Abstract
Bioremediation is an important technology for the restoration of oil-polluted environments by the use of indigenous or selected microorganisms. We analyzed the efficiency of two selected microbial consortia (A and B) inoculated in combination with inorganic fertilizer (NPK) or oleophilic fertilizer (S200 commercial) on the degradation of polycyclic aromatic hydrocarbons (PAH) by applying them in land farming treatments. Consortium A was composed of Bacillus pumilus, Alcaligenes faecalis, Micrococcus luteus, and Enterobacter sp.; consortium B was composed of B. pumilus, Enterobacter sp., and Ochrobactrum anthropi. Land treatment was performed in separated plots and the evolution of biodegradation rates was followed for 7 months.
Treatment with NPK and inoculation with consortium A efficiently reduced the n-alkane hydrocarbons. In contrast, the application of S200 C and inoculation with consortium B reduced the hydrocarbon removal capacity of polluted soil and they did not show any advantage respected to the non-treated control plot. In addition, the results showed that inoculation with consortium A and application of the combined treatment consortium A + NPK fertilizer gave the highest percentage of PAH removal. These results suggest that the inoculation of polluted soil with consortium A, integrated by hydrocarbon-degrading bacteria and biosurfactant/bioemulsifier-producing bacteria, would be a useful method for improving hydrocarbon biodegradation. In conclusion, inoculation with a selected bacterial consortium at the beginning of a land treatment followed by treatment with NPK fertilizer is an efficient combination treatment of bioaugmentation and biostimulation for application in the bioremediation of soil polluted with hydrocarbons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioremediation is a process of transforming hazardous compounds into less hazardous/non-hazardous forms with a low input of chemicals, energy, and time. It is an approach to degrade/remove pollutants in an eco-friendly manner (Haritash and Kaushik 2009). Bioremediation is a treatment used to clean up polluted areas, including soils contaminated by petroleum hydrocarbons. It is a process whereby the natural biodegradation of petroleum hydrocarbons by indigenous soil microorganisms is accelerated. It is considered to be an environmentally acceptable way of eliminating oils and fuel because the majority of hydrocarbons in crude oils and refined products are biodegradable, and hydrocarbon-degrading microbes are ubiquitous (Kanaly and Harayama 2000).
The bioremediation of a pollutant and the rate at which this is achieved depends on the environmental conditions, the number and type of microorganisms, nature, and the chemical structure of the chemical compound being degraded. Thus, to devise a bioremediation system, several factors need to be addressed and explored.
Land farming is a generic term coined to define the controlled application of bioremediation to surface soil. The technology is a direct outgrowth of the petroleum refinery practice of land treatment. Years ago, the usual removal method for several of the sludges generated in refining processes was to dump them into sandy soil, where the volume of the sludge was reduced. Land farming of hydrocarbon-contaminated soils has been demonstrated to be a safe and cost-effective treatment method for hydrocarbon waste products due to the simplicity of its design, operation and relatively high treatment efficiency (Huang et al. 2001; Marín et al. 2005).
The biodegradation of petroleum in soil ecosystems is related to the ability of microbes to degrade the contaminants. Factors affecting the biodegradation of hydrocarbons include: (1) the type and amount of hydrocarbons present, (2) the type and number of hydrocarbon-degrading microorganisms present or inoculated in contaminated soil, (3) nutrients, specially the limitation of nitrogen and phosphorous, (4) soil, temperature and pH, (5) aeration, and (6) water activity. Bioremediation is enhanced by the optimization of these factors (Margesin and Schinner 1997).
Bioaugmentation of polluted soil is often disappointing because of the low survival and activity of the inoculated degrader bacteria. The objective of this study was to evaluate the effect of two selected microbial consortia containing hydrocarbon-degrading bacteria and bioemulsifier-producing bacteria on bioremediation processes (land farming technology). We investigated whether or not the addition of inorganic NPK fertilizer or the oleophilic commercial compound S200 enhanced the natural biodegradation rate of hydrocarbons.
