Abstract
In earlier studies in the existing literature, concentration balance in the feeding solution has never been considered as an influencing factor when the studies were carried out under several feeding conditions to examine effects of different carbon sources on the enhanced biological phosphorus removal (EBPR) process. For a better understanding of a stable operation of an EBPR reactor, it is thought that effects of the concentration balance need to be combined and evaluated with effects of the type of carbon sources in EBPR studies. Therefore, this study was aimed at determining the effect of concentration balance on the performance stability and the phosphorus and glycogen dynamics of a sequencing batch reactor (SBR). In this study, the SBR operation was divided into two main stages. In the first stage, two different ratios of the total concentration of monovalent (M) to divalent (D) cations (in milliequivalent per liter, meq/L) (7.6 and 1.5, 30 days of operation for each) was applied in the feeding solution to investigate the effect of the concentration balance on the performance stability of the SBR. During this stage, sodium acetate was used as the sole carbon source. To investigate the effect of the type of carbon source on the EBPR process under the condition of constant M/D ratio, sodium acetate was used as the sole carbon source during the first half of the reactor operation of 120 days, and, then, the carbon source was abruptly switched to glucose in the second stage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The main concern for municipal wastewater treatment plants is to reduce the amount of organic matters and nutrients such as nitrogen (N) and phosphorus (P) up to a certain permitted level because effluents containing excess amount of organic matters and nutrients may pose threat to the delicate balance of the ecosystem in the receiving waters (Ahmed 2007). Under typical conditions where all factors are conducive to the growth of algae, additions of P to an ecological system are more likely to lead to accelerated growth of algae than the addition of any other nutrients (Datta 2010). Therefore, in order to meet desired nutrient ratios in effluents, removal of P as well as carbon and N is required to conserve the water quality in the receiving waters. Removal of nutrients via biological methods is a cost-effective and environmentally sound alternative to chemical processing of wastewaters (Tunçal et al. 2008). Biological P removal is performed through a process commonly known as enhanced biological phosphorus removal (EBPR). This process has been used for decades to remove P from municipal wastewaters. Mechanisms that underlie in the EBPR process have been well explained in the literature (Mino 2000; Oehmen et al. 2005, 2007; Lu et al. 2006; Zhou et al. 2008). In addition, a short list of the literature studies focusing on the major operational factors that affect the EBPR process was also given by Jeon et al. (2001).
The EBPR process allows facilities to meet water quality goals while minimizing chemical consumption and sludge production that result from the chemical P removal (Datta 2010). The quality of effluents that are discharged from wastewater treatment plants using biological methods highly depends on the efficiency of solid-liquid separation processes. These processes in a clarifier can be achieved by good bioflocculation (Kara et al. 2008). It is accepted that bioflocculation mechanism lies in the abilities of microorganisms to stick to each other and to nonbiological particles existing in the wastewater. In this mechanism, exocellular biopolymers (polysaccharide and protein) that originate from microbial activity and carry net negative charges at neutral pH values form bridges between microorganisms. Divalent cations such as calcium (Ca2+) and magnesium (Mg2+) interact with these negatively charged polymers to form a network that allow the microorganisms to adhere to each other (Jenkins et al. 2004). Monovalent cations also play an important role in this mechanism because of their potential effects on activated sludge (AS) characteristics (Kara et al. 2008).
The effects of cations on AS characteristics have been widely investigated in the literature (Park 2002; Nguyen et al. 2008; Fang et al. 2009; Li et al. 2014). Higgins and Novak (1997) showed that the settling and dewatering properties of the AS deteriorated when the ratio of the concentration of monovalent cations (Na+) to the concentration of divalent cations (Mg2+ and Ca2+) in the feeding solution was higher than 2 to 1 (meq/L). However, the deterioration was reversed when the concentration of Mg2+ and Ca2+ ions increased up to a certain level, resulting in a decrease in the M/D ratio below 2 to 1. Based on these results, the researchers suggested that the minimum concentration of each of Ca and Mg ions should be in the range of 0.7–2.0 meq/L. Another result of the study showed that the concentration of Na+ ions higher than 5 meq/L resulted in an increase in the sludge volume index (SVI) and effluent total suspended solid concentration when Ca and Mg were added to feeding solution in equimolar concentrations of 3 meq/L. Especially, the SVI could not be measured accurately when the concentration of Na+ ions was 20 meq/L due to deflocculation and flotation problems occurring in the reactor. These results clearly suggest that the composition of any feeding solution, which will be formed by taking the concentration of each of the cations into account for a given M/D ratio, plays a key role for a stable reactor operation resulting from achieving good AS characteristics.
In our preliminary studies, where the effect of the type of carbon source on the EBPR process was investigated without taking a proper concentration balance into account in the feeding solution, an unexpected result was obtained showing that both of P and ammonium-N were removed above 95 % in the anaerobic phase, where P was expected to be released into the bulk solution. A severe settling problem also occurred in the reactor. After seeking a way to solve these problems based on comprehensive literature surveys, it was concluded that the obtained results mainly originated from the concentration imbalance in the feeding solution. It was found out that the concentration imbalance resulted from accidental addition of Mg2SO4·7H2O (source of Mg2+ ions) in excess amount, which possibly caused chemical precipitation of both ions as well as poor settling of the AS. As well known, if ammonium and phosphate compounds existing in the medium react with magnesium ions, a hard crystalline deposit, termed as “struvite” and also known as magnesium ammonium phosphate (MAP, NH4MgPO4·6H2O), forms when there is a mole to mole to mole ratio (1:1:1) of magnesium, ammonium, and phosphate (Jaffer et al. 2002). Barat et al. (2008) found out that the high magnesium and phosphate concentrations achieved in their experiments could initiate the formation of different magnesium phosphates. They also reported according to the literature (Musvoto et al. 2000) that four possible phosphate magnesium salts can precipitate from a solution containing magnesium, ammonium, and phosphate. These are struvite (MgNH4PO4·6H2O), magnesium hydrogen phosphate trihydrate or newberyite (MgHPO4·3H2O), and trimagnesium phosphate in two states of hydration (Mg3(PO4)2·22H2O and Mg3(PO4)2·8H2O). These magnesium salts precipitate at different pH regions. Trimagnesium phosphates, which were reported to have a low precipitation rate (Mamais et al. 1994) and have never been identified in the pH range 6–9, were not considered in their study. Depending on above explanations, it was concluded that the accidental addition of magnesium into the feeding solution in excess amount resulted in the formation of MAP precipitates in the preliminary study. Consequently, it was thought that proper concentration balance remarkably affects the stable operation of an sequencing batch reactor (SBR) employed for the EBPR process.
