Abstract
The partial denitrification (PD) is a very promising process developed in the last decade, to study the comprehensive influence of influent carbon to nitrogen (C/N) on the activated sludge system under PD, six sequencing batch reactors (SBRs) were operated in parallel at C/N of 2.75, 3.30, 4.13, 5.50, 8.25 and 16.50, the nitrogen removal, phosphorus removal and sludge settleability of PD were investigated. The results showed that PD was observed treating synthetic wastewater in all the six SBRs, and the nitrite accumulation rate (NAR) was highest at C/N of 5.50 (NAR of 82.30%). However, due to the alternate inhibition of NO2−-N and free nitrous acid (FNA) produced by a limited carbon source, both the sludge settleability and phosphorus removal deteriorated. The average SVI at C/N of 8.25 was 130% lower than C/N of 3.30, and the average amount of PO43−-P released at C/N of 16.5 was 189% higher than C/N of 2.75. Kinetic analysis showed that the denitrification kinetics of PD and complete denitrification were similar, and the nitrite accumulation was caused by the difference between nitrate reduction rate and nitrite reduction rate. Variations of on-line parameters (pH and ORP) revealed that nitrite accumulation could be indicated by judging the nitrate turning point and nitrite turning point on pH and ORP curves, which provided guidance for the setup of PD.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The frequent eutrophication occurred by the excessive discharge of nitrogen and phosphorus is among the most urgent environmental issues in the world. At present, the activated sludge process is widely used in urban wastewater treatment, but the nutrients removal performance is usually limited by the low influent C/N [1]. According to the traditional theory, the complete denitrification process consists of “NO3−-N → NO2−-N → NO → N2O → N2”. Sometimes due to the carbon source deficiency, short hydraulic retention time, nitrite was accumulated during the denitrification process, which is termed as partial denitrification (PD) [2]. Anaerobic ammonium oxidation (ANAMMOX) is considered to be the most promising nitrogen removal process in the future. In the past NO2−-N required by ANAMMOX was usually provided by partial nitrification, with the rapid development of the PD process, the PD process was supposed to be a more feasible approach to provide NO2−-N for ANAMMOX [3]. Compared with complete denitrification, the application of PD-ANAMMOX technology to urban wastewater treatment plants can save about 80% of the carbon source and 50% of the aeration energy consumption [4].
In recent years, the rapid start of PD has been achieved in many types of reactors, such as AO, SBR [5, 6]. Cao et al. [7] proved that the NO2−-N accumulation rate could be controlled above 80% during the PD process. Du et al. [8] used PD coupled with the ANAMMOX process to treat wastewater, and stably operated the system for a long-term. The PD process could be affected by many factors, such as pH, hydraulic retention time, and so on [9]. Under high pH conditions, nitrite reductase is more sensitive under high pH, and its inhibitory effect is greater than that of nitrate reductase [10]. Shi et al. found that the PD system could be successfully activated by adjusting the pH to 9.0, and the NAR would be remained around 88% after the pH adjustment was stopped [11]. Jiang et al. [12] found that the accumulation of nitrite under PD was obvious when the hydraulic retention time was 18 h.
The availability of electron donor, expressed as C/N, is another key factor to determine PD performance [13]. However, the current research about PD is mostly focused on the generation mechanism and operation control at low C/N. Cao et al. [14] used domestic wastewater as carbon source to establish a PD system with a 90% NAR at C/N of 1.7. Krishna et al. [15] investigated the effect of C/N on PD, and a positive correlation between influent C/N and NO2−-N accumulation rate was observed when the C/N was in the range of 1.5–3. The C/N of urban wastewater in China fluctuated greatly [16]. Therefore, it is necessary to explore the effect of high C/N on the stability of PD. Ge et al. [17] studied the effect of carbon source and C/N (1–25) on denitrification using methanol, glucose and acetate as carbon sources. High C/N resulted in the growth of heterotrophic bacteria, which is widely investigated in denitrification [18]. In fact, most of the research on high C/N focused on denitrification, while the actual wastewater treatment plant is not only concerned with nitrogen removal but also cared about phosphorus removal and sludge settleability. Above all, it is imperative to explore the removal characteristics of each nutrient in the PD at different C/N, which helps to establish a stable and efficient PD process.
