Abstract
Silicon (Si) is one of beneficial elements for rice and is considered to enhance plant resistance to toxic metals. Nanofertilizers generally have a smaller particle size and specific characters and behaviors in soil and plants. Thus, nano-Si fertilizers may putatively have an advantage over traditional fertilizers in reducing heavy metal accumulation in rice straws and grains, but their effects still require investigation. Here, using a greenhouse pot culture experiment, we studied the effects of foliar application with organic or inorganic nano-Si on growth and yields, and heavy metal accumulation in six rice cultivars grown in soil artificially polluted with Cd, Pb, Cu, and Zn. Generally, hybrid cultivars had higher biomass and yields than conventional cultivars (P < 0.001), and nano-Si showed positive effects on at least four cultivars (P < 0.001). The average spike weight of six cultivars increased to 25.3 and 24.8 %, respectively, by inorganic and organic nano-Si. Hybrid cultivars generally had higher Cd concentrations in roots, shoots, and grains than conventional cultivars. In most cases, both organic and inorganic nano-Si reduced concentrations (P < 0.01) and bio-concentration factor (BCF) of the heavy metals in grains and decreased the translocation factor (TF) of heavy metals from roots to shoots and/or from shoots to grains, and the most pronounced effects were found on Cd. The average grain Cd concentration decreased to 27.1 and 23.8 %, respectively, by inorganic and organic nano-Si. In general, inorganic nano-Si had more pronounced effect than organic nano-Si on both rice growth and heavy metal accumulation. This present study firstly showed that nano-Si had positive effects on the growth and yields of rice grown in multi-metal-polluted soil and potentially reduced heavy metal accumulation in rice, especially the toxic Cd in grains. However, both rice cultivar and chemical form of Si fertilizers should be taken into account to develop efficient nano-Si fertilizers for preventing heavy metal-contaminated rice grains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Harmful effects of toxic heavy metal pollution have been widely recognized. Heavy metal pollution in agricultural fields generally results in soil quality degradation, crop yield reduction, and poor quality of agricultural products and thus poses significant health hazards to human and animals through food chain (Gupta and Gupta 1998; Singh et al. 2011). The farmlands contaminated by various heavy metals have reached 20 Mha in China, and most of them are still cultivated due to the serious cropland shortage (Chen 2007). Rice is the staple food for over half of the global population (Hawksworth 1985) and for about 60 % of the population in China (Yang et al. 2006). Unfortunately, rice has been identified as a particular crop with high heavy metal content in its grains (Chaney et al. 2004; Pan and Gong 2006; Zhao et al. 2010). Certainly, heavy metal accumulation in plants is a function of complex interaction among soil, plant, and environmental factors. Numerous studies have shown that there are remarkable differences in heavy metal accumulation in grains, shoots, and roots of different rice cultivars (Li et al. 2003; Liu et al. 2003, 2013, 2007). A significant genotypic variation in the Cd, Cr, As, Ni, and Pb concentrations in grains of nine rice genotypes was observed (Cheng et al. 2006). Therefore, both cultivar selection and environmental factors should be taken into account to develop effective techniques for preventing heavy metal-contaminated rice grains.
Silicon (Si) has been proved to be beneficial for many plant species, particularly for graminaceous plants, such as rice (Epstein 1999). The role of Si in clean and safe rice production has attracted continuous interest in recent decades. Increasing evidences have shown that Si can enhance the resistance and/or tolerance in rice plants to toxic metals, such as Cd, Pb, Cr, Zn, Al, and As (Gu et al. 2012; Liu et al. 2015; Syu et al. 2015; Tripathi et al. 2012; Wu et al. 2013; Zeng et al. 2011). In recent years, the applications of nanomaterials including nanofertilizers have shown a critical role in global food production, food security, and food safety (Servin et al. 2015). Compared with traditional fertilizers, nanofertilizers may be absorbed by plants more rapidly and completely (Mousavi and Rezaei 2011). The bioavailability of traditional Si fertilizers applied into soil is generally low because they are hardly to dissolute, or easily adsorbed by soil organic matters and minerals (Liu et al. 2009), while the foliar application with nano-Si fertilizers with smaller particle size makes them easily penetrate leaves and form a thick silicated layer on leaf surface, leading to higher utilization by rice plants. Previous studies from our group and others have shown that foliar application with nano-Si significantly increased the dry weight of grains and shoots in rice grown in Cd-contaminated soil while decreased the Cd concentration in the grains and shoots (Liu et al. 2009; Wang et al. 2015). However, only one cultivar and one single metal/silicon fertilizer were involved in these studies. In fact, most polluted farmlands used for rice production in China contain various metals, and a variety of rice cultivars are generally grown. The effects of nano-Si on heavy metal accumulation by different rice cultivars grown in multi-metal-contaminated soil still remain unclear. In the present study, our aims were (1) to investigate the effects of two forms of nano-Si on heavy metal accumulation by different rice cultivars and (2) to compare their ability to accumulate heavy metals of different rice cultivars grown in a multi-metal-contaminated soil.
