Abstract
Nano-silicon (Si) may be more effective than regular fertilizers in protecting plants from cadmium (Cd) stress. A field experiment was conducted to study the effects of nano-Si on Cd accumulation in grains and other organs of rice plants (Oryza sativa L. cv. Xiangzaoxian 45) grown in Cd-contaminated farmland. Foliar application with 5~25 mM nano-Si at anthesis stage reduced Cd concentrations in grains and rachises at maturity stage by 31.6~64.9 and 36.1~60.8%, respectively. Meanwhile, nano-Si application significantly increased concentrations of potassium (K), magnesium (Mg), and iron (Fe) in grains and rachises, but imposed little effect on concentrations of calcium (Ca), zinc (Zn), and manganese (Mn) in them. Uppermost nodes under panicles displayed much higher Cd concentration (4.50~5.53 mg kg−1) than other aerial organs. After foliar application with nano-Si, translocation factors (TFs) of Cd ions from the uppermost nodes to rachises significantly declined, but TFs of K, Mg, and Fe from the uppermost nodes to rachises increased significantly. High dose of nano-Si (25 mM) was more effective than low dose of nano-Si in reducing TFs of Cd from roots to the uppermost nodes and from the uppermost nodes to rachises. These findings indicate that nano-Si supply reduces Cd accumulation in grains by inhibiting translocation of Cd and, meanwhile, promoting translocation of K, Mg, and Fe from the uppermost nodes to rachises in rice plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence of high levels of heavy metals in the environment is a potential threat to human health and ecosystems (Goix et al. 2014; Leveque et al. 2014; Uzu et al. 2011). Cadmium (Cd) is one of the most toxic heavy metals (ATSDR 2011). Cd ion tends to accumulate in rice plants even at a relatively low content in soil (Cao et al. 2014; Wei and Yang 2010; Liu et al. 2017). Most of Cd ions in rice grains are remobilized from vegetative organs during grain-filling period via the phloem and xylem in rice plants (Kobayashi et al. 2013; Tanaka et al. 2007; Uraguchi et al. 2009). Cd ions are translocated from roots to the upper nodes of shoots and finally into rachises of rice panicles (Fujimaki et al. 2010). A lot of Cd and other elements are enriched in rachises of rice panicles, and low-Cd-accumulation cultivars generally display lower Cd content in rachises and grains than those of high-Cd-accumulation cultivars (Liu et al. 2017). When low-affinity cation transporter (OsLCT1) in the uppermost nodes and leaf blades of rice plants was knockdown, the knockdown plants accumulated much lower Cd in the grains than the control plants (Uraguchi et al. 2011). Therefore, intervascular Cd transfer at the uppermost node appears to be critical for grain Cd accumulation. Improving the inhibition capacity of the uppermost node to Cd ions based on agronomic management practices and genetic manipulation is the most promising approach to produce rice grains with permissible Cd content in contaminated farmland.
Silicon (Si) is the second most abundant element in soils and many plants are able to accumulate high concentration of Si in shoots. High silica contents in plant tissues activate physical and biochemical defense mechanisms for increased stress tolerance (Adrees et al. 2015; Wang et al. 2015). Cd toxicity in rice plants could be alleviated by the presence of Si in the cell walls and in the readjustment of cell redox homeostasis (Dehghanipoodeh et al. 2016; Farooq et al. 2016; Liu et al. 2009; Sharma and Dietz 2009; Tripathi et al. 2012). A hemicellulose-bound form of Si with net negative charges was suggested to be responsible for inhibition of Cd uptake in rice cells (Ma et al. 2015). In addition to absorption of Si from soil solution around root system, rice plants are able to take in Si by leaves. Adsorption and internalization of nutrients via the cuticle of plant leaves and penetration of nutrients via stomatal pores in leaves are the two major steps involved in foliar nutrient uptake (Shahid et al. 2017). Moreover, surface treatments such as Si can modify the photosynthetic characteristics of plants (Dehghanipoodeh et al. 2016; Maghsoudi et al. 2016).