Therefore, this study initiated the PD process by adjusting the influent nitrate concentration to prepare a series of C/N. From the sludge structure, denitrification kinetics, the effects of influent C/N on nitrogen removal, phosphorus removal and filamentous bacteria inhibition under PD condition were discussed. Finally, combined with parameters such as ORP and pH, it is hoped to provide theoretical reference and technical guidance to control the PD process.
Materials and methods
Wastewater and inoculated sludge
The synthetic wastewater with the following composition was used: 663.80 mg·L−1 of CH3COONa·3H2O, 166.90 mg·L−1 of NH4Cl, 18.80 mg·L−1 of KH2PO4, 375 mg·L−1 of NaHCO3, 40 mg·L−1 of CaCl2·2H2O, 80 mg·L−1 of MgSO4, and 0.30 ml·L−1 of micro-element solution [19]. The chemicals were analytical purity supplied by Kermel (99.9%). The synthetic wastewater quality was around 330 mg·L−1 of COD, 45 mg·L−1 of NH4+-N and 4.30 mg·L−1 of PO43−-P. The inoculated sludge was taken from the anoxic tank of an anaerobic/anoxic/oxic (AAO) WWTP (Zhengzhou, Henan province, China) which showed well nitrogen and phosphorus removal performances (both removal efficiencies were bigger than 85%). Its settleability was poor due to the overgrowth of filamentous bacteria. The sludge volume index (SVI) and mixed liquid suspended solids (MLSS) were 360 ml·g−1 and 2000 mg·L−1, respectively. The inoculated sludge was washed by pure water three times. Each time the pure water and the inoculated sludge were thoroughly mixed for 1 min. After settling for 30 min, the supernatant was discharged.
Experimental operation
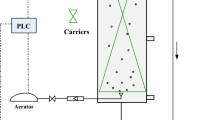
The study was carried out in 6 SBRs. Each SBR was made up of lucite with 12 L working volume, 200 mm diameter and 700 mm working height (Fig. 1). The influent COD was all controlled at 330 mg·L−1. The concentration of NO3−-N were 120, 100, 80, 60, 40, 20 mg·L−1, respectively (C/N are 2.75, 3.30, 4.13, 5.50, 8.25 and 16.50, respectively). The temperature was maintained at 21 ± 1˚C. Each cycle consisted of 5 min feeding (3L), 30 min anoxic stirring, 120 min aeration, 30 min sedimentation, 30 min discharge and idle phase. Sludge retention time (SRT) was controlled at 12.5 days by withdrawing the mixture (100 ml) at the end of the aerobic phase. Each day 3 cycles were operated. Each SBR operated for 105 cycles. Water samples were taken at 0.5 h intervals and immediately filtered with a 0.45 μm filter. Each sample was tested three times, and the average value was calculated for analysis.
Analytical methods
Samples were collected each day for COD, NH4+-N, NO2−-N, NO3−-N, MLSS, SV, SVI and mixed liquid volatile suspended solids (MLVSS) measurements, all parameters were measured according to the Standard Methods [20]. Among them, NH4+-N adopted Nessler's reagent spectrophotometry, NO2−-N adopted N-(1-naphthyl)-1,2-diaminoethan e dihydrochioride spectrophotometry, and NO3−-N adopted thymol spectrophotometry. MLSS, SV, SVI and MLVSS adopted gravimetric method. Sludge morphology was observed with an OLYPUS BX51 optical microscope; DO, pH, ORP and temperature values were recorded using WTW level 2 pH meter and pH/oxi340i meter (WTW Company, Germany). The effluent pH, DO and ORP were measured at the end of anoxic phase.