2 Materials and Methods
2.1 Preparation of Organic and Inorganic Nano-Si Solutions
Two types of nano-Si solutions including inorganic (Si-A) and organic (Si-B) forms were prepared according to the processes (Wang et al. 2015) after slight modification: (1) in Si-A, 0.7166 g Na2SiO3 was firstly dissolved in 475 mL distilled water and then 10 mL ethanol was added and stirred for 0.5 h after mixing. A mixed solution of 10 mL ethanol and 5 mL Tween 80 was slowly dropped into aforementioned solution and then fully stirred for 2 h. (2) In Si-B, 0.55 mL tetraethyl orthosilicate was mixed with 475 mL distilled water and 10 mL ethanol in a beaker and then a mixed solution of 10 mL ethanol and 5 mL Tween 80 was slowly dropped into it and then fully stirred for 2 h. The concentrations of Si in both solutions were 2.5 mM, and the pH values were adjusted to 5.5 using HCl (0.1 M) or NaOH (0.1 M) solutions. The particle sizes of the two types of nano-Si were about 60 nm, determined by a light-scattering size analyzer (Beckman N5, USA). All the solutions were newly prepared at room temperature.
2.2 Soil
Soil samples were collected from a paddy field in Guangdong Province, China. The samples were sieved through a 2-mm sieve and air-dried for 3 days. Then, soil was artificially contaminated with Cu (250 mg kg−1 of soil) as CuSO4, Zn (200 mg kg−1 of soil) as ZnSO4, Pb (100 mg kg−1 of soil) as Pb(NO3)2, and Cd (5 mg kg−1 of soil) as CdCl2·4H2O. Basal fertilizers 100 mg kg−1 N as urea, 80 mg kg−1 P, and 100 mg kg−1 K as KH2PO4 were applied to the soil. After mixing it with heavy metals and fertilizers, the soil was equilibrated for 30 days, undergoing 5 cycles of saturation with deionized water and air-drying. The soil pH was measured by 0.01 mol L−1 CaCl2 at a 1:5 ratio (w/v) using a pH meter. The selected physical and chemical properties (Table 1) were measured by the method from an agricultural and chemical analysis method of soil (Lu 2000).
2.3 Experimental Procedure
Six mostly widely grown cultivars (C-1, C-2, C-3, C-4, C-5, and C-6 representing cultivars Youyou128, Zhongjiuyou207, Peizashuangqi, Zhe9248, TeB, and Yuexiangzhan, respectively) of rice (Oryza sativa L.) provided by the Rice Research Institute of Guangdong Agricultural Academy were used in the present study. The former three cultivars are hybrid cultivars, and the latter three ones are conventional and high-consuming quality cultivars. Uniform seeds were surface-sterilized with 0.5 % NaClO solution, rinsed thoroughly with deionized water, and then placed onto a wet filter paper in a white porcelain dish in the dark at 25 °C for germination. After germination, three seedlings were transplanted in each pot (30 cm i.d. × 45 cm height) filled with 10 kg air-dried soil. Each cultivar had 12 replicates, four for Si-A, four for Si-B, and the other four for the control. All pots were arranged randomly in a non-environment-controlled greenhouse. The pot soil was maintained under flooded conditions (with 2–3 cm of water above soil surface) during the whole growth period.