Nanotechnology in agriculture and food production is extensively studied in recent years. Nanofertilizers may be absorbed by plants rapidly and completely (Deshpande et al. 2017; Mousavi and Rezaei 2011). Foliar application with nano-Si significantly decreased Cd accumulation in grains and shoots in rice plants under Cd stress (Liu et al. 2009; Wang et al. 2015). Si addition improved plant nutrition, enhanced nutrition use efficiency, but inhibited Cd transport from roots to shoots (Liu et al. 2013; Li et al. 2013; Wang et al. 2015). Coprecipitation of Cd with Si in metabolically less active tissues, especially in the endodermis cell wall, pericycle, xylem, and phloem, is likely to block the uptake and transport of Cd (Adrees et al. 2015; da Cunha and do Nascimento 2009; Shi et al. 2005). In addition, Si increases the binding of Cd to cell wall and compartmentation of more Cd into vacuoles (Rogalla and Romheld 2002; Shi et al. 2005), which also limits Cd mobility in plant tissues.
Although many studies showed that Si reduced the uptake and accumulation of Cd in the shoots of rice, the efficiency of foliar application with nano-Si to inhibit Cd accumulation in grains of rice plants under field environment was unclear. In the present study, solutions with different dosage of nano-Si were prepared and sprayed onto the leaves of rice plants grown in Cd-contaminated field. Concentrations of Cd and six other elements in grains, rachises, and other vegetative organs were determined. Possible mechanism of nano-Si to reduce Cd accumulation in grains was analyzed.
Materials and methods
Plant materials and experiment design
The field trials were carried out in northeastern part of Hunan province, China (N: 28° 42′, E: 112° 51′). A paddy rice field with average Cd concentration of 0.79 mg kg−1 in winter of 2015 was selected to represent medium Cd-contaminated alluvial soils along Xiangjiang River. The fundamental chemical properties of the topsoil (0~20 cm) at the maturity stage of rice plants in middle July 2016, were pH 5.51, organic matter content 41.54 g kg−1, cation exchange capacity 19.55 cmol kg−1, and Cd content 0.69 g kg−1 (Table 1). The seeds of rice (Oryza sativa L. cv. Xiangzaoxian 45) were provided by Xiangyin Seed Company, Hunan. Seeds were planted in March and seedlings were transplanted in late April. A randomized block design with three replications was constructed in the field. The plot area was 10.0 m2 with 4.0-m length and 2.5-m width. Field managements were as same as those used in local production. Weeds were controlled by chemical herbicide treatment.
In the middle of June 2016 when most tillers of rice plants were flowering, 40 mL nano-Si solution with 250, 500, and 1250 mM Si was diluted with 2 L water in the field. Three kinds of nano-Si solutions with 5, 10, or 25 mM Si were individually spread on the leaf surface of rice plants in each plot with a hand-held sprayer bottle. The control plants were sprayed with 2 L water from a local well. At maturity stage, three plants with roots and topsoil (0–20 cm) were dug out by a shovel from the center of each plot. After naturally dried at ambient temperature, topsoil was separated with roots manually. Then, harvested plants were separated into grains; rachises; flag leaves including sheathes, uppermost nodes (first nodes), and internodes (first internodes); second leaves, second nodes, and internodes; lower part of stalks; and roots. These tissues were washed with deionized water three times and then dried for 72 h at 70 °C.
Preparation of nano-silicon
Nano-Si was prepared using Na2SiO3 according to the published methods (Liu et al. 2009; Wang et al. 2015) with slight modification. Briefly, 35.53 g Na2SiO3·9H2O was dissolved in 475 mL distilled water, then 10 mL ethanol was added and stirred for 0.5 h after mixing. A mixed solution of 10 mL ethanol and 5 mL Tween 80 was slowly dropped into the abovementioned solution and then stirred for 2 h to obtain 250 mM Si. Nano-Si solutions with 500 or 1250 mM Si were prepared according to the same method. The average diameter of the nano-Si was in the range of 60~100 nm, which was determined by a Nano-Laser Particle Sizer (Winner 800, Jinan, China). All the solutions were freshly prepared at room temperature.