Materials and methods
Bacterial consortia
Two bacterial consortia (A and B) containing approximately 109 viable cells ml−1 of each strain were used in the land farming treatments. Consortium A was a mixed population of four bacterial strains: Bacillus pumilus 28-11, Alcaligenes faecalis 212-2, Micrococcus luteus 212-4, and Enterobacter sp. 214-6. Consortium B was a mixed population of three bacterial strains: B. pumilus 28-11, Enterobacter sp. 214-6, and Ochrobactrum anthropi AD2. Strains B. pumilus, A. faecalis, M. luteus, and Enterobacter sp. were isolated in our laboratory from solid crude oil waste samples collected from the clean up of oil storage containers. All strains grew in culture media supplemented with a wide number of hydrocarbons. Furthermore, strains A. faecalis, M. luteus and Enterobacter sp showed the capacity to synthesize bioemulsifiers and strain B. pumilus showed surfactant activity during its exponential growth phase (Calvo et al. 2002, 2004; Toledo et al. 2007).
Strain O. anthropi AD2 was isolated from activated sludge samples from the wastewater treatment plant of the oil refinery in Puertollano (Repsol YPF). This bacterium was isolated and characterized in our laboratory and it showed the capacity to degrade oil hydrocarbons and to produce bioemulsifiers (Calvo et al. 2008).
The characteristics of the microorganisms used in this study are summarized in Table 1.
Identification and phylogenetic affiliation of the included strains
The five strains included in this study were identified by analysing the sequence of the gene encoding 16 S rRNA (16S rDNA) (Toledo et al. 2006; Calvo et al. 2008). Primers fD1 and rD1 were used to amplify almost the full length of the 16S rRNA gene from each strain (Weisburg et al. 1991). The reactions were run in a Perkin Elmer GeneAmp PCR system 2400. The amplification products were purified by agarose gel electrophoresis. Sequence data were analyzed using the GCG Wisconsin Package v. 10.1 programs (Genetics Computing Group; Madison, Wisconsin. USA). The BLASTN and FASTA v. 3.3t07 programs were used for preliminary sequence identity analysis (Pearson and Lipman 1988). The Clustal X v. 1.81 program was used for sequence alignment.
Land farming assays
The soil utilized in our study was a typical xerorthent with a loamy texture, containing 50% sand, 30% silt, and 20% clay. The chemical composition of the soil was as follows: organic matter 3.75%; pH (water) 7.8; N–NO3 −, 50 mg kg−1; inorganic phosphorous, 20 mg kg−1; potassium, 80 mg kg−1. The physical and chemical characteristics of the soil were analyzed by using the techniques described by Bremner (1982), Olsen and Sommers (1982), and the Soil Conservation Service (1975).
The land farming treatments were performed in three separated plots of 150 m2. There was a slight slope across the plots to the dump of 1% for drainage. To avoid subsurface contamination, a liner system was built with an impermeable surface of 1.5-mm high-density polyethylene (HDPE) and a Geotextile floor for drainage. To protect the liner against tilling and aeration equipment, a 20 cm layer of clean sand was spread over the Geotextile floor. After the construction of the treatment unit, hydrocarbon-contaminated soil from the Refinery of Repsol (Puertollano, Spain) was added to each plot to reach a final TPH concentration of 20,000 mg kg−1. The soil was tilled weekly and irrigated at 50–80% water holding capacity.
Each land farming treatment plot was divided into six subplots of 25 m2 in order to evaluate the effects on hydrocarbon biodegradation by the following treatments: NPK fertilizer (plot B), oleophilic compound S200 C (plot C), bacterial consortium A (plot D), bacterial consortium B (plot E), and NPK fertilizer plus bacterial consortium A (plot F). Land farming treatment without any supplementation was also included as the control (plot A). The control plots were also tilled and irrigated.
