M., Purswani, J., González-López, J., & Pozo, C. (2016). An extractive membrane biofilm reactor as alternative technology for the treatment of methyl tert-butyl ether contaminated water. Biotechnology Progress, 32(5), 1238–1245. https://doi.org/10.1002/btpr.2311.
ITOPF. (2014). Eliminación de hidrocarburos y desechos. The International Tanker Owners Pollution Federation Limited. Available in (http://www.itopf.com/uploads/translated/TIP9_SPDisposalofOilandDebris.pdf).
Jurecska, L., Barkács, K., Kiss, É., Gyulai, G., Felföldi, T., Törő, B., et al. (2013). Intensification of wastewater treatment with polymer fiber-based biofilm carriers. Microchemical Journal, 107, 108–114. https://doi.org/10.1016/j.microc.2012.05.028.
Krivorot, M., Kushmaro, A., Oren, Y., & Gilron, J. (2011). Factors affecting biofilm formation and biofouling in membrane distillation of seawater. Journal of Membrane Science, 376(1–2), 15–24. https://doi.org/10.1016/j.memsci.2011.01.061.
Leyva-Díaz, J. C., González-Martínez, A., González-López, J., Muñío, M. M., & Poyatos, J. M. (2015). Kinetic modeling and microbiological study of two-step nitrification in a membrane bioreactor and hybrid moving bed biofilm reactor–membrane bioreactor for wastewater treatment. Chemical Engineering Journal, 259, 692–702. https://doi.org/10.1016/j.cej.2014.07.136.
Liu, F., Zhao, C.-C., Xia, L., Yang, F., Chang, X., & Wang, Y.-Q. (2011). Biofouling characteristics and identification of preponderant bacteria at different nutrient levels in batch tests of a recirculating cooling water system. Environmental Technology, 32(7–8), 901–910. https://doi.org/10.1080/09593330.2010.517220.
Pandey, P., Pathak, H., & Dave, S. (2016). Microbial ecology of hydrocarbon degradation in the soil: a review. Research Journal of Environmental Toxicology, 10(1), 1–15. https://doi.org/10.3923/rjet.2016.1.15.
Percival, S. L., Malic, S., Cruz, H., & Williams, D. W. (2011). Introduction to biofilms. In S. Percival, D. Knottenbelt, & C. Cochrane (Eds.), Biofilms and veterinary medicine (pp. 41–68). Berlin: Springer. https://doi.org/10.1007/978-3-642-21289-5_2.
Pintor, A. M. A., Ferreira, C. I. A., Pereira, J. C., Correia, P., Silva, S. P., Vilar, V. J. P., et al. (2012). Use of cork powder and granules for the adsorption of pollutants: a review. Water Research, 46(10), 3152–3166. https://doi.org/10.1016/j.watres.2012.03.048.
Radwan, S. S., Al-Hasan, R. H., Salamah, S., & Al-Dabbous, S. (2002). Bioremediation of oily sea water by bacteria immobilized in biofilms coating macroalgae. International Biodeterioration & Biodegradation, 50(1), 55–59. https://doi.org/10.1016/S0964-8305(02)00067-7.
Rekadwad, B. N., & Khobragade, C. N. (2015). A case study on effects of oil spills and tar-ball pollution on beaches of Goa (India). Marine Pollution Bulletin, 100(1), 567–570. https://doi.org/10.1016/j.marpolbul.2015.08.019.
Rodríguez-Calvo, A., Silva-Castro, G. A., Uad, I., Robledo-Mahón, T., Menéndez, M., González-López, J., & Calvo, C. (2017). A comparative study of adhesion by bacterial isolates of marine origin. International Biodeterioration & Biodegradation, 123(Supplement C, 87–95. https://doi.org/10.1016/j.ibiod.2017.03.031.
Rodriguez-Valera, F., Ruiz-Berraquero, F., & Ramos-Cormenzana, A. (1981). Characteristics of the heterotrophic bacterial populations in hypersaline environments of different salt concentrations. Microbial Ecology, 7(3), 235–243. https://doi.org/10.1007/BF02010306.
Röling, W. F. M., Milner, M. G., Jones, D. M., Lee, K., Daniel, F., Swannell, R. J. P., & Head, I. M. (2002). Robust hydrocarbon degradation and dynamics of bacterial communities during nutrient-enhanced oil spill bioremediation. Applied and Environmental Microbiology, 68(11), 5537–5548.