Measurement of Cd, K, Ca, Mg, Zn, Fe, and Mn concentrations
Soil and plant samples were prepared according to methods described as before (Liu et al. 2017). Each soil sample was ground and filtered by a sieve with 100 meshes. About 0.20 g soil was weighed and put into a Teflon beaker and then digested by concentrated HNO3, hydrofluoric acid, and 30% H2O2 (v:v:v was 7:2:1) using a digestion instrument DigiBloc ED54 (LabTech, Beijing, China). Plant samples were cut into small pieces. About 0.50 g plant tissues were digested by concentrated HNO3 and 30% H2O2 (v:v was 7:1). Concentrations of Cd, K, Ca, Mg, Fe, Zn, and Mn in the different soil and plant samples were determined by using inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500a, USA).
Statistical analysis
One-way ANOVA with multiple comparisons by Duncan’s test (P < 0.05) was employed to evaluate differences among treatments. Translocation factor (TF) was calculated from the ratio of elemental concentrations in different parts of the plant (Shi et al. 2015). Two-way ANOVA was used to analyze the significance of correlation coefficient between Cd concentrations in different organs at P < 0.05 or 0.01.
Results
Effects of nano-Si on Cd concentrations in different organs of rice plants
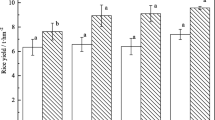
There were great differences in Cd concentrations among different organs of rice plants. The highest Cd concentration was found in roots, which was about 7.0 mg kg−1. Among aerial organs of above ground, the uppermost nodes displayed highest Cd concentration (4.5~5.5 mg kg−1). Cd concentration in grains was much lower than that in rachises, and Cd in internodes was generally lower than that in nodes (Fig. 1). Foliar application with 5.0~25.0 mM nano-Si efficiently reduced Cd concentrations in grains and rachises by 31.6~64.9 and 36.1~60.8%, respectively. Inhibition efficiency of nano-Si with 25.0 mM on Cd accumulation in grains was significantly greater than that of nano-Si with 5.0~10 mM (Fig. 1a). High concentration of nano-Si even inhibited Cd accumulation in flag leaves. After foliar application with 25.0 mM nano-Si, Cd concentrations in flag leaves, uppermost internodes, and nodes were significantly decreased by 27.4, 20.3, and 18.6%, respectively (Fig.1b). However, Cd concentrations in roots, lower part of stalks, second leaves, and nodes were not influenced by nano-Si application.
Effect of nano-silicon on Cd concentrations in grains and rachises (a); flag leaves, uppermost internodes, and nodes (b); second leaves, second internodes, and nodes (c); and lower part of stalks and roots (d) of rice plants grown in Cd-contaminated field. Si0, Si1, Si2, and Si3 represent treatments of foliar application with 0, 5, 10, and 25 mM nano-silicon at anthesis stage, respectively. Different letters above columns indicate significant difference at P < 0.05 among treatments
Effects of nano-Si on concentrations of essential elements in grains and rachises
Concentrations of some essential nutrients (K, Mg, Ca, Mn, Fe, Zn) in grains and rachises of rice plants were measured in this experiment. Results showed that application of the nano-Si had significant influence on macro-elements but little effect on micro-elements in grains and rachises. Potassium was the most abundant cation in rachises and grains of rice plants (Table 2). Concentration of K+ in rachises was in the range of 14.16~24.50 g kg−1. Only a small portion of K+ was transported into grains. Magnesium was the second abundant cation in rachises and grains. Rice plants accumulated greatly higher concentration of Mg2+ in grains (1.14~1.57 g kg−1) than that in rachises (0.66~1.45 g kg−1). Concentrations of Ca2+, Mn2+, Fe2+, and Zn2+ were in the range of 25.35~88.61 mg kg−1 in rice grains, which were much lower than the concentrations of K+ and Mg2+. Concentrations of Ca2+, Mn2+, Fe2+, and Zn2+ in grains were greatly lower than that in rachises. Foliar application with 5~25 mM nano-Si caused a significant increase of K+ concentration in grains by 13.24~32.72% and in rachises by 24.93~73.02%. Concentrations of Mg2+ were also increased by 8.06~26.61% in grains and by 18.18~119.70% in rachises. Among the micro-elements, only Fe2+ concentrations were increased by 14.24~50.77% in grains and by 7.31~56.41% in rachises after foliar application with 5~25 mM nano-Si. Foliar nano-Si supply imposed very little effects on the accumulation of Ca2+, Mn2+, and Zn2+ in rice grains and rachis.