Besides the concentration balance, the type of carbon source is another important factor affecting stable operation of an EBPR reactor. According to Hollender et al. (2002), more attention must be paid to the type of carbon source in the wastewater and its effect on the EBPR due to the heterogeneous organic matter in wastewater which often reaches the treatment plant without complete acid fermentation. Shen and Zhou (2016) reported that the performance of EBPR can be affected by available carbon source types in the wastewater that may induce different functional microbial communities in the process because carbon sources play an important role in the alteration of polyphosphate accumulating-organisms (PAOs) and glycogen-accumulating organisms (GAOs) in the process. It was also reported that the majority of the studies on the EBPR have focused on the metabolism of acetate since short-chain fatty acids (SCFAs) are believed to be the favorable substrates for the EBPR (Zengin et al. 2009), and the fate of different substrates other than acetate need to be further evaluated since domestic wastewater contains no acetate and very low SCFAs (Yağcı et al. 2003). Among these organic substrates, glucose has gained considerable attention due to its potential detrimental effect on the EBPR process. The reason why researchers have selectively focused on the effect of glucose on the EBPR process is that glucose may cause unexpected changes in the dynamics of internally stored compounds such as polyphospate (poly-P), glycogen, and polyhydroxyalkanoate (PHA), which have been reported as essential compounds of the EBPR sludge (Jeon et al. 2001).
Depending on all above explanations, it can be clearly understood that the improper concentration balance in feeding solutions as well as the type of carbon source may lead to instable operation of an EBPR reactor. However, it has never been reported in the literature so far that the concentration balance was taken into consideration as an influencing factor when feeding solutions were prepared in the studies that were aimed at examining the effect of different carbon sources on the EBPR process and carrying out under several feeding conditions. It is believed that effects of the concentration balance should be combined and evaluated with effects of the type of carbon source in EBPR studies for a better understanding of a stable operation of an EBPR reactor. The main objectives of the present study are to (i) determine the effect of the concentration balance on the characteristics of the EBPR sludge and the reactor stability, (ii) determine the effect of the type of carbon source used on the phosphorus and glycogen dynamics of the EBPR system, and (iii) explain the changes in metabolic pathways of the EBPR process.
2 Materials and Methods
2.1 Seeding Sludge, Experimental Setup, and Reactor Operation
The seeding sludge was collected from oxic zone of an activated sludge tank in Lara Advanced Wastewater Treatment Plant (Antalya, Turkey). After the sludge was collected, it was almost immediately transported to the laboratory and homogenized, and then 2.5 L sludge was transferred into the SBR.
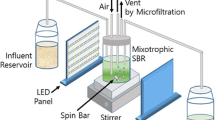
A laboratory-scale SBR (BioFlo 110, New Brunswick Scientific Co., U.S.A.) having 7.5 L of total volume was operated with a working volume of 5.0 L and four 6-h cycles per day under alternating anaerobic-aerobic conditions. A schematic representation of operational components of the SBR is shown in Fig. 1. The cycle time consisted of (i) anaerobic phase (120 min), (ii) aerobic phase (180 min), (iii) settling phase (40 min), and (iv) effluent withdrawal phase (20 min).
Schematic representation of operational components of the SBR (1 primary control unit, 2 pumps, 3 dO2/pH control module, 4 power controller, 5 glass vessel, 6 heat blanket, 7 thermowell, 8 pH sensor, 9 dO2 sensor, 10 agitation motor, 11 rotameter, 12 acid addition, 13 base addition, 14 nitrogen gas stream, 15 electrical supply, 16 vacuum filtration unit, 17 air compressor, 18 air stream, 19 sampling syringe, 20 solution A, 21 solution B, 22 two-sided peristaltic pump, 23 waste sludge withdrawal stream, 24 effluent collection beaker, 25 one-sided peristaltic pump, 26 effluent stream)
In each cycle, 2.5 L of the feeding solution (synthetic wastewater) was fed to the SBR during the first 10 min of the anaerobic phase. After the synthetic wastewater was treated, 2.5 L of effluent was drained from the reactor at the end of the settling period, resulting in a hydraulic retention time of 12 h. Thus, 2.5 L of settled sludge was remained in the resultant suspension. Adjusting of a given value for agitation, temperature, dissolved oxygen (dO2), and pH was done automatically through control modules of the reactor. Agitation was not allowed during the filling period. It was started after the first 10 min of the anaerobic phase and carried out at a constant speed of 150 rpm until the end of the aerobic phase. A two-sided peristaltic pump was used for influent and effluent streams. In order to strip out any residual oxygen from the reactor, high-purity nitrogen gas was sparged at a flow rate of 3 L/min for 3 min right after the filling period and the beginning of the agitation. Aeration was done by fine bubble diffusers at the reactor bottom by an air pump with an on/off controller to maintain the desired dO2 saturation (minimum 35 %) in the reactor, where air was supplied at a constant flow rate of 3 L/min. The dO2 saturation was measured by an oxygen sensor (Hamilton, FDA 325). The reactor was operated at a constant temperature of 22 °C and a pH value of 8.0 ± 0.1 during anaerobic and aerobic phases. To adjust the pH value, addition of 1 N HCl or 1 N NaOH solutions to the reactor was done by a four-pump module of the reactor. The pH value was measured by a pH sensor (Mettler Toledo, 325NBS), and the temperature was measured by a thermowell. Solid retention time (SRT) of 10 days was maintained by withdrawing 125 mL of mixed liquor from the reactor just before the end of the aerobic phase in each cycle, if no solid loss occurred during the withdrawal phase. To not make ineffective control modules of the reactor and the other experimental devices (peristaltic pumps, air pump, programmable logic controllers, magnetic stirrers, etc.) by unexpected power outages, an uninterrupted electrical supply of 3 kVA power was used.
2.2 Feeding Solution
The composition of the feeding solution is shown in Table 1. The solution was fed to the reactor as two separate solutions named solution A (0.4 L) and solution B (2.1 L) at the beginning of the feeding phase. Both of these solutions were prepared as five-cycle stock solutions (solution A 2 L and solution B 10.5 L) as required. In stock solution B, a trace element solution of 4 mL per liter was included. The trace element solution was prepared as 1 L of stock solution and added to stock solution B as required. Peptone and yeast extract solutions were also prepared as ten-cycle stock solutions (500 mL) in one Erlenmeyer flask. After preparation, the mixture was autoclaved at 121 °C for 15 min and added to the reactor with a known volume at the end of the filling phase. Amounts of peptone, yeast extract, nitrification inhibitor, and trace metals in the feeding solutions, of which the concentrations were kept constant during the experimental studies (Table 1), were adjusted as described in Lu et al. (2006). Amounts of the other compounds in Table 1, of which the concentrations were variable due to adjusting of the calculated M/D ratio in the feeding solutions, are described in the next sections.
