Abstract
Plant communities around penguin rookeries were studied during the Antarctic summer. Antarctic hair grass Deschampsia antarctica was examined. The abundance of this grass around penguin rookeries demonstrated a characteristic distribution. Eight UV screens were installed on a transect leading away from the rookeries. Nitrate reductase activity was measured below and outside of the screens. The activity varied between the measurement sites and also changed with the distance from rookeries. Fifteen cycles of nitrate reductase activity were conducted during the experiment. The highest values of nitrate reductase in the leaves of plants protected from UV radiation, which occurred in well-fertilized sites close to the rookery, reached 743.1 (SD = 789.7) nmol of nitrite synthesized g−1 of dry mass h−1; the lowest occurred in the poorly fertilized sites 77.2 (SD = 41.2) nmol g−1 of dry mass h−1. Nitrate reductase activity in unprotected plants growing in ambient conditions reached up to 843.8 (SD = 894.5) in well-fertilized sites and 159.5 (SD = 257.6) nmol g−1 of dry mass h−1, respectively. The greatest abundance of D. antarctica occurred in the middle of the transect. The influence of ultraviolet radiation caused the induction of NR activity in poorly fertilized sites, but this effect was not visible in sites that were well fertilized. Total nitrogen concentrations in the plant tissues varied between 1.4 % in poor sites and 3.4 % in sites that were situated close to the rookeries. In addition, the concentrations of total nitrogen in the soil varied between the sites and ranged from 0.2 to 3.3 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Antarctica has some of the most difficult conditions for plant growth on Earth; 98 % of the continent and the surface of the Antarctic islands are covered by glaciers. Ice-free habitats only occur as isolated patches of ground scattered around the Antarctic continent, along the continent coasts and a relatively few are also found inland. Human activity is low in Antarctica and therefore the local anthropogenic impact on the environment is minimal. The conditions for vegetation growth are difficult due to the low temperatures, gusting winds, the small amount of soil, and high UV radiation due to the depletion of stratospheric ozone that occurs there (Xiong and Day 2001) due to global human activity. As a result, the Antarctic ecosystems are periodically exposed to enhanced solar UV-B. The enhanced UV-B radiation that is a result of the depletion of stratospheric ozone may cause damage to plants, although the protective mechanisms of plants can mitigate these effects. It is remarkable that research on these consequences is so scanty (Rozema et al. 2001). Antarctic soils are usually poor in nutrients, but in certain locations there is a high nutrient input from sea animals such as seals and sea birds (Tatur 2002). Penguin rookeries play an important role in the circulation of organic matter between the sea and land (Pietr et al. 1983). These communities, especially those that have existed in the same place for a long time, create specific conditions around them. Accumulated penguin guano changes the nutrient balance in the soil and may also change the metabolism of plants that exist there. It is known that a specific zonation of plant communities exists around the rookeries. It has been described that the plant composition and coverage of a particular species changes rapidly with an increasing distance from the rookery. The sites nearest to a rookery are mainly dominated by green algae Prasiola crispa and some individuals of vascular Deschampsia antarctica may also occur. D. antarctica begins to dominate further from a rookery and with increasing distance from a rookery Colobathus quitansis begins to appear. The lichen Usnea antarctica is dominant at great distances from the rookeries and individuals of D. antarctica and Colobanthus quitensis may occur (Smykla et al. 2006, 2007).
Nitrogen is known to be one of the growth-limiting factors in terrestrial ecosystems (Lindberg et al. 1986). A high concentration of nitrogen compounds may have negative impact on the growth dynamics and development of plants, even though nitrogen compounds are known to be the most abundant factor that influences terrestrial ecosystems (Lindberg et al. 1986). It is known that human activity may have a harmful influence on the environment, which is mainly due to industrial and traffic pollution. Nitrogen species are an important part of that pollution (Krywult and Bytnerowicz 1997), and the most of terrestrial plants are sensitive to the presence of these species in the environment (Norby 1989). These harmful species occur in high concentrations in the unique Antarctic ecosystem. Therefore, it can be assumed that plants that grow there have developed specific physiological adaptations (Barcikowski et al. 2005).