Two nano-Si solutions (200 mL) were sprayed individually onto the leaves of rice seedlings grown in the artificially contaminated soil three times. The application was carried out at the stage of seedling (7 days after transplanting), tilling (35 days after transplanting), and flowering (70 days after transplanting), respectively. The control plants were sprayed with the same quantity deionized water containing only ethanol and Tween 80.
The shoots, roots, and grains of rice were harvested soon separately after maturity and then washed with tap water and finally with distilled water. The prepared samples were oven-dried at 60 °C for 72 h. The chaff of the grains was removed. The dry samples of shoots, roots, and grains (without husk, the grains here are substantially brown rice) were weighed and then ground in a carnelian mortar for further chemical analysis.
2.4 Sample Analysis
Heavy metal concentrations in plant samples were determined by ICP-AES (Perkin Elmer Optima 3300 DV) after strong acid digestion [4:1 concentrated HNO3 and HClO4 (v/v)].
2.5 Data Analysis
We used either a one-way ANOVA or a two-way ANOVA with the statistical software SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). To compare the significance among all the different treatments, we used Tukey’s multiple-range test (P < 0.05) following one-way ANOVA. To analyze the effect of interactions between cultivars and nano-Si treatments at P < 0.001, 0.01, or 0.05, a two-way ANOVA was used. Pearson correlation coefficients were calculated to evaluate the strength of the relationship between heavy metal concentrations in grains, shoots, and roots.
According to Bose and Bhattacharyya (2008), translocation factor (TF) and bio-concentration factor (BCF) were calculated.
Translocation factor is the ratio of metal concentration in aerial parts and metal concentration in plant root, i.e., TF = C shoot / C root, where C shoot is the concentration in plant’s aerial part and C root is the concentration in plant root.
BCF = Trace element concentration in plant tissues (mg kg−1) at harvest / Initial concentration of heavy metals in soil (mg kg−1).
3 Results
3.1 Plant Biomass and Yield
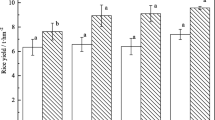
Generally, hybrid cultivars had higher biomass, spike number, and weight than conventional cultivars (Table 2). C-1 had the highest biomass and yield when no nano-Si was applied. Application with different nano-Si showed various effects on different cultivars. Biomass responded positively in C-2, C-3, and C-6 with Si-A and in C-1 with Si-B, while it was not affected in C-4 and C-5 (Table 2). The greatest increment by Si-A was observed in C-2. Both Si-A and Si-B did not change the weight per 100 grains and the spike number of all the cultivars. Spike weight increased in C-2, C-5, and C-6 with Si-A and in C-4, C-5, and C-6 with Si-B, and the average spike weight of six cultivars increased to 25.3 and 24.8 %, respectively, by Si-A and Si-B. On the whole, C-2 was the cultivar with highest biomass and yield and the most positive response to Si-A. Biomass, weight per 100 grains, and spike weight per plant were all significantly affected by cultivar and nano-Si, but only biomass was significantly affected by the interactions between them (Table 2).
3.2 Heavy Metal Concentration in Plants
Cd concentration in roots was higher than that in other tissues, following an order of roots > shoots > grains (Table 3). In most cases, when no nano-Si was applied, hybrid cultivars had higher Cd concentrations in roots, shoots, and grains than conventional cultivars, and C-6 had the lowest Cd concentrations, especially in grains and shoots. Generally, compared with the controls, the application with Si-A and Si-B decreased the grain Cd concentration in most cultivars. The average grain Cd concentration decreased to 27.1 and 23.8 %, respectively, by inorganic and organic nano-Si. Application with Si-A and Si-B decreased the shoot Cd concentrations in conventional cultivars (except C-6 with Si-B), and for hybrid cultivars, significant effects were only observed in C-2 with Si-A and in C-3 with Si-B. However, the application with Si-A and Si-B decreased the root Cd concentrations in all cultivars except C-4, and the effects were more pronounced in hybrid cultivars. Cultivar, nano-Si, and the interactions between them all had significant effects on Cd concentrations in grains, shoots, and roots (Table 3).