Three doses of 1.5 kg of NPK inorganic fertilizer were added to plots B and F at 0, 1 and 2 months of treatments. The inorganic fertilizer used in our study was a commercial NPK (18:8:17) product obtained from Agroblem S.L. (Spain). Plot C received three doses of 1 l of the bioremediation agent S200 C (IEP Europe S.L.) at 0, 1, and 2 months.
Inoculation with cell suspensions of the bacterial consortia (A and B), was accomplished by a backpack pump dispenser which released the suspensions onto the soil via a peristaltic pump. The microorganisms were grown as pure cultures in Nutrient Broth (NB) medium with the following composition (g l−1): glucose, 10; yeast extract, 5; proteose-peptone, 5; and NaCl, 5. Bacteria were cultivated at 32°C for 5 days under aerobic conditions on a rotatory shaker (2.5 Hz). The cells were harvested by centrifugation (10,000×g, 10 min) and resuspended in sterile saline solution (0.9% NaCl) to yield cell suspensions of each strain of approximately 109 colony-forming-units ml−1 (CFU ml−1). Plots D and F received 10 l of consortium A containing equal amounts of cell suspensions of B. pumilus, A. faecalis, M. luteus and Enterobacter sp. Plot E received 10 l of consortium B containing equal amounts of B. pumilus, Enterobacter sp. and O. anthropi. Plots D, F and E were newly inoculated after 2 months.
Enumeration of culturable bacteria in soil
Three separate samples per subplot from each land farming treatment were taken at 1, 2, 3, and 7 months for enumeration of aerobic heterotrophic bacteria. One tenth of serially diluted soil samples were plated on 1:10 diluted Trypticase Soy Agar (TSA, Difco) as previously reported by Sánchez-Peinado et al. (2008). Triplicate plates were incubated at 28°C for 48 h before the colonies were counted. Data were reported as CFU g−1 dry soil.
TPH, n-alkanes, and PAH determinations
Total petroleum hydrocarbons (TPH) were extracted from the soil samples with a mixture of hexane: acetone 1:1 and determined by gravimetric analysis according to Aguilera-Vázquez et al. (2001). Analyses of n-alkanes and polycyclic aromatics hydrocarbons (PAH) were performed on the hexane: acetone extract using a Hewlett-Packard 6890 GC system equipped with a HP-5-MS-capillary column (30 m × 0.32 mm I.D.). Helium (1.6 ml min−1) was used as the carrier gas. The determinations were performed using the following temperature program: 40°C held for 1 min isothermal, heating rate 4°C min−1 up to 310°C, final temperature held for 1.5 min. Injector and detector temperatures were 250 and 300°C, respectively. N-alkanes and PAH were detected using a mass detector 5872 (Hewlett-Packard) and the library utilized was Wiley 275.
Production and characterization of bioemulsifiers
Production and preliminary characterization of the chemical composition of the bioemulsifiers synthesized by A. faecalis, M. luteus, Enterobacter sp., and O. anthropi were performed according to Calvo et al. (2008); 500-ml Erlenmeyer flasks containing 100 ml of NB medium were inoculated with 1 ml of a 24-h culture of microorganisms grown in the same medium. After incubation at 32°C for 7 days, the cultures were centrifuged at 36,000 g in a Sorval RC-5B refrigerated centrifuge at 4°C for 60 min. The supernatants obtained were precipitated with 96% ethanol at 4°C. The precipitated biopolymers were dissolved in distilled water, dialyzed against distilled water for 24 h, lyophilized, and weighed. Total protein and carbohydrate content of the exopolymers were determined as described by Bradford (1976) and Dubois et al. (1956), respectively.
Emulsification assays and surfactant activity test
The emulsifying activity of the biopolymers synthesized by strains A. faecalis, M. luteus, Enterobacter sp., and the O. anthropi AD2 strain was tested using a modified version of the method previously described by Cooper and Goldenberg (1987). Test tubes (105 × 15 mm) were amended with 3.0 ml of exopolymer diluted in distilled water (0.1%, weight/volume) and 3 ml of a hydrophobic substrate (n-octane, xylene, toluene, mineral oil, or crude oil). The tubes were vigorously vortexed and left to stand for 24 h. The emulsifying activity was expressed as the percentage of the total height occupied by the emulsion. The surfactant activity was determined by measuring the surface tension with a Krüs K11 digital tensiometer, using a plate method (Barathi and Vasudevan 2001).