Nitrate reductase (NR), the enzyme that is responsible for reducing nitrate into nitrite, plays a key role in the nitrogen metabolism pathway. The induction of this enzyme mobilizes a whole metabolic pathway of nitrate reduction and assimilation that through nitrite reductase is responsible for reducing nitrite to ammonia and glutamine synthetase which incorporate ammonia into amino acids (Berg et al. 2007). The synthesis of all of these enzymes and then their activity are energy-consuming processes (Norby et al. 1989). The permanent exposure of these plants to the presence of nitrogen species can cause the continuous activation of this metabolic pathway, which in over-fertilized conditions may have a negative influence on energy balance of plants. This may cause a decrease in the growth dynamics of plants (Norby et al. 1989; Krywult and Bytnerowicz 1997). Although this enzyme is substrate inducible, its activity is dependent on many other factors such as temperature, plant water status, the intensity of light and ultraviolet radiation, acidity, the presence of ammonia, etc. (Norby 1989). In spite of that, it was found that NR activity may be used as a factor to indicate the saturation of nitrogen species in the environment (Krywult et al. 1996), although the enzyme activity differs between plant species (Krywult and Klich 2000; Krywult et al. 2002). Enzymes demonstrate a higher level activity in the presence of species such as nitrate, nitrogen oxides and nitric acid vapor (Krywult et al. 1996; Krywult and Bytnerowicz 1997). It was also found that light and ultraviolet radiation induce enzyme activity (Krywult and Bytnerowicz 1997; Sinha et al. 1998).
The NR activity, total amount of nitrogen in the plants that were studied and soil from all of the sites were measured during this research.
The aims of this study were as follows:
-
1.
Does the gradient of the concentration of nitrogen compounds at an increasing distance from penguin rookeries have an impact on the NR activity in plants?
-
2.
Does the total nitrogen content of plants correlate with the activity of NR?
-
3.
Is NR activity dependent on exposure to solar ultraviolet radiation?
2 Material and Methods
2.1 Study Area
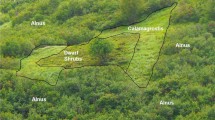
The research was conducted on King George Island (South Shetland Islands, Maritime Antarctic) near the Polish Research Station “Henryk Arctowski” (62°10′ S, 58°28′ W) within the Antarctic Specially Protected Area No. 128 Western Shore of Admiralty Bay (Fig. 1).
Topographic map of the SSSI No. 8, King George Island, West Antarctica (Pudełko 2003). Plots from UV8 to UV3 represent the transects set up due to the decreasing fertility gradient that was influenced by the rookery of Gentoo penguins. Plot UV1 represents a fertilized place close to the rookery of the Adelie penguins that is always well moistened. Plot UV2 was situated on the relicts of ornithogenic soil below an abandoned penguin rookery
King George Island, the largest in the South Shetland archipelago, is located between latitude 61°50′−62°15′ S and long. 57°30′−59°01′ W. The island lies approximately 770 km south-east of Cape Horn, from which it is separated by Drake Passage and about 160 km north of Trinity Peninsula, the northernmost part of the Antarctic Peninsula. The cold climate with mean annual temperature of −1.7 °C (2.4° in January and −6.8° in July), high humidity (84 %) with a strong oceanic influence and high precipitation (530 mm) is typical of the northern part of the Maritime Antarctic. A major climatic feature of this area is the strong katabatic winds which often reach hurricane force (Kejna 1999). Despite the harsh environmental conditions, ice-free areas, which constitute only about 10 % of the island, provide conditions favourable for supporting a relatively diverse terrestrial biota.
The vegetation of the island is typical for the Maritime Antarctic. It is almost exclusively cryptogamic, comprising mostly lichens, mosses, liverworts, algae and cyanobacteria, with the vascular flora being represented by only two native species, the Antarctic hair grass D. antarctica Desv. (Poaceae) and the Antarctic Pearlwort C. quitensis (Kunth) Bartl. (Caryophyllaceae). The most extensive vegetation is found in the sheltered areas of the west shore of Admiralty Bay, the largest embayment of the island. This south-facing bay is enclosed on three sides by mountain ranges, which vary in height from 150 to 680 m, and which afford good protection from the prevailing north-westerly winds. The ice-free areas on the west shore of Admiralty Bay are significant in that they are one of the richest botanical areas in the entire Antarctic (Ochyra 1998; Krzewicka and Smykla 2005).