The majority of Pb accumulated in roots, and much less was found in shoots and the lowest in grains (Table 4). Without nano-Si application, C-5 had the lowest grain Pb concentration while C-4 had the lowest root Pb concentration. Compared with the controls, significant lower Pb concentrations in grains were observed in C-3, C-4, and C-6 with Si-A and in C-1, C-3, and C-6 with Si-B. Shoot Pb concentrations decreased in C-5 with Si-A or Si-B while increased in C-1, C-2, C-3, and C-4 with Si-B. Root Pb concentrations were significantly higher in all the cultivars (except C-3) applied with Si-B and in C-1, C-2, C-3, and C-6 with Si-A. Cultivar, nano-Si, and the interactions between them all had significant effects on Pb concentrations in grains, shoots, and roots (Table 4).
Like Pb, Cu was accumulated mainly in roots, and much less was found in shoots and grains (Table 5). Without nano-Si application, Cu concentrations in grains and roots were highest in C-2 while lowest in C-6. Compared with the controls, significant lower Cu concentrations in grains were found in C-2 and C-5 with Si-A or Si-B. Shoot Cu concentrations decreased in C-1 with Si-A or Si-B while increased in C-3 with Si-A and did not change significantly in other cultivars. Both Si-A and Si-B had no significant effects on root Cu concentrations in all the cultivars based on one-way ANOVA results. Cultivar, nano-Si, and the interactions between them all had significant effects on Cu concentrations in grains and shoots, while interactions showed no significant effects on root Cu concentrations.
Zn was accumulated mainly in shoots and roots, and much less was present in grains (Table 6). Without nano-Si application, all the cultivars had similar Zn concentrations in grains, while C-1 had the highest Zn concentrations in shoots and the lowest in roots. Compared with the controls, significant lower Zn concentrations in grains were found in C-1 and C-2 with Si-A and in C-3, C-4, C-5, and C-6 with Si-B. In most cases, shoot Zn concentrations increased in hybrid cultivars by Si-A or Si-B but decreased in conventional cultivars. Root Zn concentrations increased in C-1 and C-3 by Si-A or Si-B while decreased in C-5 and C-6. Cultivar, nano-Si, and the interactions between them all had significant effects on Zn concentrations in grains, shoots, and roots (except cultivar on grain Zn) (Table 6).
3.3 Correlation between Heavy Metal Concentration in Grains, Shoots, and Roots
Pearson correlation analysis showed that Cd concentrations in grains correlated positively with those in shoots (P < 0.01, R = 0.781) and in roots (P < 0.01, R = 0.654), while Pb concentrations in grains correlated negatively with those in roots (P < 0.01, R = −0.683). There were significant correlations between Zn concentrations in shoots and in roots (P < 0.05, R = 0.522), while no significant correlations existed between Cu concentrations in grain, shoots, and roots.
The correlation between different heavy metals in the same plant tissue was also calculated. There were positively significant correlations between shoot Cd and Cu concentrations (P < 0.05, R = 0.472), grain Cd and Cu concentrations (P < 0.01, R = 0.736), grain Cd and Zn concentrations (P < 0.01, R = 0.677), and grain Cu and Zn concentrations (P < 0.05, R = 0.536) while negative correlations between root Pb and Cd concentrations (P < 0.05, R = −0.570).
3.4 BCF and TF of Heavy Metals
BCF of Cd, Pb, and Cu in different plant tissues all varied from the order of roots > shoots > grains, while BCF of Zn was highest in shoots, followed by roots and grains sequentially (Table 7). The BCF of heavy metals in grains varied from the order of Cd > Zn ≈ Cu > Pb in an average for nearly all the treatments. The BCF varied from the order Cd > Cu > Zn > Pb in roots, while from the order of Cd > Zn > Cu > Pb in shoots, on average for all treatments. Compared with the controls, the application with Si-A and Si-B both decreased the BCF of the four heavy metals in grains, and the most pronounced effects were observed on Cd. For most cultivars, the BCF of Cd in grains decreased at about 30 % by Si-A or Si-B. Application with Si-A and Si-B also significantly decreased the BCF of Cd in shoots and roots but displayed diverse effects on other heavy metals.