The denitrification kinetics is described by the Monod equation and was calculated using Eq. (1) [21]. Nitrate reduction rates (RNO3−-N reduction) were calculated using Eq. (2). Nitrite accumulation rates (RNO2−-N accumulation) were calculated using Eq. (3). Nitrite reduction rates (RNO2−-N reduction) were calculated using Eq. (4). The NAR was calculated using Eq. (5).
where V is the specific denitrification rates of NO3−-N and NO2−-N at concentration C, mg·(g·d)−1; Vm is the maximum specific denitrification rates of NO3−-N and NO2−-N, mg·(g·d)−1; Ks is the half-saturation constant, mg·L−1.
where CNO3−-N0 and CNO2−-N0 is the initial NO3−-N or NO2−-N concentration, [mg·(L·min)−1]; CNO3−-Nt and CNO2−-Nt is the NO3−-N or NO2−-N concentration at time t (corresponding to the nitrate turning point of pH and ORP), [mg·(L·min)−1].
where CNO2−-N and CNO3−-N are the concentrations of NO2−-N and NO3−-N at the end of anoxic period, mg·L−1.
Results and discussion
Effect of C/N ratio on PD
Theoretically, to denitrify 1 g of NO3−-N to NO2−-N will consume 1.14 g COD. However, due to the assimilation effect 3.45 g COD will actually be consumed [1.14/(1 − YH) g COD, YH = 0.67] [22]. According to the variation trend of NAR, the experiment can be divided into two stages, the initiation stage of PD (NAR gradually increased) and the maintenance stage of PD (NAR remained stable) (shown in Fig. 1). When the C/N was 2.75 and 3.30, the average NAR of the second stage (40–105 d, 30–105 d) was 79.29% and 72.22%, respectively (Fig. 2a, b). Compared with the C/N of 2.75, the system entered the second stage faster. The results showed that the growth and metabolism of denitrifying bacteria were inhibited when carbon sources were insufficient [1]. The start time of the second stage was close at C/N of 4.13 and 5.50 (Fig. 2c, d), the average NAR increased from 56 and 76% in the first stage (0–18 d) to 81.31% and 82.30% in the second stage (19–105 d), respectively. When the C/N was 8.25 and 16.50, the systems directly came into the PD process (Fig. 2e, f). It had been reported that PD was prone to occur when the C/N was lower than 7 [16]. In this study, the PD was obvious when C/N was less than 5.50, and it would be inhibited when C/N was bigger than 8.25. The results showed that the increase of C/N would be harmful to the PD. A high NO2−-N accumulation PD sludge was obtained at high C/N of 5.50, which was much higher than previous reported values at low C/N of 2.5 [23]. The discrepancy showed that limited carbon source during PD was not the key factor for high NAR, and higher NAR could be achieved within a wide range of organic carbon concentrations.
Effect of C/N on phosphorus removal and sludge settling
The variations of phosphorus uptake and phosphorus release under different C/N were monitored (Fig. 3). When C/N was 2.75 and 3.30, the average PO43−-P concentrations in anoxic effluent were 11.81 and 12.59 mg·L−1, respectively. Since the carbon source was insufficient, the phosphorus-accumulating bacterias (PAOs) were disadvantaged in the competition with denitrifying bacterias [24]. Therefore, phosphorus removal performance was almost disappeared. When the C/N was bigger than 4.13, because of the inhibition of free nitrous acid (FNA) on PAOs, the phosphorus removal performances were different (Fig. 3a, b). Zeng et al. [25] found that PAOs would consume a large amount of polyhydroxyalkanoate (PHA) for detoxification when FNA was bigger than 0.0068 mg·L−1. In this study, the FNA concentration was just in this range. In addition, the PO43−-P concentrations in aerobic effluent under different C/N were 8.6 ± 2.5 mg·L−1, which was similar to the PO43−-P concentration of the influent. This suggested that the NO2−-N accumulated by PD seriously inhibited the activity of PAOs. Zhou et al. [26] found that the phosphorus removal performance of complete denitrification reactors was linearly related to the C/N, and the high C/N did not inhibit the release of phosphorus. However, the phosphorus release ability was inhibited at all the six C/N in this study. With the increase of C/N, the attenuate inhibition of PAOs was observed. The average amount of released PO43−-P at C/N of 16.50 was 189% higher than C/N of 2.75. The high accumulation of nitrite was the key factor for the difference between traditional phosphorus removal and PD phosphorus removal.