Statistical analyses
The microbiological and chemical parameters were calculated from the values obtained in each measurement of the triplicate samples. Differences between biological and chemical analysis in the different soil samples were tested by multivariate analysis. The statistical significance was evaluated at the Tukey P < 0.05. All statistical analyses were carried out using the SPSS 15.0 software (SPSS Inc. Chicago, EE.UU).
Results and discussion
Oily wastes are often expensive to store and remediate. The bioremediation of land contaminated with hydrocarbons has been demonstrated to be a safe and cost-effective technology due to its simplicity of design, operation, and efficacy (Admon et al. 2001; Prince et al. 2002; Namkoong et al. 2002). Microbes are primary agents for the degradation of hydrocarbon contaminants in soil. However, it is well known that an individual organism normally only metabolizes a limited range of hydrocarbon substrates. Whereas, microbial consortia of mixed populations with overall broad enzymatic capacities are able to degrade complex mixtures of hydrocarbons (Leahy and Colwell 1990).
In this research, we studied the capacity of two microbial consortia, A and B, to remove n-alkanes and polycyclic aromatic hydrocarbons (PHA) from oil-contaminated soil using land farming technology. The microorganisms of the consortia belonged to the collection at our laboratory and were chosen because they are hydrocarbon-degrading microorganisms or because they produce biosurfactant and bioemulsifier polymers (Calvo et al. 2002, 2008; Toledo et al. 2006, 2007).
There are a number of bacterial species which have been isolated from different environments and found to be capable of degrading hydrocarbons (Benton et al. 2005). Acclimatization of these species can serve as the key for enhanced degradation. The induction of the degradation capacity by exposing the microbes to higher levels of pollutants may, at times, result in genetic adaptations/changes responsible for higher rates of removal. In this sense, the microorganisms included in this study might be well adapted to strongly polluted habitats in extreme environments due to their origin (Calvo et al. 2004; Toledo et al. 2006, 2007). Consequently, they had to be good candidates for use in bioremediation processes.
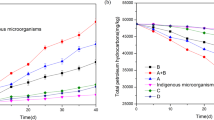
In the land farming assays, the total number of aerobic heterotrophic bacteria in the soil plots was determined at 1, 2, 3, and 7 months of treatment in order to evaluate the effect of different bioremediation treatments on culturable microbial populations. Leahy and Colwell (1990) reported that hydrocarbon degradation in soil is correlated with the total number of microbiota present in these habitats. The mean count during the land farming treatments ranged between 106 and 107 CFU g−1 of soil (Table 2). When treated soils (plots B, C, D, E and F) were compared with control soil (plot A) an increase in the culturable bacterial populations during the first month of treatment was observed. In this sense, positive statistically significant differences were showed P < 0.05. This stimulatory effect indicated that the application of inorganic NPK fertilizer (plots B and F), the oleophilic product S200 C (plot C) and bacterial consortia A (plot D and F) and B (plot E) onto hydrocarbon-contaminated soils can produce a prompt and efficient stimulation of total microbial populations. These data are consistent with the degradation kinetics of TPH and n-alkanes found in plots B, D and F, indicating a positive correlation between both parameters (Figs. 1, 2, 3, 4). In contrast, the application of S200 C increased indigenous microbial populations but it did not enhance hydrocarbon degradation, suggesting that the soil microorganisms consumed the S200 C as a more reliable substrate for growth than the hydrocarbons. After this period the number of microorganisms was similar in all plots except in plot E inoculated with consortium B, where a slight decrease in the microbial population was detected after the second month of treatment. The lower number of heterotrophic bacteria detected in plot E could be associated with the accumulation of some toxic compounds from the hydrocarbon pollutants and/or starvation of some nutrients. However, further studies are needed to confirm this suggestion.