The TF of heavy metals from roots to shoots generally varied from the order of Zn > Cd > Cu > Pb on average (Table 8), while the TF from shoots to grains varied generally from the order of Cu > Pb > Cd > Zn (except for a few exceptions). In most cases, the application with Si-A and Si-B decreased the TF of Pb and Cu from roots to shoots, the TF of Pb and Cd from shoots to grains, and the TF of Zn from shoots to grains in C-1, C-2, C-3, and C-4.
4 Discussion
Numerous studies have found that a wide difference exists among rice cultivars/genotypes in their ability to accumulate heavy metals in grains, shoots, and roots (Cheng et al. 2006; Li et al. 2003; Liu et al. 2003). Our present results confirmed that different cultivars accumulated various amounts of heavy metals in their roots, shoots, and grains, particularly of Pb in roots (with a large coefficient of variation, see Table 4). However, significant genotype-environment interactions of the concentrations of heavy metals existed in grains of rice (Cheng et al. 2006). In addition to the genetic character, environmental factors may alter the availability of heavy metals in soil and the metabolic pattern of plants and thus affect the heavy metal uptake and accumulation in rice. Sometimes, high yields and low toxic metal content cannot be easily achieved simultaneously. For example, the hybrid cultivar Zhongjiuyou207 had the highest biomass and yield but also accumulated the highest Cd in grains (Table 3).
BCF and TF reflect the ability of plants to take up heavy metals from soil and to transport them from roots to aerial parts. The present BCF and TF results showed that among the four heavy metals, Pb was most easily absorbed from soil but most hardly transported from roots to shoots. Consequently, Pb was accumulated mainly in roots, and Pb concentrations in shoots and grains were the lowest compared with other heavy metals. We found significant variations in TF of different cultivars, and the TF of Pb from shoots to grains was larger than the TF from roots to shoots. However, grain Pb concentrations negatively correlated (P < 0.05) with root but not with shoot Pb concentrations. These may indicate the uptake and translocation of Pb may mainly vary with cultivars. The BCF and TF of Cd from roots to shoots and from shoots to grains were overall quite high compared with the other three metals, which can explain why Cd concentration was relatively high in rice grains even though it was low in soil. The easier translocation of Cd within rice plants may be due to the strong affinity of Cd ions for sulfhydryl groups of several compounds and phosphate groups involved in plant metabolism (Pahlsson 1989).
The beneficial role of Si in stimulating the plant growth and development of rice and the protection against the stress of toxic metals have been extensively recognized. The present study firstly confirmed that application with nano-Si (inorganic or organic forms) increased the biomass and yields of four rice cultivars grown under combined pollution of Cd, Pb, Cu, and Zn, and in most cases, the application with Si-A or Si-B reduced the concentrations of heavy metals in rice, especially toxic Cd in grains. These indicate a substantial alleviation of heavy metal toxicity in rice by nano-Si and may provide an effective approach to control toxic metal contents in rice grains.
Interestingly, in most cases, the application with nano-Si all decreased the BCF and TF of heavy metals in rice, especially of Cd, which was in accordance with many results achieved using non-nano-Si (Tripathi et al. 2012; Zeng et al. 2011). In addition to its nutritional function, Si can alleviate toxic metal stress in higher plants via various mechanisms (Liang et al. 2007). Si is a structural component of plants’ cell walls (Epstein 1999). The co-precipitation with Si in apoplast is thought to be responsible for the amelioration of heavy metal toxicity in plants (Horst et al. 1999; Iwasaki et al. 2002). Not like application into soil, the foliar application with Si is unlikely to change the soil characters, so immobilization of metal ions in growth media must not be involved in the present study. The lower root Cd concentration can be partly attributed to the growth promotion effect by nano-Si (Liu et al. 2009). However, the lower shoots/roots and grains/roots Cd ratios cannot be solely explained by “dilution effect.” Root-to-shoot Cd translocation via the xylem is considered the major and common physiological process in determining the Cd accumulation level in shoots and grains of rice plants (Uraguchi et al. 2009). Si significantly reduced the transport of the apoplastic fluorescence tracer PTS (trisodium-8-hydroxy-1,3,6-pyrenesulfonate) from roots to shoots (Shi et al. 2005), suggesting that the deposition of Si in the vicinity of the endodermis maybe partially blocked the apoplast bypass flow across the roots and restrained the apoplastic transport of Cd. Additionally, remobilization of Cd in leaf blades also contributes to grain Cd accumulation (Rodda et al. 2011), and thus, Si sprayed onto leaves may enter leaves and increase Cd sequestration to cell wall and decrease the potential for further redistribution. Excess heavy metals generally cause the formation of reactive oxygen species that damage membrane permeability and function, while Si reduces membrane permeability and improves its structure and stability (Liang et al. 2007), thus limiting the transport of heavy metals.