These ice-free areas on the west shore of Admiralty Bay also provide a breeding ground for large populations of Pygoscelis penguins: Adelie (Pygoscelis adelie), Gentoo (Pygoscelis papua), and Chinstrap (Pygoscelis antarctica). The entire population of penguins nesting in this area ranges between 30,000–50,000 pairs (Tatur 2002). The presence of these colonies and their attendant nutrient supply is crucial in determining the distribution and abundance of the terrestrial vegetation (Tatur et al. 1997; Smykla et al. 2006, 2007) as well as the functioning of the entire terrestrial ecosystem around the bay (Tatur 2002; Barcikowski et al. 2005).
More detailed descriptions of this area have already been presented in several publications (Rakusa-Suszczewski 1993; Ochyra 1998; Beyer and Bölter 2002).
2.2 Experimental Design
Eight study sites were selected around colonies of Adelie and Gentoo penguins for the UV exclusion field experiment (Fig. 1). Vegetation was surveyed on each site and the percentage of the coverage of vegetation was recorded. In general, the experimental study sites were distributed on a transect representing increasing distances from active penguin colonies. Particular sites represented plant assemblages corresponding to the zonations of vegetation that were related to different degrees of the influence of a penguin colony (see Smykla et al. 2006, 2007 for details). In addition, one of the study sites was established on a relict penguin colony. A detailed description of the sites that were studied is given in Table 1.
Two plots with relatively homogenous vegetation representing a plant assemblage characteristic for a particular vegetation zone were established at each study site. Then, the ultraviolet exclusion chambers were placed over one of the two established plots and the second plot represented the ambient conditions at each study site. The individual treatment chambers placed over the UV experimental plots consisted of aluminum frames (1.20 × 1.20 m) covered with a clear acrylic filter 2.00 mm thick. In addition, two acrylic curtains were added to the chamber sides that were exposed to the NW and NE to avoid the penetration of the solar radiation below the screens. The screens were adjusted approximately 40 cm above ground level in order to avoid the greenhouse effect and to allow proper air ventilation and moisturizing. The filters excluded UV radiation below the 350-nm waveband and absorbed less than 8 % of the visible photosynthetic active radiation. The spectral characteristic of the filters and their relative wavelength transmission were tested using a spectrometer in the laboratory. The results are illustrated on Fig. 2.
Green tissues of plants were collected during the Antarctic summer period on sunny days at around noon. Samples were taken from under and outside the screen simultaneously.
2.3 Experimental Plots Description
Eight plots in the study area were selected (Fig. 1). Six plots were situated in relation to the gradient of the decreasing influence of a penguin rookery and two additional plots, UV1 and UV2, were set up. Plot UV1 was set up close to a rookery, in a place that was always well moistened. Plot UV2 was situated on the relicts of a penguin colony on old ornithogenic soil. A detailed description of the sites that were studied is shown in Table 1.
2.4 NR Activity
NR activity is typically assayed in vivo by measuring nitrite production in tissue that has been vacuum infiltrated with a buffered nitrate solution (Downs et al. 1993). For this study, a NR assay was adapted from a number of studies (Jaworski 1971; Al Gharbi and Hipkin 1984; Norby et al. 1989) with our own modifications (Krywult and Klich 2000; Krywult et al. 2002).
The measurements were taken from green leaves of D. antarctica that were collected inside the UV exclusion experiment chambers and in the control plots outside the UV chambers at the same time. At each plot several leaves were collected from a few different grass specimens to form a composite sample for each plot. The leaves from the UV experiment plots were collected from grass growing in the central part of the chamber in order to avoid collecting leaves that were exposed to any direct solar radiation. The experimental period and NRA measurements continued through the austral summer season. The sampling and measurements were only carried out on sunny days between the hours of 11 a.m. and 1 p.m. of the solar time.
Immediately after collection, the leaves were dissected into ca. 2-mm segments and placed into test tubes with a buffer solution. The leaf tissue was then subjected to vacuum infiltration (with a manually operated vacuum pump) at 0.33 atm. for 10 min and incubated in the buffer for 4 h at 20 °C in the dark. The composition of the incubation buffer was 0.1 M KNO3, 0.1 M K2HPO4, and 0.6 % 1-propanol, adjusted to pH 7.5 with HCl.