2.3 Sampling
Samples of 125 mL of the total volume were periodically collected once in every 2 days at the beginning of the cycle startup (25 mL), at the end of the anaerobic phase (50 mL), and at the end of the aerobic phase (50 mL) while the suspension in the reactor was mixing. Sampling time during the cycle where filtrate samples and/or mixed liquor samples were collected is shown in Table 2. The analysis of chemical oxygen demand (COD), ammonium nitrogen (NH4-N), nitrite nitrogen (NO2-N), nitrate nitrogen (NO3-N), and orthophosphate (PO4-P) was done through the filtrate samples, and the analysis of mixed liquor suspended solids (MLSS), mixed liquor volatile suspended solids (MLVSS), SVI, and glycogen was done through the mixed liquor samples. Intracellular total P (TP) analysis was done through both the mixed liquor and filtrate samples.
In order to maintain the targeted SRT, no additional mixed liquor was withdrawn after the sampling period was completed. For SVI analysis, 1 L of the mixed liquor sample was withdrawn just before the end of the aerobic phase in the sampling cycle while the suspension was still mixing. After the settling period for the SVI analysis was completed, the supernatant in the Imhoff cone was drained and the sludge that remained in the cone was transferred into the reactor before the next cycle was started. Hence, potential solid loss resulting from the SVI analysis was prevented.
2.4 Analytical Procedures
Analyses were performed immediately after the samples were collected. MLSS, MLVSS, and SVI were determined according to standard methods (APHA 1999). Glycogen was determined as detailed in Jenkins et al. (2004). For this analysis, a mixed liquor sample of 3 mL with a known MLSS concentration was centrifuged at 4000 rpm for 5 min. After the subsequent procedures that were described in Jenkins et al. (2004) were completed, 0.1 mL of the resultant suspension was added in a glass culture tube and diluted to 1 mL with distilled water. Then, the remaining procedures were carried out. COD concentrations were determined according to the standard methods as described in the Sect. 5220D. The absorbance readings were done at 600 nm for COD concentrations higher than 100 mg/L and at 420 nm for COD concentrations <90 mg/L in a spectrophotometer (WTW, spektroFlex 6100). Heating of culture tubes was carried out in a thermoreactor (WTW, CR 2200) at 150 °C for 2 h. Concentrations of NH4-N, NO2-N, NO3-N, and PO4-P were measured colorimetrically by using spectrophotometer test kits of Merck Millipore (114752 for NH4-N, 114776 for NO2-N, 109713 for NO3-N, and 114848 for PO4-P) and 1-cm light path quartz cuvettes.
Hydrolysis procedure for intracellular TP determination was carried out according to nitric acid-sulfuric acid method (4500-P B.4) as described in the standard methods (APHA 1999). PO4-P concentration in the resultant solution which was obtained after the hydrolysis procedure was determined colorimetrically with the Merck test kit (114848). For the hydrolysis procedure, 2 mL of mixed liquor sample and filtrate sample was individually added into Pyrex tubes. Then, 1 mL concentrated H2SO4 and 5 mL concentrated HNO3 were added into each tube. The mixtures were digested in a digestion rack at 250 °C to a volume of 1 mL and then continued until the solution becomes colorless to remove HNO3. The remaining solutions were cooled and then transferred into an Erlenmeyer flask of 100 mL. Twenty milliliters of distilled water and one drop of phenolphthalein indicator was added into the flasks, and the mixture was titrated with 1 N NaOH solution until producing a faint pink tinge. After the titration, one drop of concentrated H2SO4 was added into the flasks to neutralize the solutions. Then, the solutions were transfferred into a 100-mL volumetric flask and the final volume was adjusted to 100 mL with distilled water. After this procedure, the PO4-P concentration of the solutions was determined colorimetrically in a spectrophotometer (WTW, spektroFlex 6100) by using the Merck test kit (114848). To calculate the PO4-P concentration (TP concentration) in the mixed liquor and filtrate samples, the following equation was used:
where C P is the PO4-P concentration of the digested sample (mg/L); C is the PO4-P concentration of the mixture with 100 mL of the final volume (mg/L); V S is the volume of the digested sample (mL); and V T is the volume of the final solution (mL).
Intracellular TP content of the samples which were collected at the end of anaerobic and aerobic cycles was expressed as percentage content of the known MLSS concentration. To calculate the TP content, the following equation was used:
where TP is the intracellular TP content of the sample collected at the end of the anaerobic or the aerobic cycle; \( {C}_{P_{DML}} \) is the PO4-P concentration of the digested mixed liquor sample (mg/L); \( {C}_{P_{DF}} \) is the PO4-P concentration of the digested filtrate sample (mg/L); and MLSS is the MLSS concentration of the sample collected at the end of the anaerobic or the aerobic cycle (mg/L).
2.5 Experimental Studies
Experimental studies were carried out under two main stages. In the first stage, the SBR was operated under two different M/D ratios (7.6 and 1.5, 30 days of operation for each ratio) in order to determine the effect of the concentration balance on the characteristics of the EBPR sludge and the performance stability of the SBR. During this stage, sodium acetate was used as the sole carbon source. The seeding sludge was changed after the first operation period of 30 days was completed. The concentration (mg/L) profile of the compounds and the concentration of the cations (in meq/L) are shown in Table 3.
In the second stage of the study, the SBR was continuously operated for 120 days under the conditions of the constant M/D ratio determined in the first stage. In the first 60 days of the operation period, sodium acetate of 500 mg COD/L was used as the sole carbon source to favor the proliferation of PAOs in the AS. After this period, the carbon source was abruptly switched from sodium acetate to glucose (500 mg COD/L) to determine the effect of the type of carbon source on the P and glycogen dynamics of the EBPR process. The concentration (mg/L) profile of the compounds and the concentration of the cations (in meq/L) are shown in Table 4. The entire reactor operation was completed by using the initial seeding sludge.
When the stock feeding solutions (solutions A and B) were prepared, the COD/N/P ratio was kept constant at a value of 100/8.3/4 during all experimental studies. Therefore, the concentration of the source of C, N, and P remained constant in all prepared feeding solutions and thus the compounds for the monovalent cations were added into the reactor in the same concentration at the beginning of any cycle startup during all experimental studies.