The application with Si-A and Si-B also decreased the TF of Pb, Cu, and Zn (four cultivars) from roots to shoots and/or from shoots to grains, showing that co-precipitation may be an important mechanism responsible for lower heavy metal concentrations in grains. Si-assisted Zn tolerance of rice was considered mainly due to the reduction of uptake and translocation of excess Zn, and a stronger binding of Zn in the cell wall of less bioactive tissues might also contribute to some degree (Gu et al. 2012). Another study has shown that amendment with calcium silicate can restrain the transfer of Cu from rice root to stem (Li et al. 2009). Therefore, although Cu and Zn are essential elements required for plants, the mechanisms of Si in alleviating their toxicity in rice may be similar when they are in excess demand.
We also found that nano-Si was more effective in reducing Cd than other metals in grains. Obviously, Cd, Pb, Cu, and Zn may differ from each other in their uptake and transport by rice plants. For example, Cd had higher BCF and TF from roots to shoots and from shoots to grains than the other three metals and thus might co-precipitate with Si more easily, which may explain its most pronounced response to nano-Si. On the other hand, Cd, Pb, Cu, and Zn are all absorbed by plants as divalent cations and the co-precipitation site with Si may be also similar, and thus, the competition between them is likely to take place during their uptake and translocation under nano-Si treatment. Actually, a negative correlation between Pb and Cd content in roots was observed in our results. However, positive correlations existed between grain Cd, Cu, and Zn contents. Thus, Si may produce different effects because of the complicated interactions between them, which requires further investigation.
Overall, Si-A had a more pronounced effect than Si-B on rice biomass and yields. This may be explained by that inorganic Si is more easily absorbed and utilized by rice than the organic form. Two-way ANOVA results show a significant interaction between cultivar and nano-Si on heavy metal accumulation, confirming variations exist in the effects of nano-Si on different cultivars. Not surprisingly, different cultivars differ in their requirement and utilization of Si and thus respond differently to Si supply. Undoubtedly, when developing an approach to control heavy metals in grains, genetic characteristics of rice cultivars must be taken into account.
Because of their smaller size and specific characters and behaviors in soil and plants, nano-Si fertilizers may have an advantage over traditional fertilizers in improving crop yields and quality. In our present results, the foliar application with nano-Si had a positive role both in improving rice growth and in reducing heavy metal contents in grains, indicating a promising use in future rice production. We have found various mechanisms such as decreased heavy metal accumulation and partitioning in shoots, stimulated antioxidant systems, increased GSH content, and photosynthesis rate in the rice plants applied with nano-Si (Wang et al. 2015); however, so far, very little has been known on the application and molecular mechanisms of nano-Si fertilizers in plant growth and resistance, which need more comprehensive greenhouse and field studies.
5 Conclusion
Here, our present results provided the first evidence that foliar application with nano-Si increased the biomass and yields of rice grown in soil polluted with Cd, Pb, Cu, and Zn and reduced the concentrations of heavy metals in rice, especially the toxic Cd in grains. In most cases, both inorganic and organic nano-Si reduced concentrations and BCF of the heavy metals in grains and decreased the TF of heavy metals from roots to shoots and/or from shoots to grains, and the most pronounced effects were found on Cd. The co-precipitation with Si in the apoplast may be the main mechanism responsible for the alleviation of heavy metal toxicity to rice plants and the reduction of accumulation in grains. This may present an effective approach using nano-Si fertilizer to control toxic metal content in rice grains.