Effects of nano-Si on the distribution of elements in rice plants
Distribution of K, Mg, Fe, and Cd in organs was apparently different and influenced by foliar application with nano-Si (Fig. 2). When rice plants were grown in Cd-contaminated field, Cd ions were greatly enriched in roots. Among aerial organs, nodes accumulated much higher Cd than internodes and leaves (Fig. 2). Each essential element displayed its specific distribution model. For example, the highest concentration of K was found in the uppermost internodes, and the highest Mg was in second leaves under flag leaves. Concentration of Fe in rice roots was as high as 74.12~78.60 g kg−1, but only in the range of 0.11~0.93 g kg−1 in the organs of stalks. Foliar application with nano-Si promoted the accumulation of K and Mg in grains and rachises significantly. Accumulation of Fe in grains and rachises was promoted only by high concentration of nano-Si (25 mM). Meanwhile, Cd accumulation in grains and rachises was significantly inhibited. High concentration of nano-Si displayed greater influence on the distribution of K, Mg, Fe, and Cd in grains and rachises than low concentration of nano-Si. Concentrations of K, Mg, and Fe in flag leaves, first internodes, and first nodes were not influenced by nano-Si supply, but concentrations of Cd in these organs decreased by 25 mM nano-Si supply. Cd concentration in grains was significantly correlated with Cd in rachises, r = 961**. Foliar application with nano-Si at anthesis stage had little effect on the accumulation of these ions in organs below the uppermost node.
Distribution model of Ca in vegetative organs was different from that of Mn and Zn. Leaves and lower part of stalks accumulated much more Ca than other organs. High concentration of Mn was found in second leaves, but high concentration of Zn was found in the uppermost and second internodes (Table S1). Foliar application with nano-Si had little effects on their distribution in vegetative organs.
Effects of nano-Si on translocation factors of elements in rice plants
Concentration of Cd ions in organs reflects their remobilization potential from one organ to the others. TFs were calculated from the ratio of Cd concentrations in different organs of rice plants. TFs of Cd ions from rachises to grains (TFs(Grains/Rachises)) were greatly lower than TFs from the uppermost nodes to rachises (TFs(Rachises/Nodes)) and TFs from roots to the uppermost nodes (TFs(Nodes/Roots)) (Fig. 3a). Foliar application with nano-Si had little effect on the TFs of Cd from rachises to grains, but significantly decreased TFs of Cd from the uppermost nodes to rachises as well as from roots to the uppermost nodes. TF(Nodes/Roots) of K was as high as 13.20, but TFs(Grains/Rachises) and TFs(Rachises/Nodes) of K were only in the range of 0.15~0.51 (Fig. 3b). Nano-Si supply significantly increased TFs of K from the uppermost nodes to rachises, but had little effect on TFs(Grains/Rachises) and TFs(Nodes/Roots). TFs(Grains/Rachises) of Mg significantly decreased with doses of nano-Si supply (Fig. 3c). However, foliar application with nano-Si increased TFs(Rachises/Nodes) of Mg but had little effect on TFs(Nodes/Roots) of Mg. Most of Fe were remained in root tissues and TFs of Fe between different organs were less than 0.33 (Fig. 3d). Nano-Si supply increased TFs(Rachises/Nodes) of Fe on a small scale. TFs of Ca, Mn, and Zn from rachises to grains were in the range of 0.19~0.50. Foliar supply with nano-Si had little effects on TFs of Ca, Mn, and Zn between different organs.