In order to adjust the concentration of the divalent cations calculated in Tables 3 and 4, two factors were taken into account when the feeding solutions were prepared: (i) the constant COD/N/P ratio and (ii) the absence of Na+ ions in glucose differently from sodium acetate. Since these factors did not allow changing the concentration of each of the monovalent cations, only the concentration of each of the divalent cations was changed in order to adjust the calculated concentrations.
3 Results and Discussion
3.1 The First Stage: Effect of the Concentration Balance on the Performance Stability of the SBR
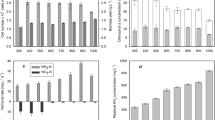
In this stage, performance stability of the SBR was evaluated according to the results of the MLSS and SVI analyses (both of the parameters were determined from the samples that were collected at the end of the aerobic phase. Figure 2 shows the effect of the concentration balance on the performance stability of the SBR. According to the results, SVI increased gradually from an initial value of 125 to 440 mL/g and MLSS decreased gradually from an initial value of 2700 to 1000 mg/L at the end of the operation period when the M/D ratio was 7.6. These results indicated that both of the settling performances of the AS and the MLSS concentrations decreased gradually during 30 days of operation. The rapid decrease in the settling performance of the AS, especially after the 14th day, resulted in gradual decrease in the MLSS concentration since the sludge did not settle properly at the end of the settling period. The decrease in the MLSS concentration was attributed to the loss of the biomass that still remained unsettled in the effluent fraction of the bulk solution when effluent was withdrawn. Therefore, MLSS concentration decreased proportionally since the sludge could not be withdrawn properly. That means the SBR could not be operated under the targeted SRT value since the sludge of the desired volume could not be withdrawn from the SBR. Due to these unwanted results, operation of the SBR was stopped.
When the SBR was operated under the condition of the M/D ratio of 1.5, better results were obtained for SVI and MLSS parameters. As seen on Fig. 2, the SVI value decreased from an initial of 130 to 70 mL/g and the MLSS concentration varied in the range of 2950–2750 mg/L during this stage. These results indicated that the settling performance of the AS improved and the MLSS parameter was not influenced adversely. As a comparison of the obtained results, it can be concluded that performance of the SBR decreases when the M/D ratio in the feeding solution exceeds a certain value and performance stability of the SBR deteriorates if the unwanted cases become severe.
3.2 The Second Stage: Effect of the Type of Carbon Source on the EBPR Process
3.2.1 AS Characteristics
The combined effect of the concentration balance and the type of carbon source on the sludge characteristics was determined according to the MLSS, MLVSS, and SVI values that were measured at the end of aerobic periods and the ratio of MLVSS to MLSS (MLVSS/MLSS) parameters. As seen on Fig. 3, the SVI decreased from an initial value of 152 to 36 mL/g for the sodium acetate-fed reactor at the end of 60 days of operation. When glucose was used as the sole carbon source, the SVI did not change significantly and the values varied between 35 and 43 mL/g during the subsequent operation of 60 days. During the entire operation period, the SVI decreased gradually in the first 40 days and then became stable. These results imply that the settling performance of the AS was not influenced adversely by the type of the carbon source during the entire reactor operation. Similar results were also obtained by some researchers investigating the sludge granulation in an EBPR system (Dülekgürgen et al. 2003; Wu et al. 2010; Ong et al. 2012). In these granule-based EBPR studies, the low SVI values obtained were attributed to an excellent sludge settling performance. As a result of the good settling performance achieved in the present study, the reactor was operated robustly without any solid loss during the withdrawal phases, resulting in a negligible change in the MLSS concentration. The minimum and maximum MLSS concentrations were found to be 2423 and 2560 mg/L for the sodium acetate-fed reactor, respectively, and 2204 and 2483 mg/L for the glucose-fed reactor, respectively. Zengin et al. (2009) showed that the MLSS gradually decreased from an initial concentration of 3000–4000 to 2000 mg/L after 29 days of operation with a remarkable deterioration in EBPR activity. A sludge bulking problem occurred after day 35, and the reactor operation was ended at day 54 since the SBR operation could not be recovered. In the mentioned study, the relation between the carbon source (glucose) and the bulking problem was attributed to the fermentation products that were detected in significant amounts at the end of the latter phase of the reactor operation. Results of the microscopic examinations showed the presence of some filamentous organisms that could be the cause of the bulking problem due to the probable stimulation of these organisms by the fermentation products. In the present study, such problems were not observed when sodium acetate or glucose was used as the sole carbon source in the EBPR system.
Jenkins et al. (2004) reported that AS flocs are made up of biological (bacteria, fungi, protozoa, and metazoa) and nonbiological materials (organic and inorganic particulates), and AS suspended solids contain from 60 to 90 % volatile materials in the absence of chemical addition. Hence, the MLVSS/MLSS ratio may be a good indicator to estimate the organic material content of the sludge. The results for the ratio of the concentration of MLVSS to MLSS were found to be 79 % for the sodium acetate-fed reactor and 77 % for the glucose-fed reactor. Similar result was also obtained by Zengin et al. (2009). The results of the MLVSS/MLSS ratio obtained in the present study indicate that the AS flocs were typical in terms of the organic material content during the entire operation period.
The results obtained for the AS characteristics clearly show that taking the proper concentration balance into account had a great importance on the AS characteristics in order to achieve a stable reactor operation without any solid-liquid separation problem when the reactor was fed with different carbon sources.
3.2.2 NH4-N, NO2-N, and NO3-N Profiles
NH4-N, NO2-N, and NO3-N measurements were performed regularly during the entire reactor operation for both of the carbon sources. NH4-N concentrations were measured to determine whether N or P was chemically removed via precipitation due to the possible interaction between PO4-P, NH4, and Mg2+ ions. NO2-N and NO3-N concentrations were measured to determine whether nitrification occurred in the reactor. The results of NO2-N and NO3-N measurements were lower than the minimum detectable concentration limit for these parameters (data not shown). These results suggest that nitrification did not occur in the EBPR system when sodium acetate or glucose was used as the sole carbon source. The results obtained from NH4-N measurements showed that the initial NH4-N concentration at the cycle startup did not change significantly during both of the anaerobic and aerobic phases in both EBPR systems (data not shown). This finding implies that Mg2+ ions were included in the feeding solution with a sufficient concentration resulting in no removal of NH4-N or PO4-P by chemical precipitation. This means that the amount of Mg2+ ions included in the composition of both feeding solutions (Table 4) did not exceed the stoichiometric amount required for the chemical precipitation. Based on the results, it is suggested that changes in the NH4-N concentration profile as well as in the NO2-N and NO3-N concentration profiles may be monitored as control parameters during the EBPR process.