The variations of SVI under different C/N are shown in Fig. 4. It could be seen that sludge settleability deteriorated under low C/N condition. SVI was lower when the influent C/N were 8.25 and 16.50, while it increased when the influent C/N were 2.75, 3.30, 4.13, and 5.50. Casey et al. [27] found that NO was an intermediate product during denitrification, and it was only accumulated in the floc bacterias rather than filamentous bacterias. In addition, the FNA also showed an inhibition effect on the microorganisms [28]. When C/N were 2.75, 3.30 and 5.50, the average concentrations of NO2−-N and FNA were 51.63, 42.73, 28.73 and 0.11, 0.091, 0.061 mg·L−1, respectively, which were both in the range to deteriorate sludge settleability (NO2−-N ≥ 28.73 mg·L−1, FNA ≥ 0.061 mg·L−1) [29]. When C/N were 8.25 and 16.50, the average concentrations of NO2−-N and FNA were 5.27 and 3.41 mg·L−1 and 0.011 and 0.0072 mg·L−1, respectively. The average SVI was the lowest at C/N of 8.25, which was 130% lower than C/N of 3.30. This suggests that the sludge settleability improved when the concentrations of NO2−-N and FNA are low. Meanwhile, when the influent C/N was bigger, more organics would be adsorbed and uptaken by floc bacterias, and the sludge settleability would be improved [29].
Effect of C/N on PD kinetics
There are two kinds of electron acceptors in the denitrification process, the variations of NO3−-N and NO2−-N under different C/N were shown in Table 1, the reduction rates of NO3−-N (RNO3−-N reduction) were bigger than those of NO2−-N (RNO2−-N reduction) under different C/N, which was the main reason for the accumulation of NO2−-N [RNO3−-N reduction ≈ (2–10) RNO2−-N reduction]. The RNO2−-N reduction at high C/N was bigger than that at low C/N. It maybe the activity of nitrite reductase was low in the presence of high nitrate because competition for the electron with nitrate reductase. In other words, nitrate is preferred as an electron acceptor over nitrite when both nitrate and nitrite were available for microorganisms. Based on Monod kinetic equation, NO3−-N almost decreased linearly during NO2−-N accumulation, and RNO3−-N reduction was close to the Vmax-NO3−-N reduction. When the concentration of NO3−-N was lower than the KsNO3−-N reduction, RNO3−-N reduction gradually decreased with the decrease of NO3−-N. Conversely, RNO2−-N reduction increased with the increase of NO2−-N before reached the concentration of VmaxNO2−-N reduction. NO2−-N concentration would reach the peak value when the RNO3−-N reduction and RNO2−-N reduction were the same. The VmaxNO3−-N reduction at different C/N were 2.53, 2.50, 2.61, 3.07, 3.11 and 3.20 d−1, respectively, which were higher than the VmaxNO3−-N reduction reported by Wang et al. [30] at 0.71 d−1. This shows that the PD system of this study can achieve high nitrite accumulation in a shorter time. With the decrease of initial NO3−-N concentration, VmaxNO3−-N reduction gradually increased, and KsNO3−-N reduction gradually decreased, which was the same as observed by Cao et al. [14] during the complete denitrification process. It could be concluded that the denitrification kinetics of NO3−-N under PD condition is consistent with that of complete denitrification.