After incubation, the enzyme activity was terminated by adding 1 % sulphanilamide in 8 % HCl. The concentration of synthesized nitrite in the incubation buffer was determined colorimetrically upon diazotization and the formation of azo dye following the addition of 0.02 % N-(1-naphthyl)ethylenediamine-dihydrochloride to the reaction mixture (Keeney and Nelson 1982). Optical density was measured colorimetrically after 10 min at 540 nm using a spectrometer (Shimadzu UV-120). A mixture of the incubation buffer with 1 % sulphanilamide in 8 % HCl and 0.02 % N-(1-naphthyl)ethylene-diamine-dihydrochloride in the same proportions as those used in creating the diazo compound was used as a blank. All of the chemicals were supplied by Merck (Germany). The leaf samples were removed from the test tubes and weighed after oven-drying to a constant weight at 60 °C. NR activity was calculated on the basis of a calibration curve for KNO2. The results were expressed as the amount of nitrite synthesized in nanomoles per gram of plant tissue dry weight per hour.
2.5 Total Nitrogen Analysis
The total nitrogen concentrations in the D. antarctica leaves and soil samples were determined using the Kiejdahl method. The samples for those analyses were collected at the end of the summer season. The collected samples were dried at room temperature and shipped to Poland for the analyses. Once in the lab, the grass leaves were washed with double distilled water to remove any contamination from their surface. Then, the leaves were oven-dried at 60 °C to a constant weight and homogenized to a fine powder. The soil samples were first oven-dried and then sieved to a particle size of 1 mm. After that, the samples were mineralized in concentrated sulphuric acid with CuSO4 addition at 440 °C the total nitrogen amount was analyzed using Kieltec 2300 Foss Tecator. The results were expressed as the percentage of N-content per gram of sample dry weight.
2.6 Statistical Analyses
The differences in NR activity between protected and nonprotected plants were examined using the paired-samples Wilcoxon test (Sokal and Rohlf 1995). The Spearman rank correlation test was applied to analyze the relationship between total nitrogen concentrations and the total average value of NR activity in D. antarctica leaves. The accepted level of significance was p <0.05 throughout entire work. All statistics were calculated using the R language and environment (R Development Core Team 2011).
3 Results
The NR activity in D. antarctica tissues varied significantly among the study sites and between the treatments. The mean NR activity in the D. antarctica leaves ranged 160–3146 and 31–2,340 nmol g−1 DWh−1 in grass growing in ambient conditions and under the UV exclusion chambers, respectively. In general, the values of NR activity showed a decreasing trend along the gradient of the decreasing influence of a penguin colony. The highest values of NR activity were recorded in plants growing within active penguin colonies (sites UV1 and UV8) and in plots located close to the colonies (sites UV7 and UV6). Plants growing on the relict colony (site UV2) also had high enzyme activity values, whereas plants growing on sites at a distance from the influence of penguin colonies had the lowest values of enzyme activity (sites UV5, UV4, and UV3) (Figs. 3 and 4).
It is interesting that D. antarctica occurred in all of the sites that were studied (well, moderate and poorly fertilized). This may show that plants demonstrate specific adaptations to environments that are either rich or poor in nitrogen forms.
UV treatment did not show a uniform pattern of influence on the NR activity. On sites located within active and relict penguin colonies, the enzyme activity in the grass leaves was higher in the UV exclusion plots than in the ambient plots but the differences were not statistically significant. There was no evidence of any difference in NR activity between plants growing in ambient conditions and those that were protected. However, on sites that were at a distance from penguin colonies that had little or no influence from the colonies, the enzyme activity showed a different relationship. It was lower in the UV exclusion plots than in the ambient plots however, the difference was statistically different on only two sites (UV6 and UV3).
Total nitrogen concentrations in plant leaves differed between the sites. The lowest values were found in plants growing on the sites that were most distant from penguin colonies (plots UV4 and UV3, 1.4 and 1.5 % of dry mass of leaves, respectively). The highest values were recorded in plants growing on sites located within active penguin colonies (plots UV8 and UV1) and in plants growing on the relict penguin colony (plot UV2), which ranged up to 3.9 % (Fig. 5). Total nitrogen concentrations in D. antarctica leaves correlated with the total average value of NR activity in the respective plots: rs = 0.31; p = 0.009, and this dependence was significant only for UV protected plants (Fig. 6). Concentrations of the total nitrogen in soil ranged from 0.09 to 3.08 %. Although the highest values of the total nitrogen were found in soil from a penguin colony (site UV8) and although sites that were at a distance from penguin colonies demonstrated low values, the decreasing trend was not statistically significant. This can probably be explained by the high degree of variability in the data related to substrate type and by biogens being washed away by water run-offs on some of the sites that were studied. There was also no significant relationship in the total nitrogen concentration between the soil and the grass leaves.