3.2.3 COD Removal and P Dynamics
The effect of the type of carbon source on the COD concentration profile and the COD removal efficiency during anaerobic and aerobic phases is shown in Table 5 and Fig. 4. In the case of the sodium acetate usage, a great portion of the COD concentration at the cycle startup decreased significantly during the anaerobic phase, and the concentration continued to decrease a little bit more in the subsequent aerobic phase (Table 5). A similar trend was also observed when glucose was used as the sole carbon source. The result for the COD removal efficiency showed that the COD removal during the anaerobic phase was nearly 45 % at the beginning of the reactor operation and the removal gradually increased up to 85 % in the first 34 days of the 60 days of the reactor operation with sodium acetate and then the removal remained almost stable (Fig. 4). The removal in the aerobic phase varied between 27 and 58 % in the first 34 days and then varied between 14 and 28 % after the anaerobic removal became stable. In the case of the glucose usage, the COD removal was almost stable during the anaerobic phase with varying values in the range of 76–84 %, and the removal during the aerobic phase varied between 19 and 35 % during this operation period. By considering that nitrification did not occur in the EBPR process and depending on the obtained results for the COD, it can be suggested that a great portion of both of the carbon sources could be utilized by fermentative bacteria during the anaerobic phase. This implies that both of the carbon sources were sustainably converted to volatile fatty acids during the anaerobic phase in order to help PAOs to survive and/or proliferate under anaerobic conditions.
Effect of the type of carbon source on the COD removal efficiency during anaerobic and aerobic phases. (SA(AN) and SA(AE): removal efficiency with sodium acetate during anaerobic and aerobic phases, respectively. G(AN) and G(AE): removal efficiency with glucose during anaerobic and aerobic phases, respectively)
The effect of the type of carbon source on the PO4-P concentration profile, the PO4-P release efficiency during anaerobic phases, and the PO4-P removal efficiency during aerobic phases is shown in Table 5 and Fig. 5. According to the results, switching of the carbon source from sodium acetate to glucose caused different trends on the PO4-P dynamics between anaerobic and aerobic phases. The main difference was that sodium acetate usage resulted in an enhancement in both the anaerobic PO4-P release and the aerobic PO4-P removal, whereas glucose usage resulted in a reduction in both of these parameters (Fig. 5). In the case of the feeding with sodium acetate, the released PO4-P during the anaerobic phase was removed completely during the subsequent aerobic phases (Table 5). In addition, the PO4-P release increased gradually from an initial value of 37 to 355 % during the first 38 days of the 60 days of the operation period and then it almost became stable after that time (Fig. 5). This means that the PO4-P release efficiency increased by 9.5-fold in the first 38 days. Also, the PO4-P removal gradually and consistently increased from 52 to 93.2 % during the entire operation period (Fig. 5). In this period, the maximum PO4-P concentration at the end of the anaerobic phase was measured as 101.3 mg/L and the minimum PO4-P concentration at the end of the aerobic phase was measured as 4.6 mg/L. All these results related to the PO4-P dynamics clearly indicate that sodium acetate improved the PO4-P release and removal. This means that PAOs released the intracellular P to the bulk solution in the form of PO4-P and, thus, they efficiently depleted their poly-P pool in the anaerobic phase and efficiently took up most of PO4-P into the poly-P pool in the subsequent aerobic phase to replenish the poly-P pool. However, when glucose was used as the sole carbon source, a deterioration was observed in the PO4-P dynamics. According to the results, both the anaerobic PO4-P release efficiency and the aerobic PO4-P removal efficiency decreased gradually and consistently during the entire operation period of 60 days. The decrease was from 346 to 62 % for the anaerobic PO4-P release and from 92 to 65 % for the aerobic PO4-P removal. At day 120, the release was 0.62-fold more at the end of the anaerobic phase compared to the cycle startup. Combining these results with the results of the COD removal (Fig. 4), it can be concluded that glucose was utilized as the carbon source by a group of microorganisms, but, unlike sodium acetate, it caused some changes in metabolic activities of PAOs in terms of the utilization of P. Similar results were also obtained by some researchers (Cech and Hartman 1990; Cech et al. 1993; Akın and Uğurlu 2001; Hollender et al. 2002; Zengin et al. 2009; Gebremariam et al. 2012).
In the present study, the increase in the P release and removal activity in the case of sodium acetate feeding and the decrease in the same dynamics in the case of glucose feeding led the researchers to think that glucose as the sole carbon source was absolutely detrimental for the EBPR process, where PAOs became the dominant species in the AS under the condition of the sodium acetate feeding, since it caused a change in metabolic pathways for P utilization.
3.2.4 Ratio of Prel/CODrem and Prel/Pup
Barat et al. (2008) suggested that the ratio of the amount of P released during anaerobic phases to the amount of acetate taken up by microorganisms (Prel/ACup) is a typical parameter for an EBPR system, and this parameter is a good metabolic indicator included in the acetate uptake under anaerobic conditions. In the present study, this indicator was linked with the ratio of the amount of the released P (Prel) to the amount of decrease in the COD concentration (CODrem) during anaerobic phases (Fig. 6). The results showed that the ratio gradually increased from an initial value of 0.159 to 0.315 until the 34th day and then it gradually decreased up to a value of 0.218 until switching of the carbon source from sodium acetate to glucose. After switching of the carbon source, the ratio continued to decrease from 0.208 to 0.060 during the remaining operation period. It has been reported according to the literature review (Barat et al. 2008) that the ratio of Prel/ACup varies between the range of 0.03 and 0.8 g P per gram of COD, and this ratio tends to favor the growth of PAOs or GAOs, meaning that any variation in this ratio results in a change in the metabolic pathways of these microorganisms for the utilization of P and/or carbon sources. As a comparison, the results for the ratio of Prel/CODrem obtained in the current study were in good agreement with that reported by Barat et al. (2008).
The occurrence of P release to the bulk solution during the anaerobic phase as well as the removal of COD until the 34th day (Figs. 4 and 5) and the increase in the Prel/CODrem ratio by 2-fold in the 34th day (Fig. 6) revealed that the P release occurred much more than the COD removal. This was evident that the metabolic pathways of PAOs proceeded as expected under the condition of sodium acetate feeding due to the occurrence of the regular release and removal of P simultaneously with the utilization of the carbon source, implying that the fraction of PAOs increased gradually in the EBPR sludge. The reason for the decrease in value after the 34th day, especially in the case of the glucose feeding, was the gradual decrease of the P release during the anaerobic phase while the COD removal remained almost stable (Fig. 4). This result supports that PAOs were not able to deplete their poly-P pools completely although glucose was efficiently utilized as the carbon source during the anaerobic phase. Thus, the poly-P pools, where a replenishment was expected by taking up all the released P during the aerobic phase, were only replenished with a limited P uptake. As a result, this limited P uptake caused a gradual decrease in the PO4-P removal efficiency during the aerobic phase (Fig. 5). These findings further suggest that metabolic activities of PAOs changed even after glucose was used as the sole carbon source.