pH and ORP as control parameters
The variations of NO3−-N and NO2−-N in the PD process under different C/N could be indicated by pH and ORP. As shown in Fig. 5a and b, when C/N were 2.75 and 3.30, the concentrations of NO3−-N and NO2 −-N changed linearly. When C/N were 4.13, 5.50, 8.25, the NO2−-N reached the peak value at 5, 10, and 15 min, respectively (Fig. 5c–e). Correspondingly, the nitrate turning point appeared on the pH and ORP curves, indicating that denitrification with NO3−-N as the electron acceptor was finished. As shown in Fig. 5 (f), when C/N was 16.50, the turning points of nitrate and nitrite appeared at 3 min and 25 min, respectively. The curves of ORP and pH could be divided into three stages by nitrate turning point and nitrite turning point. The differences of slope in ORP curves are mainly caused by the different electric potential of NO3−-N/NO2−-N and NO2−-N/N2. The ORP curve dropped sharply after the nitrite turning point, which indicated the system altered from anoxic state to anaerobic state [31]. No alkalinity was produced during NO3−-N was reduced to NO2−-N, but it was produced when NO2−-N was reduced to N2. The pH curve slowly increased before the turning point of nitrate. The reason was CH3COONa as the only carbon source was alkalescence, and OH− would be generated with the consumption of CH3COOH [24]. After the turning point of nitrite, facultative anaerobic bacteria began to produce short-chain fatty acids, and the pH curve decreased rapidly [32]. Moreover, with the increase of C/N, the nitrate turning point appeared earlier, and the nitrite turning point was prone to appear. The nitrate turning points in this study all appeared before 15 min, which were earlier than those reported by Du et al. [33]. It shows that this study has a higher nitrite accumulation rate, and it is reasonable for WWTP to choose the turning points on the curves of pH and ORP to control the PD process. According to the turning points of the pH curve (deceleration point, infection point) and the two turning points of the ORP curve (nitrate turning point, nitrite turning point), the process of PD can be clearly controlled.
Conclusion
This research explored the characteristics of nutrients removal and sludge settleability under partial denitrification. The FNA and NO2−-N produced would deteriorate both sludge settleability and phosphorus removal. When the C/N was in the range of 2.75–5.50, the PD phenomenon was obvious. Sludge settleability deteriorated because floc bacterias would be inhibited by the intermediate products produced by insufficient denitrification. And phosphorus removal would deteriorate as PAOs could not get enough carbon source to release PO43−-P. When the C/N was in the range of 8.25–16.50, the inhibition effect decreased. The denitrification kinetics of PD and complete denitrification were similar, and the nitrite accumulation was caused by the difference between nitrate reduction rate and nitrite reduction rate. When the influent quality is stable, monitoring the turning points of pH and ORP curves will help to regulate the PD process. Further study on the changes of microbial populations at different C/N would help researchers to understand, apply and/or maintain the PD. For this purpose, pure or highly enriched cultures with identified metabolic pathways and functional genes would be required.
References
Zou J, Pan J, Wu S et al (2019) Rapid control of activated sludge bulking and simultaneous acceleration of aerobic granulation by adding intact aerobic granular sludge. Sci Total Environ 674:105–113. https://doi.org/10.1016/j.scitotenv.2019.04.006
Du R, Peng Y, Cao S et al (2016) Mechanisms and microbial structure of partial denitrification with high nitrite accumulation. Appl Microbiol Biotechnol 100(4):2011–2021. https://doi.org/10.1007/s00253-015-7052-9
Ma B, Wang S, Cao S et al (2016) Biological nitrogen removal from sewage via anammox: recent advances. Bioresour Technol 200:981–990. https://doi.org/10.1016/j.biortech.2015.10.074