Correlation between the total average of NR activity (in nanomoles per gram dry mass per hour) in D. antarctica leaves recorded during the entire time period of the experiment and the total amount of nitrogen in D. antarctica leaves collected at the end of experiment (in percentage of plant mass). The enzyme activity is presented as nanomoles of nitrite synthesized per gram of dry mass of leaves per hour and the total nitrogen amount is shown as the percentage of nitrogen in dry mass of tissue
4 Discussion and conclusions
The results obtained during this study demonstrate a high diversity of NR activity on the plants among the plots that were studied. The highest values of enzyme activity in D. antarctica correspond with high concentrations of nitrogen compounds on the plots. The chemical composition of ornithogenic soils in the Admiralty Bay area has been relatively well examined (Pietr et al. 1983; Tatur and Myrcha 1984; Tatur et al. 1997; Juchnowicz-Bierbasz and Rakusa-Suszczewski 2002). All of the research conducted in previous years indicated that the nitrogen compounds that exist here are mainly in a reduced form, especially in plants growing close to a penguin rookery. However, D. antarctica demonstrated a high enzyme NR activity in these areas. This may suggest impossibility of excluding these metabolic pathways due to the presence of nitrate in the soil and also precipitation, or on the automatic activation of this reaction, which some authors have suggested (Norby 1989). These may have a negative influence on the energy balance and growth dynamics of the plants that were studied. This deduction appears to confirm deployment and percentage of the coverage of D. antarctica on plots that were studied. D. antarctica was present on all of the examined plots but in different proportions. The following relation was found. The percentage of coverage of hair grass on the plots that were studied was the lowest in both the well and the poorest fertilized sites (Smykla et al. 2006, 2007). The activities of the enzyme NR in these plots were high and low, respectively. The NR activity reached medium values in the plots where the percentage of coverage was the highest (Figs. 3 and 4). In the opinion of the authors, this indicates that this is the optimal habitat for this species. It was concluded that hair grass needs a moderate but constant influx of nitrate for optimal development. It was found that the mineralization of penguin guano occurs quickly in this area and that biogens are quickly transported within the area both by running water and through atmospheric deposition (Pietr et al. 1983; Tatur and Myrcha 1984; Nędzarek and Rakusa-Suszczewski 2007; Zhu et al. 2009). Moreover, these mechanisms are similar in the entire maritime Antarctic, which was confirmed by the results obtained by other authors (Allen et al. 1967; Juchnowicz-Bierbasz 1999; Rankin and Wolf 2000; Zubel 2005; Zhu et al. 2011). Therefore, biogens of an ornithogenic origin are available even at a far distance from rookeries. However, the differences in the soils and precipitation form a strong gradient with an increasing distance from rookeries (Juchnowicz-Bierbasz and Rakusa-Suszczewski 2002; Rakusa-Suszczewski 2003). The results obtained during this study are also in agreement with this finding.
The influence of ultraviolet radiation on NR activity was most visible on some poorly fertilized plots. Although the induction of NR activity was statistically significant only on plots UV3 and UV6 (Table 2), this trend was also visible on the other poorly fertilized plots (Figs. 3 and 4). This may suggest a subservient role of UV as a signal factor in proportion to the presence of nitrate. On the poorly fertilized plots, factors other than UV signaling or distressing factors such as drought, temperature changes, different exposure to winds and the presence of other nutrients may prevent any clear identification of the influence of UV. Many authors have identified factors that are known to have a strong influence on changes in NR activity (Remmler and Cambell 1986; Norby et al. 1989; Foyer et al. 1998; Krywult et al. 2002; Strohm 2007; Krywult et al. 2008). It was found that exposure to UV-B radiation caused a decrease in the growth rate of D. antarctica. Leaves on plants exposed to UV-B were denser, thicker, and had higher concentrations of photosynthetic and UV-B absorbing pigments, although the rates of photosynthetic gas exchange on a leaf area basis were not affected by exposure to UV-B and cannot explain these reductions in growth (Xiong and Day 2001). On the other hand, it was found that a single ultraviolet radiation in controlled laboratory conditions is a factor that can induce NR activity (Sinha et al. 1998). The fact that the area that was studied has a large spatial variability may also be of major importance. The results of the soil analysis did not demonstrate any trend through the investigated transect (Fig. 7). It is possible that the investigated area resembles a mosaic. D. antarctica, which grows on all of the plots that were studies is distinguished by its ability to adapt (Barcikowski et al. 1999). This ability does not ensue from genetic diversity; it was found that this species demonstrates a low genetic variability (Holderegger et al. 2003). Antarctic hair grass demonstrates a high anatomical diversity (Chwedorzewska et al. 2008). In addition, D. antarctica demonstrates a higher level of sucrose and fructans as compared with other gramineae (Zuniga et al. 1996). It was also found that Antarctic hair grass has the ability to successfully compete with soil microbes and moss for proteinaceous nitrogen at an early stage of decomposition (Hill et al. 2011). All of these processes, which are connected with the efficient acquisition of nitrogen compounds, may be the key to the evolutionary success of Antarctic hair grass.