Effects of nano-Si on translocation factors (TFs) of Cd (a), K (b), Mg (c), and Fe (d) between grains, rachises, uppermost nodes, and roots of rice plants. Si0, Si1, Si2, and Si3 represent foliar application with 0, 5, 10, and 25 mM nano-Si, respectively. Different letters denote significant difference among treatments (P < 0.05)
Discussion
About 60~90% of total carbon in the rice panicles at harvest is derived from photosynthesis after heading, while 80% or more of nitrogen in the panicles at harvest is absorbed before heading and remobilization from top three leaves (Mae 1997; Zhang et al. 2003). Other nutrients stored in vegetative organs are also remobilized to flow into grains (Bahrani and Joo 2010; Zhao et al. 2006). Rachis is the organ to connect stem with grains. Nutrients in leaves and roots are finally transported to the developing grains through rachises in panicles of rice. High Cd concentration in rachises is closely correlated with high Cd in rice grains (Liu et al. 2017). Therefore, conjoint effects of foliar fertilizers on the photosynthetic characteristics and water using efficiency will certainly influence the Cd concentration in rachises. Results in this experiment proved that the concentrations of Cd and other elements in rachises of rice plants were significantly affected by foliar application with nano-Si at anthesis stage.
Foliar application of macro- and micro-nutrients has been proven beneficial to plant development. Many factors such as plant morphological characteristics and meteorological conditions affect the uptake of nutrients with foliar application (Shahid et al. 2017). Some leaf characteristics such as stomatal density, roughness, and epicuticular waxes may affect deposition of heavy metals on leaf surface. Apparently, nanometer scale particles have more chance to penetrate into leaf tissues. After nano-Si was sprayed onto rice leaves at the anthesis stage, Cd content in both grains and rachises at maturity stage was significantly decreased (Fig. 1). It indicates that foliar application with nano-Si can inhibit the movement of Cd from vegetative organs to panicles of rice plants. Unlike application into soil, foliar application with nano-Si directly increases the content of Si in leaf tissues. High Si concentration may promote the coprecipitation of Cd with Si in metabolically less active tissues, especially in the endodermis cell wall, pericycle, xylem, and phloem (Adrees et al. 2015; da Cunha and do Nascimento 2009; Shi et al. 2005). As a result, foliar Si supply ameliorates Cd toxicity by immobilization of Cd ions in leaves and stalks in rice plants.
There are complicated interactions between Cd and plant nutrients. Many membrane transporters and channels are permeable to many essential cations like Ca2+, K+, Mg2+, and unessential Cd2+ as well (Pinto and Ferreira 2015; Sarwar et al. 2010). Cd could interfere with plant growth and cause cell death by inhibiting uptake of essential nutrients. Improving mineral nutrition is one of the roles for Si to alleviate Cd toxicity in rice plants (Tripathi et al. 2012; Farooq et al. 2016; Wang et al. 2015). Our previous study found that rice grains accumulated a lot of K and Mg. The concentration of K was in the range of 0.73~3.84 g kg−1 and Mg in the range of 0.47~5.34 g kg−1 in rice grains, depending on cultivars and planting sites (Liu et al. 2017). In this experiment, concentrations of K+ increased from 2.72 to 3.61 g kg−1 and Mg2+ from 1.24 to 1.57 g kg−1 in grains after foliar application with nano-Si at anthesis stage. However, Si supply increased Fe2+ content in rice grains on a small scale (3.61~12.87 mg kg−1) and imposed very little effects on the accumulation of Ca2+, Mn2+, and Zn2+ in rice grains. It means that Si, as a beneficial plant nutrient, primarily promotes the accumulation of macronutrients in grains. The enhancement effects of Si on the accumulation of K and Mg in rachises seems greater than that in grains, as their translocation factors from rachises to grains decrease with Si supply.