Tri Le, Peng B, Su C et al (2019) Nitrate residual as a key parameter to efficiently control partial denitrification coupling with anammox. Water Environ Res 91(11):1455–1465. https://doi.org/10.1002/wer.1140
Cao S, Li B, Du R et al (2016) Nitrite production in a partial denitrifying upflow sludge bed (USB) reactor equipped with gas automatic circulation (GAC). Water Res 90:309–316. https://doi.org/10.1016/j.watres.2015.12.030
Zhang Z, Zhang Y, Chen Y (2020) Recent advances in partial denitrification in biological nitrogen removal: from enrichment to application. Bioresour Technol 298:122444. https://doi.org/10.1016/j.biortech.2019.122444
Cao S, Wang S, Peng Y et al (2013) Achieving partial denitrification with sludge fermentation liquid as carbon source: the effect of seeding sludge. Bioresour Technol 149:570–574. https://doi.org/10.1007/s00253-015-7052-9
Du R, Cao S, Niu M et al (2017) Performance of partial-denitrification process providing nitrite for anammox in sequencing batch reactor (SBR) and upflow sludge blanket (USB) reactor. Int Biodeterior Biodegrad 122:38–46. https://doi.org/10.1016/j.ibiod.2017.04.018
Du R, Peng Y, Ji J et al (2019) Partial denitrification providing nitrite: opportunities of extending application for anammox. Environ Int 131:105001. https://doi.org/10.1016/j.envint.2019.105001
Lu H, Chandran K, Stensel D (2014) Microbial ecology of denitrification in biological wastewater treatment. Water Res 64:237–254. https://doi.org/10.1016/j.watres.2014.06.042
Shi L, Du R, Peng Y (2019) Achieving partial denitrification using carbon sources in domestic wastewater with waste-activated sludge as inoculum. Bioresour Technol 283:18–27. https://doi.org/10.1016/j.biortech.2019.03.063
Jiang C, Xu S, Wang R et al (2018) Comprehensive assessment of free nitrous acid-based technology to establish partial nitrification. Environ Sci-Wat Res Technol 4(12):2113–2124. https://doi.org/10.1039/c8ew00637g
Wang Z, Ji Y, Yan L et al (2020) Simultaneous anammox and denitrification process shifted from the anammox process in response to C/N ratios: performance, sludge granulation, and microbial community. J Biosci Bioeng 130(3):319–326. https://doi.org/10.1016/j.jbiosc.2020.04.007
Cao S, Du R, Li B et al (2017) Nitrite production from partial-denitrification process fed with low carbon/nitrogen (C/N) domestic wastewater: performance, kinetics and microbial community. Chem Eng J 326:1186–1196. https://doi.org/10.1016/j.cej.2017.06.066
Krishna Mohan TV, Nancharaiah YV, Venugopalan VP et al (2016) Effect of C/N ratio on denitrification of high-strength nitrate wastewater in anoxic granular sludge sequencing batch reactors. Ecol Eng 91:441–448. https://doi.org/10.1016/j.ecoleng.2016.02.033
Chen H, Liu S, Yang F et al (2009) The development of simultaneous partial nitrification, ANAMMOX and denitrification (SNAD) process in a single reactor for nitrogen removal. Bioresour Technol 100(4):1548–1554. https://doi.org/10.1016/j.ibiod.2016.10.047
Ge S, Peng Y, Wang S et al (2012) Nitrite accumulation under constant temperature in anoxic denitrification process: the effects of carbon sources and COD/NO(3)-N. Bioresour Technol 114:137–143. https://doi.org/10.1016/j.biortech.2012.03.016
Ding F, Liang D, Wu Y et al (2020) Effect of C/N on partial nitrification in an MBBR at low temperature. Environ Sci-Wat Res Technol 6:3391–3399. https://doi.org/10.1039/D0EW00654H
Van d A A G, De Bruijn P, Robertson LA et al (1996) Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiol 142(8):2187–2196. https://doi.org/10.1099/13500872-142-8-2187
State Environmental Protection Administration of China (2002) Water and waste water monitoring analysis methods, 4th edn. Chinese Environmental Science Publishing House, Beijing
Zhang Y, Wang X, Wang W et al (2019) Investigation of growth kinetics and partial denitrification performance in strain Acinetobacter johnsonii under different environmental conditions. R Soc Open Sci 6(12):191275. https://doi.org/10.1098/rsos.191275