References
Al Gharbi, Q. A., & Hipkin, C. R. (1984). Studies on nitrate reductase on British angiosperms. New Phytologist, 97, 629–639.
Allen, S. E., Grimshaw, H. M., & Holdgate, M. W. (1967). Factors affecting the availability of plant nutrients on an Antarctic Island. Journal of Ecology, 55, 381–396.
Barcikowski, A., Łyżwińska, R., & Zarzycki, K. (1999). Growth rate and biomass production of Deschampsia antarctica Desv. in the Admiralty Bay region (South Shetland Island, Antarctica). Polish Polar Research, 20(3), 301–311.
Barcikowski, A., Łyszkiewicz, A., Loro, P., Rektoris, L., Smykla, J., Wincenciak, A., et al. (2005). Keystone species and ecosystem functioning: the role of penguin colonies in differentiation of the terrestrial vegetation in the Maritime Antarctic. Ecological Questions, 6, 117–128.
Berg, J. M., Tymoczko, J. L., & Stryer, L. (2007). Biochemia. Warszawa: PWN.
Beyer, L., & Bölter, M. (Eds.). (2002). Geoecology of Antarctic icefree coastal landscapes. Ecological Studies, 156. Berlin: Springer.
Chwedorzewska, K., Giełwanowska, I. E., & Bochenek, A. (2008). High anatomical and low genetic diversity in Deschampsia antarctica Desv. from King George Island, the Antarctic. Polish Polar Research, 29(4), 377–386.
Downs, M. R., Nadelhoffer, K. J., Melillo, J. M., & Aber, J. D. (1993). Foliar and fine root nitrate reductase activity in seedlings of four forest tree species in relation to nitrogen availability. Trees, 7, 233–236.
Foyer, C. H., Valadier, M.-H., Migge, A., & Becker, T. W. (1998). Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiology, 117, 283–292.
Hill, P. W., Farrar, J., Roberts, P., Farrell, M., Grant, H., Newsham, K. K., et al. (2011). Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nature Climate Change, 1(1), 50–53.
Holderegger, R., Stehlik, I., Smith, R. I. L., & Abbott, R. J. (2003). Population of Antarctic hair grass (Deschampsia antarctica) show low genetic diversity. Arctic, Antarctic, and Alpine Research, 35(2), 214–217.
Jaworski, E. G. (1971). Nitrate reductase assay in intact plant tissue. Biochemical and Biophysical Research Communications, 43, 1274–1279.
Juchnowicz-Bierbasz, M. (1999). Year-round changes of nutrients in fresh water bodies near Arctowski station (South Shetland Islands, Antarctica). Polish Polar Research, 20(3), 243–258.
Juchnowicz-Bierbasz, M., & Rakusa-Suszczewski, S. (2002). Nutrients and cations content in soil solutions from the present and abandoned penguin rookeries (Antarctica, King George Island). Polish Journal of Ecology, 50(1), 79–91.
Keeney, D. R. & Nelson, D. W. (1982). Nitrogen-inorganic forms. In: A. L. Page (Ed.), Methods of soil analysis, Part 2. Chemical and biological properties. Agronomy, 9(2), 643–698
Kejna, M. (1999). Air temperature on King George Island, South Shetland Island, Antarctica. Polish Polar Research, 20(3), 183–201.