Percentage of C10–C20 n-alkanes that remained in polluted soil during land farming treatment. Plot A control plot; B plot treated with NPK fertilizer; plot C treated with S200 C; D plot inoculated with consortium A; E plot inoculated with consortium B; and F plot treated with consortium A plus NPK fertilizer
Percentage of C20–C32 n-alkanes that remained in polluted soil during land farming treatment. Plot A control plot; B plot treated with NPK fertilizer, plot C treated with S200 C; D plot inoculated with consortium A; E plot inoculated with consortium B; and F plot treated with consortium A plus NPK fertilizer
Percentage of C32–C40 n-alkanes that remained in polluted soil during land farming treatment. Plot A control plot; B plot treated with NPK fertilizer; plot C treated with S200 C; D plot inoculated with consortium A; E plot inoculated with consortium B; and F plot treated with consortium A plus NPK fertilizer
Hydrocarbons analyses of the polluted soil samples showed that hydrocarbon biodegradation in terms of TPH was more superior in the plots treated with consortium A and NPK fertilizer (B, D, and F) than in the control plot A (natural attenuation). In general, these treatments increased the rate of hydrocarbon removal by following the same pattern of biodegradation: a high degradation rate was observed during the first few months of treatment in comparison to the final period, which exhibited a lower rate of biodegradation. Furthermore, this rapid rate of degradation was stimulated by consortium A inoculation. In contrast, the addition of S200 C (plot C) or consortium B (plot E) reduced the hydrocarbon removal capacity in the polluted soil with respect to the control (Fig. 1). Statistical analyses perform using SPSS 15.0 software showed positive statistical significant differences of TPH removal in plots B, D and F respect to plots A, C, and E with Tukey P < 0.05.
In the same way, the analyses of n-alkanes showed beneficial effects of both consortium A inoculation and the application of inorganic fertilizer containing nitrogen and phosphorous on the biodegradation of C10–20, C20–32 and C32–40 alkanes (Figs. 2, 3, 4). Thus, plots B, D, and F seemed to be the first plots where the degradation of C10-20 and C20-32 took place. In contrast, the addition of S200 C and bacterial consortium B negatively affected the n-alkane removal capacity of the soil. In this case, statistical significant differences were detected in C10–20 and C20–32, showing plot A, B, D, and F a P value < 0.005 at the first month of land assay. However, for C32–40 the statistical significant differences were observed at the end of treatment but with a positive effect only on plots B and D (Table 3).
Biodegradation is nature’s way of recycling wastes by breaking down organic matter or inorganic compounds into nutrients which can be used by living organisms (Gan et al. 2009). Crude oil pollution adversely affects the soil ecosystem by adding excess carbon for microbial use which might induce a limitation in soil nitrogen and phosphorous (Baker and Herson 1994). The addition of organic or inorganic nitrogen and phosphorous has been used to enhance bioremediation, thus, many authors have reported that the application of nutrients such as N and P increase bioremediation processes by increasing the microbial biomass (Benton et al. 2005; Sarkar et al. 2005). In this context, soil supplementation with inorganic fertilizers represents one of the most common stimulating agents utilized in soil bioremediation. The effectiveness of these treatments has, however, been conflicting; in many cases, the application of NPK fertilizers as the sole stimulating agent was not enough to totally recuperate a polluted soil (Cunnighan and Philp 2000) and, therefore, the effectiveness of each treatment in polluted soil needs to be evaluated.