Iacopozzi I, Innocenti V, Marsili-Libelli S et al (2007) A modified activated sludge model No 3. (ASM3) with two-step nitrification—denitrification. Environ Modell Softw 22(6):847–861. https://doi.org/10.1016/j.envsoft.2006.05.009
Gong L, Huo M, Yang Q et al (2013) Performance of heterotrophic partial denitrification under feastfamine condition of electron donor: a case study using acetate as external carbon source. Bioresour Technol 133:263–269. https://doi.org/10.1016/j.biortech.2012.12.108
Campo R, Sguanci S, Caffaz S et al (2020) Efficient carbon, nitrogen and phosphorus removal from low C/N real domestic wastewater with aerobic granular sludge. Bioresour Technol 305:122961. https://doi.org/10.1016/j.biortech.2020.122961
Zeng W, Wang A, Zhang J et al (2016) Enhanced biological phosphate removal from wastewater and clade-level population dynamics of “Candidatus Accumulibacter” under free nitrous acid inhibition: linked with detoxication. Chem Eng J 296:234–242. https://doi.org/10.1016/j.cej.2016.03.063
Zhou J, Sun Q (2020) Performance and microbial characterization of aerobic granular sludge in a sequencing batch reactor performing simultaneous nitrification, denitrification and phosphorus removal with varying C/N ratios. Bioprocess Biosyst Eng 43(4):663–672. https://doi.org/10.1007/s00449-019-02264-w
Casey TG, Wentzel MC, Ekama GA (1999) Filamentous organism bulking in nutrient removal activated sludge systems—paper 11: a biochemical/microbiological model for proliferation of anoxic-aerobic (AA) filamentous organisms. Water SA 25(4):443–451
Zhou Y, Oehmen A, Lim M et al (2011) The role of nitrite and free nitrous acid (FNA) in wastewater treatment plants. Water Res 45(15):4672–4682. https://doi.org/10.1016/j.watres.2011.06.025
Wang J, Chon K, Ren X et al (2019) Effects of beneficial microorganisms on nutrient removal and excess sludge production in an anaerobic–anoxic/oxic (A2O) process for municipal wastewater treatment. Bioresour Technol 281:90–98. https://doi.org/10.1016/j.biortech.2019.02.047
Wang Y, Sabba F, Bott C et al (2019) Using kinetics and modeling to predict denitrification fluxes in elemental-sulfur based biofilms. Biotechnol Bioeng 116(10):2698–2709. https://doi.org/10.1002/bit.27094
Foladori P, Petrini S, Andreottola G (2018) Evolution of real municipal wastewater treatment in photobioreactors and microalgae-bacteria consortia using real-time parameters. Chem Eng J 345:507–516. https://doi.org/10.1016/j.cej.2018.03.178
Jiang H, Liu G, Ma Y et al (2018) A pilot-scale study on start-up and stable operation of mainstream partial nitrification-anammox biofilter process based on online pH-DO linkage control. Chem Eng J 350:1035–1042. https://doi.org/10.1016/j.cej.2018.06.007
Du R, Peng Y, Cao S et al (2016) Characteristic of nitrous oxide production in partial denitrification process with high nitrite accumulation. Bioresour Technol 203:341–347. https://doi.org/10.1016/j.biortech.2015.12.044
Acknowledgements
We would like to acknowledge the support of Zhengzhou University via our access to research resources. Finally, we would like to thank all of the Reviewers and the handling Editor for their constructive input.
Funding
This work was supported by the [Key scientific research project plan of Henan University] under Grant [20B560018]; [Research project of Henan Science and Technology] under Grant [HNKJZK-2020-03C]; [Key R&D and Promotion Project of Henan Province] under Grant [202102310278].
Author information
Authors and Affiliations
Contributions
ZP contributed to the study conception and design. Material preparation, data collection and analysis were performed by TL, KJ, JW and NN. The first draft of the manuscript was written by TL and all authors commented on previous versions of the manuscript. NN and AL checked the English writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, Z., Lou, T., Jiang, K. et al. Characteristics of nutrients removal under partial denitrification initiated by different initial nitrate concentration. Bioprocess Biosyst Eng 44, 2051–2059 (2021). https://doi.org/10.1007/s00449-021-02582-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02582-y