Grain Cd are determined by both xylem-mediated root-to-shoot Cd translocation and phloem-mediated Cd transport in rice (Uraguchi et al. 2009; Kato et al. 2010). Shoot nodes play an important role in transferring Cd from the xylem to the phloem (Fujimaki et al. 2010). Transporters are crucial for the redirection of some minerals at nodal regions (Yamaji and Ma 2009; Kato et al. 2010). For example, the knockdown plants of a low-affinity cation transporter gene (OsLCT1), which was high expressed in nodes during the grain-ripening stage, accumulated approximately half as much Cd in the grains as did the control plants (Uraguchi et al. 2011). In this experiment, we found the Cd concentration in the uppermost node was as higher as 6.0 mg kg−1, which was more than five times higher than those in leaves and internodes. After redistribution of Cd in the uppermost nodes, Cd concentration in the uppermost internodes reduced to about 1.0 mg kg−1. Foliar application with nano-Si did not change the Cd concentration in the uppermost nodes and internodes, but significantly reduced the Cd concentrations in rachises and grains. It indicates that Si supply primarily inhibits Cd translocation from the uppermost internodes to rachises and from rachises to grains. Si from foliar application at anthesis stage may efficiently inhibit the remobilization of Cd by binding of Cd to ligands in the cell walls of the uppermost nodes and by inducing the lower expression of Cd-related transporters in the uppermost internodes and rachises (Ma et al. 2015, 2017).
Foliar-applied nutrients penetrate the cuticle into leaf free space from where they may undergo selective phloem loading stage followed by long-distance transport inside the plants (Shahid et al. 2017). Dosage of nutrients sprayed on the leaves, surface texture of leaves, and environmental conditions are the predominant factors influencing nutrient uptake by foliar pathways. With the increase of Si content in leaf cells, Si is capable of forming unstable silicates with Cd ions in the cytoplasm or inhibits Cd activity via the apoplastic pathway by covalently bonding with Cd ions in the cell walls as they diffuse through the cell walls and extracellular spaces (Adrees et al. 2015). In this experiment, Cd concentrations in grains and rachises were negatively associated with Si doses sprayed on leaves. When high concentration of nano-Si (25 mM) was applied by foliar pathway, Cd concentrations in flag leaves, uppermost internodes, and nodes were also significantly reduced. This phenomenon may have resulted from the Si deposition underneath leaf cuticles. Si can modulate the formation of subcuticular double layer and reduce water loss by transpiration of leaves (Lux et al. 2002; Hattori et al. 2005; Maghsoudi et al. 2016). Decline of transpiration of flag leaves during grain-filling period would reduce the symplastic transport of Cd ions from lower parts of plants into flag leaves.
Conclusions
The present study demonstrates that foliar application with nano-Si can significantly inhibit Cd accumulation in grains and rachises of rice plants grown in Cd-contaminated farmland. The uppermost nodes can accumulate more than seven times higher Cd than rice grains. Translocation of Cd ions from roots to the uppermost nodes, and from the uppermost nodes to rachises is greatly inhibited with nano-Si supply. Nano-Si can efficiently promote translocation of K, Mg, and Fe from the uppermost nodes to rachises, but has little effect on remobilization of Ca, Zn, and Mn in rice plants. High dose of nano-Si is more effective than low dose of nano-Si in inhibiting remobilization of Cd ions from vegetative organs to rachises during grain-filling period. Therefore, nano-Si has the potential to be developed into a foliar fertilizer for the control of Cd accumulation in rice grains.