In this investigation, we also studied the capacity of land treatment to remove PAHs. Some PAHs have been catalogued by the International Agency for Research on Cancer (IARC) as possible or probable human carcinogens, and designated by the United States Environmental Protection Agency (USEPA) as primary contaminants (IARC 1989; USEPA 2000). The results obtained in the land treatment inoculated with consortium A clearly show the capacity of these microorganisms to stimulate PAH removal with significant differences Tukey P < 0.05 (Table 4). However, this positive effect was not observed in plots C (S200 C) and E (consortium B), we not found statistically significant differences respect with control. The percentages of PAH removal were 89.9% in the land treatment exclusively inoculated with consortium A, 87% of the PAHs were eliminated in the plot treated with NPK fertilizer plus consortium A and an 83% removal was found in plot B with NPK fertilizer alone. A similar response was found in the elimination of individual PHA, such as acenaphtilene, acenaphthene, fluorine, phenanthrene, fuorantene, and pyrene.
A degradation index (Id) was calculated using the control plot (natural attenuation) as the reference control. Our results showed that removal efficacy varied depending on the types of hydrocarbon and land treatment applied, thus, inoculation with consortium A (alone or combined with NPK fertilizer) was found to be the most efficient method according to the Id values obtained (Table 4).
Finally, cluster analysis was done to evaluate the effect of treatments in the bioremediation process. Figure 5 shows the dendogram with two groups of similarity, one of them includes consortium A treatment, consortium A plus NPK fertilizer, NPK treatment (with less than 2% of dissimilarity) and natural attenuation (with less than 5% of dissimilarity) and the second cluster links consortium B inoculation and S200 C treatment with 25% of dissimilarity respect to the first group.
Benton et al. (2005) reported that the best bioaugmentation performance can be achieved by using microorganisms that are already present in the soil by increasing their abundance; indigenous microorganisms are well adjusted to their own environment. With an increase in a specific microbial community and the addition of nutrients, this approach substantially reduces the cleanup time. The microorganisms of consortium A were isolated from waste crude oil samples collected from oil storage containers and seemed to be well adapted to oil pollution and easily degraded hydrocarbons.
In conclusion, our results show that consortium A, which contained a mixed population of four species of bacteria could be a useful tool for improving the rate of hydrocarbon biodegradation, particularly in the removal of PHA. Also, the addition of NPK inorganic fertilizer increased the bioremediation effectiveness in a very short period of time. Thus, the application of this selected bacterial consortium and an inorganic NPK fertilizer could be an efficient combined bioaugmentation and biostimulation treatment for the bioremediation of soil polluted by hydrocarbons.
References
Admon S, Green M, Avnimelech Y (2001) Biodegradation kinetics of hydrocarbons in soil during land treatment of oily sludge. Bioremediat J 5:193–209
Aguilera-Vázquez L, Soto-Cruz NO, Saucedo-Castañeda G, Gutiérrez-Rojas M (2001) A model system for composting hydrocarbon contaminated soil by using water activity and porosity as response variables. Chem Eng J 81:197–202
Baker KH, Herson D (1994) Microbiology and biodegradation. In: Baker K, Herson D (eds) Bioremediation. McGraw Hill Inc, New York, pp 9–60
Barathi S, Vasudevan N (2001) Utilization of petroleum hydrocarbons by Pseudomonas fluorescens isolated from a petroleum-contaminated soil. Environ Int 26:413–416
Benton FM, Camargo F, Okeke B, Frankenberger W (2005) Comparative bioremediation f soil contaminated with diesel soil by attenuation, biostimulation and bioaugmentation. Bioresour Technol 96:1049–1055
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Bremner JM (1982) Nitrogen. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis: part 2, chemical and microbiological properties. American Society of Agronomy, Soil Science Society of America, Madison, pp 699–708