The effect of the type of carbon source on the ratio of the amount of P release (Prel) to the amount of P uptake (Pup) is shown in Fig. 6. In the case of the sodium acetate feeding, the ratio increased rapidly in the first 10 days and then became almost stable during the next 24 days after a slight increase. The reason for the rapid increase in the first 10 days was that P release in the anaerobic phase occurred much more than P removal in the aerobic phase during this period (Table 5). In the remaining operation period with sodium acetate, the ratio did not change significantly because both the P release and removal changed with a similar trend. The maximum value was found to be 0.864 for the sodium acetate-fed system at day 38, meaning that P release occurred lesser than P removal after the EBPR system became stable. However, when glucose was used as the sole carbon source, the ratio gradually decreased from an initial value of 0.839 to 0.592 during the entire operation period, implying that the glucose feeding resulted in an apparent decrease in both P release and removal (Table 5). These results clearly suggest that sodium acetate improved the metabolic behaviors of the PAOs, whereas glucose played a detrimental role for the EBPR system.
3.2.5 Intracellular TP Content
The effect of the type of carbon source on the intracellular TP content (as percent of MLSS content) is shown in Fig. 7. According to the results, the initial intracellular TP content of the AS (2.37 %) increased during the entire operation period under the condition of the sodium acetate feeding. In this period, the maximum TP content was obtained as 5.81 and 8.22 % for the anaerobic and aerobic phases, respectively. However, when glucose was used as the sole carbon source, the intracellular TP content decreased to 3.97 and 5.33 % for the anaerobic and aerobic phases, respectively, at the end of the operation period. Gerardi (2006) reported that P content of an AS typically varies between 1 and 3 %, and this value increases above 6–7 % when the EBPR process is used. The result obtained in the current study revealed that performance of the EBPR system was in good agreement with the literature results under the sodium acetate feeding condition. On the other hand, use of glucose reduced the system performance because the TP content of the AS started to decrease after glucose was introduced to the system. The reason for the decrease was thought to be the regular sludge wasting at the end of each cycle, causing a continuous loss in TP content in the biomass. If PAOs had taken up PO4-P from the liquid phase with a higher efficiency in aerobic phases under the glucose feeding condition, then the poly-P pool would have been replenished and depleted regularly in a consecutive aerobic-anaerobic cycle. In such a case, where the metabolic pathways for P utilization could not be managed regularly by PAOs, wasting of the sludge might result in the continuous loss in the intracellular TP. Therefore, the TP content could not be recovered during the entire operation period under the glucose feeding conditions.
The results obtained for the TP content in the present study were in agreement with the literature results (Cech and Hartman 1990; Zengin et al. 2009) due to the increase of the intracellular TP content under the sodium acetate feeding condition and the decrease under the glucose feeding condition. The two main results, (i) the decrease in the P release and removal (Fig. 5) and (ii) the decrease in the intracellular TP content (Fig. 7), clearly showed that the metabolic pathways of PAOs for the P utilization were not inhibited completely under the glucose feeding condition but the P utilization was proceeded with a decreasing affinity although the substrate was consumed efficiently. This finding suggests that glucose as the sole carbon source causes a decrease in dependency of the P utilization of PAOs.
3.2.6 Glycogen Dynamics
It has been reported that external glucose can replace intracellular glycogen and serves as a reducing power and energy source for the anaerobic PHA formation, implying that the dependency on the poly-P may be reduced (Mino et al. 1998; Akın and Uğurlu 2001). Satoh et al. (1994) postulated that the “non-poly-P PHA accumulation system” may be preferred if the influent contains glucose. As a result of the study, Akın and Uğurlu (2001) showed that “non-poly-P bacteria” used glucose for energy uptake, but not poly-P for substrate uptake. Based on these explanations, it can be concluded that polyphosphate-accumulating mechanism (PAM) may be preferred by PAOs when sodium acetate is used as the sole carbon source since the reducing power and the energy are primarily supplied from the degradation of poly-P and glycogen for the anaerobic substrate uptake and PHA formation. However, when glucose is used as the sole carbon source, the reducing power and the energy are primarily and substantially supplied from the glycogen degradation for substrate consumption and PHA formation under anaerobic conditions. In this case, the glycogen-accumulating mechanism (GAM) may be preferred by PAOs.
Zhou et al. (2008) reported that glycogen utilization and its regeneration is an integrated part of the PAO metabolism and that it has been hypothesized by Schuler and Jenkins (2003) that the GAM was most likely the dominant process for energy production by PAOs under P-limiting conditions. To clarify this hypothesis, Zhou et al. (2008) made an experimental attempt to employ a metabolism of a highly enriched PAO culture in the absence or low presence of intracellular poly-P by carrying out a series of batch experiments where sodium acetate was used as the sole carbon source. Based on the results obtained from the chemical and FISH analysis and the biomass staining, the researchers showed that the anaerobic reaction stoichiometry obtained for the highly enriched PAO culture under poly-P limitation was very close to that of GAOs, and therefore, PAOs can behave as GAOs under P-limiting conditions. This hypothesis was also supported by Gebremariam et al. (2012). Based on the results of their study, especially the results obtained for the glycogen dynamics, the researchers showed that PAOs can switch their metabolism to that of GAOs when a glucose-dominated feeding solution was used. They also explained that the failure observed in the EBPR system was due to the shifting of the metabolism of PAOs from PAM to GAM rather than a shift in microbial composition.
In the current study, if GAM was the dominant metabolic pathway in the system where glucose was used as the sole carbon source, then it was expected that consumption of glycogen in anaerobic phases would be greater and P release and uptake would be less than what occurred in the system, where sodium acetate was used as the sole carbon source. Table 6 shows the effect of the type of carbon source on the glycogen dynamics. The experimental results showed that the glycogen content (as percentage of dry matter, MLSS) gradually increased from nearly 9 to 30 % during the entire operation period under the sodium acetate-feeding condition. In this period, the glycogen content at the end of the aerobic phase was measured a little bit higher than that of the anaerobic phase. Values of Δ%, describing the difference between the glycogen contents in the aerobic and anaerobic phases, revealed that glycogen that was synthesized during any aerobic phase was not consumed in excess amount during the subsequent anaerobic phase. The mean value was calculated as 19.04 % for the anaerobic phase and 24.67 % for the aerobic phase under the sodium acetate feeding condition. When glucose was used as the sole carbon source, the glycogen dynamics showed a different trend. According to the results, the mean value was calculated as 19.27 % for the anaerobic phase and 44.46 % for the aerobic phase. This result showed that glycogen was synthesized and stored much more than sodium acetate was in the aerobic phase. The result of the Δ% showed that the synthesized glycogen was consumed more when compared with sodium acetate. Also, the calculated mean value for the Δ% was obtained as 5.63 % for the sodium acetate feeding condition and 25.39 % for the glucose feeding condition. This result indicates that glycogen was consumed nearly 5-fold higher after the carbon source was switched from sodium acetate to glucose.