Krywult, M., Karolak, A., & Bytnerowicz, A. (1996). Nitrate reductase activity as an indicator of Ponderosa pine (Pinus ponderosa Dougl. ex. Laws) response to atmospheric nitrogen deposition in the San Bernardino Mountains. Environmental Pollution, 93(2), 141–146.
Krywult, M., & Bytnerowicz, A. (1997). Induction of nitrate reductase activity by nitric acid vapor in California black oak (Quercus kelloggii), canyon live oak (Quercus chrysolepis) and Ponderosa pine (Ponderosa pine) seedlings. Canadian Journal of Forest Research, 27, 2101–2104.
Krywult, M., & Klich, M. (2000). Nitrate reductase activity as an indicator of nitrate fixation and assimilation by tropical forest species on StThomas Island. Fragmenta Floristica et Geobotanica, 45(1–2), 213–220.
Krywult, M., Turunen, M., Sutinen, M.-L., Derome, K., & Norokorpi, Y. (2002). Nitrate reductase activity in some subarctic species and UV in influence in the foliage of Betula pendula Roth. seedlings. The Science of the Total Environment, 284(1–3), 149–153.
Krywult, M., Smykla, J., Kinnunen, H., Martz, F., Sutinen, M.-L., Lakkala, K., et al. (2008). Influence of solar UV radiation on the nitrogen metabolism in needles of Scots pine (Pinus sylvestris L.). Environmental Pollution, 156, 1105–1111.
Krzewicka, B., & Smykla, J. (2005). The lichen genus Umbilicaria from the neighbourhood of Admiralty Bay (King George Island, maritime Antarctic), with a proposed new key to all Antarctic taxa. Polar Biology, 28, 15–25.
Lindberg, S. E., Lowett, G. M., Richter, D. D., & Johnson, D. W. (1986). Atmospheric deposition and canopy interactions of major ions in the forest. Science, 231, 141–145.
Nędzarek, A., & Rakusa-Suszczewski, S. (2007). Nutrients and conductivity in precipitation in The Coast of King George Island (Antarctica) in relation to wind speed and penguin colony distance. Polish Journal of Ecology, 55(4), 705–716.
Norby, R. J. (1989). Foliar nitrate reductase: a marker for assimilation of atmospheric nitrogen oxides. In: Biologic markers of air-pollution stress and damage in forests (pp. 245–250). Washington DC: National Academy Press.
Norby, R. J., Weerasurija, Y., & Hanson, P. J. (1989). Induction of nitrate reductase activity in red spruce needles by NO2 and HNO3 vapor. Canadian Journal of Forest Research, 19, 889–896.
Ochyra, R. (1998). The moss flora of King George Island, Antarctica. Cracow: W. Szafer Institute of Botany, Polish Academy of Science.
Pietr, S. J., Tatur, A., & Myrcha, A. (1983). Mineralization of penguin excrements in the Admirality Bay region (King George Island, Antarctica). Polish Polar Research, 4, 97–112.
Pudełko, R. (2003). Topographic map of the SSSI No. 8, King George Island. Polish Polar Research, 24, 53 60
R Development Core Team (2011). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org/
Rakusa-Suszczewski, S. (1993). The Maritime Antarctic coastal ecosystem of Admiralty Bay. Warsaw: Department Antarctic Biological Polar Acadademical Sciences.
Rakusa-Suszczewski, S. (2003). Functioning of the geoecosystem for the West Side of Admiralty Bay (King George Island, Antarctica): Outline of research at Arctowski Station. Ocean Polar Research, 25, 653–662.
Rankin, A. M., & Wolf, E. W. (2000). Ammonium and potassium in snow around an emperor penguin colony. Antarctic Sciences, 12, 154–159.
Remmler, J. R., & Cambell, W. H. (1986). Regulation of corn leaf nitrate reductase. II >Synthesis and turnover of the enzymes activity and protein. Plant Physiology, 80, 442–447.
Rozema, J. J., Broekman, R., Lud, D., Huiskes, A. H. L., Moerdijk, T. C. V., De Bakker, N., et al. (2001). Consequences of depletion of stratospheric ozone for terrestrial Antarctic ecosystems: the response of Deschampsia antarctica to enhanced UV-B radiation in controlled environment. Plant Ecology, 154, 101–115. ISSN 1385–0237.