Calvo C, Martínez-Checa F, Toledo FL, Porcel J, Quesada E (2002) Characteristics of bioemulsifiers synthesized in crude oil media by Halomonas eurihalina and their effectiveness in the isolation of bacteria able to grow in the presence of hydrocarbons. Appl Microbiol Biot 60:347–351
Calvo C, Toledo FL, González-López J (2004) Surfactant activity of a naphthalene degrading Bacillus pumilus strain from oil sludge. J Biotechnol 109:255–262
Calvo C, Silva-Castro GA, Uad I, García FC, Laguna J, González-López J (2008) Efficiency of the EPS emulsifier produced by Ochrobactrum anthropi in different hydrocarbon bioremediation assays. J Ind Microbiol Biot 35:1493–1501
Cooper DJ, Goldenberg BG (1987) Surface active agents from two Bacillus species. Appl Environ Microb 54:224–229
Cunnighan CJ, Philp JC (2000) Comparison of biostimulation in ex-situ treatment of diesel contaminated soil. Land Contam Reclam 8:261–269
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Biochem 28:350–356
Gan S, Lau EV, Ng NK (2009) Remediation of soil contaminated with polycyclic aromatic hydrocarbons (PAHs). J Hazard Mater 172:532–549
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15
Huang XD, Glick BR, Greenberg BM (2001) Combining remediation techniques increases kinetics for removal of persistent organic contaminants from soil. ASTM Spec Tech Pub 1403:271–282
IARC (1989) Monographs on the evaluation of carcinogenic risks to humans. In: Some organic solvents, resin monomers and related compounds, pigments and occupational exposures in paint manufacture and painting, Vol. 47, Lyon, pp 237–261
Kanaly RA, Harayama S (2000) Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol 182:2059–2067
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54:305–315
Margesin R, Schinner S (1997) Effect of temperature on oil degradation by psychotropic yeast in liquid culture and soil. FEMS Microbiol Ecol 24:243–249
Marín JA, Hernández T, García C (2005) Bioremediation of oil refinery sludge by landfarming in semiarid conditions: Influence on soil microbial activity. Environ Res 98:185–195
Namkoong W, Hwang EY, Park JS, Choi JY (2002) Bioremediation of diesel-contaminated soil with composting. Environ Pollut 119:23–31
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis: part 2 Chemical and Microbiological Properties. American Society of Agronomy, Soil Science Society of America, Madison, pp 699–708
Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85:2444–2448
Prince RC, Lessard RR, Clark JR (2002) Bioremediation of marine oil spill. Oil Gas Sci Technol 58:463–468
Sánchez-Peinado M, González-López J, Rodelas B, Galera V, Pozo C, Martínez-Toledo MV (2008) Effect of linear alkylbenzene sulfonates on the growth of aerobic heterotrophic cultivable bacteria isolate from an agricultural soil. Ecotoxicology 17:549–557
Sarkar D, Ferguson M, Datta R, Birnbaum S (2005) Bioremediation of petroleum hydrocarbons in contaminated soil: comparison, and monitored natural attenuation. Environ Pollut 136:187–195
Soil Conservation Service, Soil taxonomy (1975) A basic system of soil classification for making and interpreting soil surveys, US Department of Agriculture, Washington
Toledo FL, Calvo C, Rodelas B, González-López J (2006) Selection and identification of bacteria isolated from waste crude oil with polycyclic aromatic hydrocarbons removal capacities. Syst Appl Microbiol 29:244–252
Toledo FL, González-López J, Calvo C (2007) Production of bioemulsifier by Bacillus subtilis, Alcaligenes faecalis and Enterobacter species in liquid culture. Bioresour Technol 99:8470–8475
USEPA (United States Environmental Protection Agency) (2000) Determination of polynuclear aromatic hydrocarbons in industrial and municipal wastewater EPA-600/4-00-025. Environmental Monitoring. Systems Laboratory, Cincinnati
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Acknowledgment
This study was supported by a Grant from the Commission Interministerial de Ciencia y Tecnología (REN2000-0384-P4-02) and the Ministerio de Medio Ambiente (MMA. A4872007/20-01.1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva-Castro, G.A., Uad, I., Gónzalez-López, J. et al. Application of selected microbial consortia combined with inorganic and oleophilic fertilizers to recuperate oil-polluted soil using land farming technology. Clean Techn Environ Policy 14, 719–726 (2012). https://doi.org/10.1007/s10098-011-0439-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-011-0439-0