References
Adrees M, Ali S, Rizwan M, Zia-Ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK (2015) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf 119:186–197
ATSDR (2011) 2011 priority list of hazardous substances that will be the subject of toxicological profiles (detailed data table). Agency for Toxic Substances and Disease Registry, Atlanta
Bahrani A, Joo MH (2010) Flag leaf role in N accumulation and remobilization as affected by nitrogen in a bread and durum wheat cultivars. Am J Agric Environ 8:728–735
Cao F, Wang R, Cheng W, Zeng F, Ahmed IM, Hu X, Zhang G, Wu F (2014) Genotypic and environmental variation in cadmium, chromium, lead and copper in rice and approaches for reducing the accumulation. Sci Total Environ 496:275–281
da Cunha KPV, do Nascimento CWA (2009) Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water Air Soil Pollut 197:323–330
Dehghanipoodeh S, Ghobadi C, Baninasab B, Gheysari M, Bidabadi SS (2016) Effects of potassium silicate and nanosilica on quantitative and qualitative characteristics of a commercial strawberry (fragaria × ananassa cv.‘camarosa’). J Plant Nutr 39:502–507. https://doi.org/10.1080/01904167.2015. 1086789
Deshpande P, Dapkekar A, Oak MD, Paknikar KM, Rajwade JM (2017) Zinc complexed chitosan/TPP nanoparticles: a promising micronutrient nanocarrier suited for foliar application. Carbohydr Polym 165:394–401
Farooq MA, Detterbeck A, Clemens S, Dietz K-J (2016) Silicon-induced reversibility of cadmium toxicity in rice. J Exp Bot 67:3573–3585
Fujimaki S, Suzui N, Ishioka NS, Kawachi N, Ito S, Chino M, Nakamura S (2010) Tracing cadmium from culture to spikelet: noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol 152:1796–1806
Goix S, Lévêque T, Xiong TT, Schreck E, Baeza-Squiban A, Geret F, Uzu G, Austruy A, Dumat C (2014) Environmental and health impacts of fine and ultrafine metallic particles: assessment of threat scores. Environ Res 133:185–194
Hattori T, Inanaga S, Araki H, An P, Morita S, Luxová M, Lux A (2005) Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol Plant 123:459–466
Kato M, Ishikawa S, Inagaki K, Chiba K, Hayashi H, Yanagisawa S, Yoneyama T (2010) Possible chemical forms of cadmium and varietal differences in cadmium concentrations in the phloem sap of rice plants (Oryza sativa L.) Soil Sci Plant Nutr 56:839–847
Kobayashi NI, Tanoi K, Hirose A, Nakanishi TM (2013) Characterization of rapid intervascular transport of cadmium in rice stem by radioisotope imaging. J Exp Bot 64:507–517
Leveque T, Capowiez Y, Schreck E, Xiong T, Foucault Y, Dumat C (2014) Earthworm bioturbation influences the phytoavailability of metals released by particles in cultivated soils. Environ Pollut 191:199–206
Li S, Zhang S, Ding X, Liao X, Wang R (2013) Spraying silicon and/or cerium sols favorably mediated enhancement of Cd/Pb tolerance in lettuce grown in combined Cd/Pb contaminated soil. Prog Environ Sci 18:68–77
Liu C, Li F, Luo C, Liu X, Wang S, Liu T, Li X (2009) Foliar application of two silica sols reduced cadmium accumulation in rice grains. J Hazard Mater 161:1466–1472
Liu J, Zhang H, Zhang Y, Chai T (2013) Silicon attenuates cadmium toxicity in Solanum nigrum L. by reducing cadmium uptake and oxidative stress. Plant Physiol Biochem 68:1–7
Liu Y, Zhang C, Zhao Y, Sun S, Liu Z (2017) Effects of growing seasons and genotypes on the accumulation of cadmium and mineral nutrients in rice grown in cadmium contaminated soil. Sci Total Environ 579:1282–1288
Lux A, Luxova M, Hattori T, Inanaga S, Sugimoto Y (2002) Silicification in sorghum (Sorghum bicolor) cultivars with different drought tolerance. Physiol Plant 115:87–92
Ma J, Cai H, He C, Zhang W, Wang L (2015) A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol 206:1063–1074