Sinha, R. P., Krywult, M., & Haeder, D. P. (1998). Effects of ultraviolet, monochromatic and PAR wavebend on nitrate reductase activity and pigmentation in a rice field cyanobacterium Anabaena sp. Acta Hydrobiologica, 40, 105–112.
Smykla, J., Wołek, J., Barcikowski, A., & Loro, P. (2006). Vegetation patterns around penguin rookeries at Admiralty Bay, King George Island, Maritime Antarctic: Preliminary results. Polish Botanical Studies, 22, 449–458.
Smykla, J., Wołek, J., & Barcikowski, A. (2007). Zonation of vegetation related to penguin rookeries on King George Island, Maritime Antarctic. Arctic, Antarctic, and Alpine Research, 39(1), 143–151.
Sokal, R. R., & Rohlf, F. J. (1995). Biometry: the principles and practice of statistics in biological research. 3rd ed. (p. (887pp.)). New York: W. H. Freeman and Company.
Strohm, T.O. (2007). Growth yields in bacterial denitrification and nitrate ammonification. Applied and Environmental Microbiology, pp. 1420–1424.
Tatur, A., & Myrcha, A. (1984). Ornithogenic soils on King George Island, South Shetland Islands (Maritime Antarctica). Polish Polar Research, 5(1–4), 31–60.
Tatur, A., Myrcha, A., & Niegodzisz, J. (1997). Formation of abandon penguin rookery ecosystems in the maritime Antarctic. Polar Biology, 17, 405–417.
Tatur, A. (2002). Ornithogenic Ecosystems in maritime Antarctic - formation, developmentand disintegration. In L. Beyer & M. Bolter (Eds), Geoecology of Terrestrial Antarctic Ice-Free Coastal Landscapes. Ecological Studies, 154. Berlin: Springer, pp. 161–184.
Xiong, F. S., & Day, T. A. (2001). Effect of solar ultraviolet-B radiation during springtime ozone depletion on photosynthesis and biomass production of Antarctic vascular plants. Plant Physiology, 125, 738–751.
Zhu, R., Liu, Y., Ma, E., Sun, J., Xu, H., & Sun, L. (2009). Greenhouse gas emissions from penguin guanos and ornithogenic soils in coastal Antarctica: Effect of freezing-thawing cycles. Atmospheric Environment, 43, 2336–2347.
Zhu, R., Sun, J., Liu, Y., Gong, Z., & Sun, L. (2011). Potential ammonia emissions from penguin guano, ornithogenic soils and seal colony soils in coastal Antarctica: effects of freezing-thawing cycles and selected environmental variables. Antarctic Science, 23(1), 78–92.
Zubel, P. (2005). Keystone species and ecosystems functioning: the role of penguin colonies in differentiation of the terrestrial vegetation in the Maritime Antarctic. Ecological Questions, 6, 117–128.
Zuniga, G. E., Alberdi, M., & Corcuera, L. J. (1996). Non-structural carbohydrates in Deschampsia antarctica Desv. from South Shetland Islands, Maritime Antarctic. Environmental and Experimental Botany, 36(4), 393–399.
Acknowledgments
The authors owe special thanks to Krystyna Grodzińska, Kazimierz Zarzycki, and Adam Barcikowski, whose ideas initiated this survey, for their generous encouragement and support throughout, without which it would not have been accomplished. Special thanks are also due to all the XXVI "Arctowski" expeditionary team, particularly the leader of this expedition, Paweł Loro, for their logistical aid and comradeship during field studies. The field survey was partly supported by logistical and financial aid from the Department of Antarctic Biology, Polish Academy of Sciences. We also thank Ms. Michele Simmons for English correction of the manuscript. This work was supported by the Polish Ministry of Science and Higher Education within the program “Supporting International Mobility of Scientists” edition III, project No. 2 to JS and grants Nos. 6P04F01820, 2P04F00127, and NN305376438 to MK and JS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krywult, M., Smykla, J. & Wincenciak, A. The Presence of Nitrates and the Impact of Ultraviolet Radiation as Factors that Determine Nitrate Reductase Activity and Nitrogen Concentrations in Deschampsia antarctica Desv. Around Penguin Rookeries on King George Island, Maritime Antarctica. Water Air Soil Pollut 224, 1563 (2013). https://doi.org/10.1007/s11270-013-1563-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1563-8