References
Bose, S., & Bhattacharyya, A. (2008). Heavy metal accumulation in wheat plant grown in soil amended with industrial sludge. Chemosphere, 70(7), 1264–1272.
Chaney, R. L., Reeves, P. G., Ryan, J. A., Simmons, R. W., Welch, R. M., & Angle, J. S. (2004). An improved understanding of soil Cd risk to humans and low cost methods to phytoextract Cd from contaminated soils to prevent soil Cd risks. Biometals, 17(5), 549–553.
Chen, J. (2007). Rapid urbanization in China: a real challenge to soil protection and food security. Catena, 69(1), 1–15.
Cheng, W. D., Zhang, G. P., Yao, H. G., Wu, W., & Xu, M. (2006). Genotypic and environmental variation in cadmium, chromium, arsenic, nickel, and lead concentrations in rice grains. Journal of Zhejiang University. Science. B, 7(7), 565–571.
Epstein, E. (1999). Silicon. Annual Review of Plant Biology, 50(1), 641–664.
Gu, H. H., Zhan, S. S., Wang, S. Z., Tang, Y. T., Chaney, R. L., Fang, X. H., Cai, X. D., & Qiu, R. L. (2012). Silicon-mediated amelioration of zinc toxicity in rice (Oryza sativa L.) seedlings. Plant and Soil, 350(1-2), 193–204.
Gupta, U. C., & Gupta, S. C. (1998). Trace element toxicity relationships to crop production and livestock and human health: implications for management. Communications in Soil Science & Plant Analysis, 29(11-14), 1491–1522.
Hawksworth, D. (1985). Rice diseases. UK: CMI Slough, CAB.
Horst, W. J., Fecht, M., Naumann, A., Wissemeier, A. H., & Maier, P. (1999). Physiology of manganese toxicity and tolerance in Vigna unguiculata (L.) Walp. Journal of Plant Nutrition and Soil Science, 162(3), 263–274.
Iwasaki, K., Maier, P., Fecht, M., & Horst, W. J. (2002). Effects of silicon supply on apoplastic manganese concentrations in leaves and their relation to manganese tolerance in cowpea (Vigna unguiculata (L.) Walp.). Plant and Soil, 238(2), 281–288.
Li, P., Wang, X. X., Zhang, T. L., Zhou, D.M., & He, Y. Q. (2009). Distribution and accumulation of copper and cadmium in soil–rice system as affected by soil amendments. Water, Air, Soil & Pollution, 196(1-4), 29–40.
Li, Z., Zhang, Y. L, Pan, G., Li, J., Huang, X., & Wang, J. (2003). Grain contents of Cd, Cu and Se by 57 rice cultivars and the risk significance for human dietary uptake. Environmental Science (China), 24(3), 112-115. (in Chinese)
Liang, Y., Sun, W., Zhu, Y. G., & Christie, P. (2007). Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environmental Pollution, 147(2), 422-428.
Liu, C. P., Li, F. B., Luo, C. L., Liu, X. M., Wang, S. H., Liu, T. X., & Li, X. D. (2009). Foliar application of two silica sols reduced cadmium accumulation in rice grains. Journal of Hazardous Materials, 161(2-3), 1466–1472.
Liu, J., Cai, H., Mei, C., & Wang, M. (2015). Effects of nano-silicon and common silicon on lead uptake and translocation in two rice cultivars. Frontiers of Environmental Science & Engineering, 9(5), 905–911.
Liu, J., Li, K., Xu, J., Zhang, Z., Ma, T., Lu, X., Yang, J., & Zhu, Q. (2003). Lead toxicity, uptake, and translocation in different rice cultivars. Plant Science, 165(4), 793–802.
Liu, J., Ma, X., Wang, M., & Sun, X. (2013). Genotypic differences among rice cultivars in lead accumulation and translocation and the relation with grain Pb levels. Ecotoxicology and Environmental Safety, 90, 35–40.
Liu, J., Qian, M., Cai, G., Yang, J., & Zhu, Q. (2007). Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. Journal of Hazardous Materials, 143(1), 443–447.