Saeki, D., Nagashima, Y., Sawada, I., & Matsuyama, H. (2016). Effect of hydrophobicity of polymer materials used for water purification membranes on biofilm formation dynamics. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 506, 622–628. https://doi.org/10.1016/j.colsurfa.2016.07.036.
Schrader, E. L. (1991). Remediation of floating, open water oil spills: comparative efficacy of commercially available polypropylene sorbent booms. Environmental Geology and Water Sciences, 17(2), 157–166. https://doi.org/10.1007/BF01701571.
Sezonov, G., Joseleau-Petit, D., & D’Ari, R. (2007). Escherichia coli physiology in Luria-Bertani broth. Journal of Bacteriology, 189(23), 8746–8749. https://doi.org/10.1128/JB.01368-07.
Silva-Castro, G. A., Uad, I., Gónzalez-López, J., Fandiño, C. G., Toledo, F. L., & Calvo, C. (2012). Application of selected microbial consortia combined with inorganic and oleophilic fertilizers to recuperate oil-polluted soil using land farming technology. Clean Technologies and Environmental Policy, 14(4), 719–726. https://doi.org/10.1007/s10098-011-0439-0.
Soliman, R. M., El-Gendy, N. S., Deriase, S. F., Farahat, L. A., & Mohamed, A. S. (2014). The evaluation of different bioremediation processes for Egyptian oily sludge polluted soil on a microcosm level. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 36(3), 231–241. https://doi.org/10.1080/15567036.2012.711799.
Vanysacker, L., Denis, C., Declerck, P., Piasecka, A., & Vankelecom, I. F. J. (2013). Microbial adhesion and biofilm formation on microfiltration membranes: a detailed characterization using model organisms with increasing complexity. BioMed Research International, 2013. https://doi.org/10.1155/2013/470867.
Vyas, N., Sammons, R. L., Addison, O., Dehghani, H., & Walmsley, A. D. (2016). A quantitative method to measure biofilm removal efficiency from complex biomaterial surfaces using SEM and image analysis. Scientific Reports, 6, 32694. https://doi.org/10.1038/srep32694.
Ward, O., Singh, A., & Van Hamme, J. (2003). Accelerated biodegradation of petroleum hydrocarbon waste. Journal of Industrial Microbiology & Biotechnology, 30(5), 260–270. https://doi.org/10.1007/s10295-003-0042-4.
Wei, Q. F., Mather, R. R., Fotheringham, A. F., & Yang, R. D. (2003). Evaluation of nonwoven polypropylene oil sorbents in marine oil-spill recovery. Marine Pollution Bulletin, 46(6), 780–783. https://doi.org/10.1016/S0025-326X(03)00042-0.
Yoo, S.-Y., Daud, W. M. A. W., & Lee, M.-G. (2012). Preparation of a biodegradable oil absorber and its biodegradation. Bioprocess and Biosystems Engineering, 35(1–2), 283–288. https://doi.org/10.1007/s00449-011-0613-0.
Zamani, M. M., Fallahpour, M., Yousefi Harvani, G., Khodi Aghmiuni, S., Zamani, M., Minai Tehrani, D., & Savaghebi Firrozabadi, G. (2014). Recent proportionate treatment methods for crude oil contamination evaluation of the Tehran refinery site soil. Thrita, 3(1). https://doi.org/10.5812/thrita.12113.
Zhou, D., Hai, R., & Wang, W. (2013). Novel complex fiber biofilm carrier in an anaerobic/anoxic/oxic reactor for sewage mixture treatment. Asian Journal of Chemistry, 25(12), 6943–6947. https://doi.org/10.14233/ajchem.2013.15277.
Acknowledgements
This research has been supported by Compañía Logística de Hidrocarburos S.A. and by the Environmental Microbiology Research Group RNM270 of the University of Granada, Spain. We acknowledge Mr. David Nesbitt for improving the English in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez-Calvo, A., Silva-Castro, G.A., Robledo-Mahón, T. et al. Capacity of Hydrophobic Carriers to Form Biofilm for Removing Hydrocarbons from Polluted Industrial Wastewater: Assay in Microcosms. Water Air Soil Pollut 229, 175 (2018). https://doi.org/10.1007/s11270-018-3826-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3826-x