Similar results were also reported by some researchers. Gebremariam et al. (2012), who examined the effect of the increase in glucose amount in the feeding solution on the EBPR system, found out that glycogen dynamics varied substantially compared to the control reactor fed with 100 % sodium acetate depending on the glucose fraction in the feeding solution of the test reactor. In their study, it was shown that the sludge in the test reactor showed increased production and pronounced cycling of glycogen between the anaerobic and aerobic stages. Also, simultaneous breakdown and synthesis of glycogen occurred in the test reactor under anaerobic conditions as the glucose ratio increased. However, glycogen was mainly synthesized during the aerobic stage in the control reactor. Zengin et al. (2009) showed that a low level of glycogen consumption was observed under anaerobic conditions although there was a rapid conversion of external glucose into glycogen during the good EBPR period, indicating that PAOs were the dominant microorganisms in the system. In the deteriorated EBPR period, a significant increase in glycogen consumption was observed as well as a decrease in the TP content of the sludge and the phosphate release/carbon uptake ratio. Based on their results, they suggested that the microbial dynamics changed from PAOs to GAOs after the EBPR system was deteriorated and the GAOs were responsible for the system deterioration. Hollender et al. (2002) also showed that glycogen was degraded under anaerobic conditions and accumulated under aerobic conditions. However, differently from the results of the current study, they reported that the highest glycogen concentration and the highest difference between the anaerobic and aerobic phases were reached with sodium acetate and only a low level of glycogen was stored when glucose was used as the sole carbon source. They also reported that their results contradicted with the results of the other investigations (Cech et al. 1993; Satoh et al. 1992), which postulated the storage of glycogen instead of poly-P when glucose was used in the feeding solution, causing a deterioration in the EBPR system.
In the present study, the significant increases obtained for P release with simultaneous anaerobic substrate uptake (Table 5 and Figs. 4 and 5), Prel/CODrem ratio (Fig. 6), and intracellular TP content (Fig. 7) as well as the low glycogen consumption (Table 6) during the system operation with sodium acetate clearly suggest that the dominant group of microorganisms in the EBPR sludge was PAOs and PAM took place during the system operation. On the other hand, the decreases obtained for P release (Table 5 and Fig. 5), Prel/CODrem ratio (Fig. 6), and intracellular TP content (Fig. 7) as well as the higher glycogen consumption (Table 6) during the system operation with glucose showed that the metabolism of the resident PAOs in the sludge shifted from PAM to GAM.
4 Conclusions
Experimental results showed that phosphorus release (Prel) and uptake (Pup) occurred at significant levels at the first 60 days of operation period in the SBR. However, the release and uptake deteriorated gradually during the following operation period of 60 days after glucose was introduced in the feeding solution. The major difference obtained during the entire reactor operation was that glycogen regeneration during aerobic phases and its utilization during anaerobic phases increased substantially in the glucose-fed SBR, implying that PAOs obtained the main energy by using glycogen. These results reveal that PAOs shifted their metabolic pathways from PAM to GAM after the carbon source was switched from sodium acetate to glucose. The results obtained for the SVI showed that it decreased gradually and then remained almost constant until the SBR operation was ended. This result suggests that the concentration balance calculated for a given M/D ratio and the amount of each cation included in feeding solutions should necessarily be taken into consideration to achieve a robust reactor operation during the experimental studies which will be carried out under several feeding conditions. According to the results of the NH4-N measurement, it can be concluded that this parameter can be used as a monitoring strategy to understand whether a chemical removal by precipitation occurs in the EBPR process. To clarify whether there will be a change in the microbial composition of an EBPR system dominated by PAOs when glucose or any other substrate is introduced into the feeding solution, resulting in a deterioration of the EBPR system, further studies that can be supported by results of microscopic examinations, FISH analysis, or any other biochemical parameters should be carried out.
References
Ahmed, F. (2007). Effects of cation addition on the flocculation behaviour of activated sludge at applied constant shear force. Göteborg, Sweden, Chalmers University of Technology, Deparment of Civil and Environmental Engineering, Water Environment Technology, M.Sc. Thesis
Akın, B. S., & Uğurlu, A. (2001). Enhanced phosphorus removal by glucose fed sequencing batch reactor. Journal of Environmental Science and Health, Part A, 36(9), 1757–1766.
Barat, R., Montoya, T., Borras, L., Ferrer, J., Seco, A., et al. (2008). Interactions between calcium precipitation and the polyphosphate-accumulating bacteria metabolism. Water Research, 42, 3415–3424.
Cech, J. S., & Hartman, P. (1990). Glucose induced breakdown of enhanced biological phosphate removal. Environmental Technology, 11(7), 651–656.
Cech, J. S., Hartman, P., Wanner, J., et al. (1993). Competition between poly-P and non-poly-P bacteria in an enhanced phosphate removal system. Water Environment Research, 65(5), 690–692.
Datta, T. (2010). Evaluation of feasibility, sustenance and microbial ecology of enhanced biological phosphorus removal in aerated-anoxic activated sludge systems. Utah, U.S.A., The University of Utah, Department of Civil and Environmental Engineering, Ph.D. Thesis.
Dülekgürgen, E., Ovez, S., Artan, N., Orhon, D., et al. (2003). Enhanced biological phosphate removal by granular sludge in a sequencing batch reactor. Biotechnology Letters, 25, 687–693.
Fang, F., Liu, X. W., Xu, J., Yu, H. Q., Li, Y. M., et al. (2009). Formation of aerobic granules and their PHB production at various substrate and ammonium concentrations. Bioresource Technology, 100, 59–63.
Gebremariam, S. Y., Beutel, M. W., Christian, D., Hess, T. F., et al. (2012). Effect of glucose on the performance of enhanced biological phosphorus removal activated sludge enriched with acetate. Bioresource Technology, 121, 19–24.
Gerardi, M. H. (2006). Wastewater bacteria. Williamsport, Pennysylvania: Wiley.