Lu, R. (2000). Analysis methods of soil agricultural chemistry. Beijing: China Agricultural Science and Technology Press (in Chinese).
Mousavi, S. R., & Rezaei, M. (2011). Nanotechnology in agriculture and food production. Journal of Applied Environmental and Biological Sciences, 1(10), 414–419.
Pahlsson, A. M. B. (1989). Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants: a literature review. Water, Air, & Soil Pollution, 47(3-4), 287–319.
Pan G, Gong W. (2006). Issues of grain Cd uptake and the potential health risk of rice production sector of China. Science & Technology Review, 24(5), 43-48. (in Chinese)
Rodda, M. S., Li, G., & Reid, R. J. (2011). The timing of grain Cd accumulation in rice plants: the relative importance of remobilisation within the plant and root Cd uptake post-flowering. Plant and Soil, 347(1-2), 105–114.
Servin, A., Elmer, W., Mukherjee, A., De la Torre-Roche, R., Hamdi, H., White, J. C., Bindraban, P., & Dimkpa, C. (2015). A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. Journal of Nanoparticle Research, 17(2), 92.
Shi, X., Zhang, C., Wang, H., & Zhang, F. (2005). Effect of Si on the distribution of Cd in rice seedlings. Plant and Soil, 272(1-2), 53–60.
Singh, R., Gautam, N., Mishra, A., & Gupta, R. (2011). Heavy metals and living systems: an overview. Indian Journal of Pharmacology, 43(3), 246.
Syu, C. H., Huang, C. C., Jiang, P. Y., Chien, P. H., Wang, H. Y., Su, J. Y., & Lee, D. Y. (2015). Effects of foliar and soil application of sodium silicate on arsenic toxicity and accumulation in rice (Oryza sativa L.) seedlings grown in As-contaminated paddy soils. Soil Science and Plant Nutrition, 61(1), 1–10.
Tripathi, D. K., Singh, V. P., Kumar, D., & Chauhan, D. K. (2012). Rice seedlings under cadmium stress: effect of silicon on growth, cadmium uptake, oxidative stress, antioxidant capacity and root and leaf structures. Chemistry and Ecology, 28(3), 281–291.
Uraguchi, S., Mori, S., Kuramata, M., Kawasaki, A., Arao, T., & Ishikawa, S. (2009). Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. Journal of Experimental Botany, 60(9), 2677–2688.
Wang, S. H., Wang, F. Y., & Gao, S. C. (2015). Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environmental Science and Pollution Research, 22(4), 2837–2845.
Wu, J. W., Shi, Y., Zhu, Y. X., Wang, Y. C., & Gong, H. J. (2013). Mechanisms of enhanced heavy metal tolerance in plants by silicon: a review. Pedosphere, 23(6), 815–825.
Yang, Q., Lan, C., Wang, H., Zhuang, P., & Shu, W. (2006). Cadmium in soil–rice system and health risk associated with the use of untreated mining wastewater for irrigation in Lechang, China. Agricultural Water Management, 84(1), 147–152.
Zeng, F. R., Zhao, F. S., Qiu, B. Y., Ouyang, Y. N., Wu, F. B., & Zhang, G. P. (2011). Alleviation of chromium toxicity by silicon addition in rice plants. Agricultural Sciences in China, 10(8), 1188–1196.
Zhao, K. L., Liu, X. M., Xu, J. M., & Selim, H. M. (2010). Heavy metal contaminations in a soil-rice system: identification of spatial dependence in relation to soil properties of paddy fields. Journal of Hazardous Materials, 181(1-3), 778–787.
Acknowledgments
This work was sponsored by the National Natural Science Foundation of China (41471395, 41171369), the Plan For Scientific Innovation Talent of Henan Province (154100510010), and the Innovation Team Foundation of Henan University of Science and Technology (2015TTD002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Wang, F., Gao, S. et al. Heavy Metal Accumulation in Different Rice Cultivars as Influenced by Foliar Application of Nano-silicon. Water Air Soil Pollut 227, 228 (2016). https://doi.org/10.1007/s11270-016-2928-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2928-6