Ma J, Zhang X, Wang L (2017) Synergistic effects between [Si-hemicellulose matrix] ligands and Zn ions in inhibiting Cd ion uptake in rice (Oryza sativa) cells. Planta 245:965–976. https://doi.org/10.1007/s00425-017-2655-2
Mae T (1997) Physiological nitrogen efficiency in rice: nitrogen utilization, photosynthesis, and yield potential. Plant Soil 196:201–210
Maghsoudi K, Emam Y, Pessarakli M (2016) Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J Plant Nutr 39:1001–1015. https://doi.org/10.1080/01904167.2015. 1109108
Mousavi SR, Rezaei M (2011) Nanotechnology in agriculture and food production. J Appl Environ Biol Sci 1:414–419
Pinto E, Ferreira IM (2015) Cation transporters/channels in plants: tools for nutrient biofortification. J Plant Physiol 179:64–82
Rogalla H, Romheld V (2002) Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ 25:549–555
Sarwar N, Saifullah MSS, Zia MH, Naeem A, Bibi S, Farid G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 90:925–937
Shahid M, Dumat C, Khalid S, Schreck E, Xiong T, Niazi NK (2017) Foliar heavy metal uptake, toxicity and detoxification in plants: a comparison of foliar and root metal uptake. J Hazard Mater 325:36–58
Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50
Shi X, Zhang C, Wang H, Zhang F (2005) Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 272:53–60
Shi GL, Zhu S, Bai SN, Xia Y, Lou LQ, Cai QS (2015) The transportation and accumulation of arsenic, cadmium, and phosphorus in 12 wheat cultivars and their relationships with each other. J Hazard Mater 299:94–102
Tanaka K, Fujimaki S, Fujiwara T, Yoneyama T, Hayashi H (2007) Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants (Oryza sativa L.) Soil Sci Plant Nutr 53:72–77
Tripathi DK, Singh VP, Kumar D, Chauhan DK (2012) Rice seedlings under cadmium stress: effect of silicon on growth, cadmium uptake, oxidative stress, antioxidant capacity and root and leaf structures. Chem Ecol 28:281–291
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Uraguchi S, Kamiya T, Sakamoto T, Kasai K, Sato Y, Nagamura Y, Yoshida A, Kyozuka J, Ishikawa S, Fujiwara T (2011) Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc Natl Acad Sci U S A 108:20959–20964
Uzu G, Sauvain J-J, Baeza-Squiban A, Riediker M, Hohl MSS, Val S, Tack K, Denys S, Pradere P, Dumat C (2011) In vitro assessment of the pulmonary toxicity and gastric availability of lead-rich particles from a lead recycling plant. Environ Sci Technol 45:7888–7895
Wang S, Wang F, Gao S (2015) Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ Sci. Pollut Res 22:2837–2845. https://doi.org/10.1007/s11356-014-3525-0
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107
Yamaji N, Ma J (2009) A transporter at the node responsible for intervascular transfer of silicon in rice (W). Plant Cell 21:2878–2883
Zhang C, Peng S, Laza RC (2003) Senescence of top three leaves in field-grown rice plants. J Plant Nutr 26:2453–2468
Zhao B, Wang P, Zhang H, Zhu Q, Yang J (2006) Source-sink and grain-filling characteristics of two-line hybrid rice Yangliangyou 6. Rice Sci 13:34–42
Funding
This work has been financed by the Funds for Science and Technology Innovation Project from the Chinese Academy of Agricultural Sciences (grant no. CAAS-XTCX2016018). We are grateful to Dr. Li Mu for providing help in using ICP-MS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Table S1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Chen, R., Zhang, C., Zhao, Y. et al. Foliar application with nano-silicon reduced cadmium accumulation in grains by inhibiting cadmium translocation in rice plants. Environ Sci Pollut Res 25, 2361–2368 (2018). https://doi.org/10.1007/s11356-017-0681-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0681-z