Higgins, M. J., & Novak, J. T. (1997). The effect of cations on the settling and dewatering of activated sludge: laboratory results. Water Environment Research, 69(2), 215–224.
Hollender, J., Van der Krol, D., Kornberger, L., Gierden, E., Dott, W., et al. (2002). Effect of different carbon sources on the enhanced biological phosphorus removal in a sequencing batch reactor. World Journal of Microbiology and Biotechnology, 18, 355–360.
Jaffer, Y., Clark, T. A., Pearce, P., Parsons, S. A., et al. (2002). Potential phosphorus recovery by struvite formation. Water Research, 36, 1834–1842.
Jenkins, D., Richard, M.G., Daigger, M.T., et al. (2004). Manual on the causes and control of activated sludge bulking, foaming, and other solid separation problems. Florida, U.S.A., 3rd ed. CRC Press, Taylor and Francis Group.
Jeon, C. O., Lee, D. S., Lee, M. W., Park, J. M., et al. (2001). Enhanced biological phosphorus removal in an anaerobic–aerobic sequencing batch reactor: effect of pH. Water Environment Research, 73(3), 301–306.
Kara, F., Gurakan, G. C., Sanin, F. D., et al. (2008). Monovalent cations and their influence on activated sludge floc structure. Biotechnology and Bioengineering, 100(2), 231–239.
Li, H., Wen, Y., Cao, A., Huang, J., Zhou, Q., et al. (2014). The influence of multivalent cations on the flocculation of activated sludge with different sludge retention times. Water Research, 55, 225–232.
Lu, H., Oehmen, A., Virdis, B., Keller, J., Yuan, Z., et al. (2006). Obtaining highly enriched cultures of Candidatus Accumulibacter phosphates through alternating carbon sources. Water Research, 40, 3838–3848.
Mamais, D., Pitt, P. A., Cheng, Y. W., Loiacono, J., Jenkins, D., et al. (1994). Determination of ferric chloride dose to control struvite precipitation in anaerobic sludge digester. Water Environment Research, 66(7), 912–918.
Mino, T. (2000). Microbial selection of polyphosphate-accumulating bacteria in activated sludge wastewater treatment processes for enhanced biological phosphate removal. Biochemistry-Moscow, 65(3), 341–348.
Mino, T., Van Loosdrecht, M. C. M., Heijnen, J. J., et al. (1998). Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Research, 32, 3193–3207.
Musvoto, E. V., Wentzel, M. C., Ekama, G. A., et al. (2000). Integrated chemical–physical processes modelling-II. Simulating aeration treatment of anaerobic digester supernatants. Water Research, 34(6), 1857–1867.
Nguyen, T. P., Hilal, N., Hankins, N. P., Novak, J. T., et al. (2008). The relationship between cation ions and polysaccharide on the floc formation of synthetic and activated sludge. Desalination, 227, 94–102.
Oehmen, A., Zeng, R. J., Yuan, Z., Keller, J., et al. (2005). Anaerobic metabolism of propionate by polyphosphate-accumulating organisms in enhanced biological phosphorus removal systems. Biotechnology and Bioengineering, 91(1), 43–53.
Oehmen, A., Lemos, P. C., Carvalho, G., Yuan, Z., Keller, J., Blackall, L. L., Reis, M. A. M., et al. (2007). Advances in enhanced biological phosphorus removal: from micro to macro scale. Water Research, 41, 2271–2300.
Ong, Y. H., Chua, A. S. M., Lee, B. P., Ngoh, G. C., Hashim, M. A., et al. (2012). An observation on sludge granulation in an enhanced biological phosphorus removal process. Water Environment Research, 84(1), 3–8.
Park, C. (2002). Cations and activated sludge floc structure. Virginia, U.S.A.,Virginia Polytechnic Institute and State University, Department of Environmental Engineering, M.Sc. Thesis
APHA (American Public Health Association) (1999). Standard methods for the examination of water and wastewater. Washington D.C., 20th ed.
Satoh, H., Mino, T., Matsuo, T., et al. (1992). Uptake of organic substrates and accumulation of polyhydroxyalkanoates linked with glycolysis of intracellular carbohydrates under anaerobic conditions in the biological excess phosphate removal process. Water Science and Technology, 26, 933–942.
Satoh, H., Mino, T., Matsuo, T., et al. (1994). Deterioration of enhanced biological phosphorus removal by the domination of microorganisms without polyphosphate accumulation. Water Science and Technology, 30, 203–211.
Schuler, A. J., & Jenkins, D. (2003). Enhanced biological phosphorus removal from wastewater by biomass with different phosphorus content, part I: experimental results and comparison with metabolic models. Water Environment Research, 75(6), 485–498.
Shen, N., & Zhou, Y. (2016). Enhanced biological phosphorus removal with different carbon sources. Applied Microbiology and Biotechnology, 100, 4735–4745.
Tunçal, T., Pala, A., Uslu, O., et al. (2008). Investigation of enhanced biological phosphorus removal characteristics in İzmir Wastewater Treatment Plant. İstanbul Teknik Üniversitesi Dergisi E: Su Kirlenmesi Kontrolü, 18(1), 17–31 (in Turkish).
Wu, C. Y., Peng, Y. Z., Wang, S. Y., Ma, Y., et al. (2010). Enhanced biological phosphorus removal by granular sludge: from macro to micro scale. Water Research, 44, 807–814.
Yağcı, N. O., Taşlı, R., Artan, N., Orhon, D., et al. (2003). The effect of nitrate and different substrates on enhanced biological phosphorus removal in sequencing batch reactors. Journal of Environmental Science and Health, Part A, 38(8), 1489–97.
Zengin, G. E., Artan, N., Mino, T., et al. (2009). The effect of glucose on the enhanced biological phosphorus removal in a sequencing batch reactor. İstanbul Teknik Üniversitesi Dergisi E: Su Kirlenmesi Kontrolü, 19(1–2), 63–73 (in Turkish).
Zhou, Y., Pijuan, M., Zeng, R. J., Lu, H., Yuan, Z., et al. (2008). Could polyphosphate-accumulating organisms (PAOs) be glycogen-accumulating organisms (GAOs)? Water Research, 42, 2361–2368.
Acknowledgments
This study was funded by the Scientific Research Project Commission of Süleyman Demirel University, Isparta, Turkey (project no. 2202-D-10). The financial assistance of the study is thankfully acknowledged by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yazıcı, H., Kılıç, M. Effect of the Concentration Balance in Feeding Solutions on EBPR Performance of a Sequencing Batch Reactor Fed with Sodium Acetate or Glucose. Water Air Soil Pollut 227, 389 (2016). https://doi.org/10.1007/s11270-016-3080-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3080-z