Abstract
Waste fluorescent lamps containing a significantly high quantity of rare earth metals have great potential to be an unconventional source of critical metals if exploited efficiently for resource recovery. Therefore, the present study dealt with the selective leaching of red phosphor rare earths from waste fluorescent lamps. The parametric effects of the acid media and their concentrations, addition of H2O2, pulp density, temperature, and time were studied. The results revealed that 2.0 M HCl with 5 vol.% H2O2 yielded 100% yttrium and more than 95% europium compared to only 92% and 96% yttrium and 89% and 91% europium while using H2SO4 and HNO3, respectively. The green phosphor compounds Ce0.67Tb0.33MgAl11O19 and (La0.65Ce0.15Tb0.2)PO4 were undissolved in a residual mass that can be handled separately. Kinetics data followed logarithmic rate law, and the chemically-controlled mechanism was indicated by the values of apparent activation energy (i.e., Ea(Y), 87.8 kJ/mol and Ea(Eu), 54.1 kJ/mol).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because of their distinct properties, like optics, electronic configuration, and magnetic and catalytic activities, the rare earth metals (REMs; a group of 15 lanthanides plus scandium and yttrium) are increasingly being used in modern technology applications.1 An array of clean and renewable energy devices, advanced electronics, low-energy fluorescent bulbs, permanent magnets, and petrocatalysts have established REMs as critical raw materials for modern high-tech products.2,3 It has been estimated that the demand for REMs reached 150,000 tons by the end of 2018, and that it is continuously increasing.4 Currently, China is producing about 90% of the total REMs,5 hence, fulfilling domestic REMs demand is a serious challenge for other countries. On the other hand, a large volume of end-of-life materials containing REMs, discarded as waste materials, are usually sent to landfills. Such a linear practice for using natural resources is unsustainable in terms of environmental pollution, and also because of the loss of the value of the REMs.6 The worldwide awareness towards green economy development plans, namely, Korea Green New Deal,7 the European Green Deal,8 the US Green New Deal,9 etc., has resulted in looking for a rapid transition towards the resource recycling of critical raw materials, in particular, the REMs.
Fluorescent lamps are one of the focused-on candidates for efficient recycling of REMs, as they contain a significant number of various phosphors. Red phosphors (Y2O3:Eu3+), green phosphors (LaPO4:Ce3+, Tb3+) and (Ce, Tb)MgAl11O19, and blue phosphors (BaMgAl10O17:Eu2+) are commonly used in fluorescent lamps that emit cool white light with low-energy consumption.10 As an estimate, the fluorescent lamps represent ~ 80% of the total lighting waste within waste electrical and electronic equipment in 18 European countries,11 which is a potential reservoir of yttrium (87 t), lanthanum (12 t), cerium (10 t), europium (6 t), and terbium (4.4 t).12 In general, the REMs’ content of phosphors is about 23 wt.%, which is several folds higher than the exploitable primary ores of the REMs. Therefore, resource recovery of REMs from waste fluorescent lamps containing a significantly high quantity of critical metals has an excellent potential to be a major unconventional source to be exploited. Efficient recycling of waste fluorescent lamps can effectively mitigate the supply and demand gaps and promote the sustainable use of natural resources.13,14
Hydrometallurgy is commonly used to extract individual REMs’ oxide from mineral concentrates, which involves leaching, separation, purification, and recovery through precipitation.15 Therefore, it is also largely applied to the recycling of waste fluorescent lamps.16,17 In order to resource the recovery of REMs, their dissolution in suitable lixiviants that carry out impurity (base metals) removal followed by the mutual separation of the REMs, using solvent extraction,18 precipitation,19 supercritical fluid extraction,20 ion exchange,21 and ionic liquid,22 are practiced. The products can also be deoxidized to the metallic state instead of using the usual molten salt electrolysis and metallothermic reduction.23,24 Leaching of red phosphors containing yttrium and europium has been widely studied in different acidic solutions; however, the efficiency achieved was on average < 95% and from a less complex matrix.25 In the case of sulfuric acid leaching, the formation of gypsum may prevent the leaching progress due to surface passivation. To overcome this issue, Resende and Morais26 used a two-step leaching of red phosphors. In the first step, red phosphor was leached in conc. H2SO4 followed by dynamic leaching in water, yielding an efficiency > 96%. Looking at the criticality of REMs, a simple processing route is desirable with a greater efficiency of metal extraction.
Therefore, the leaching behavior of REMs from waste fluorescent powder was investigated by dissolving in different acid media with/without adding an oxidant. The effects of acid concentration, H2O2 addition, temperature, time, and pulp density were examined to achieve the selective and enhanced leaching of yttrium and europium by keeping green phosphors undissolved in the residue. The leaching kinetics of both these rare earths is particularly highlighted while establishing the dissolution mechanism. It should be noted that the present study was limited to the selective leaching of red phosphor REMs (yttrium and europium), while the residual green phosphor materials of a refractory nature will be treated separately.
Experimental
An amount of 10 kg of waste fluorescent lamps was collected from a local source at Jeonju (South Korea) and ball-milled to collect a stock sample of 100-mesh particle size after a sieving operation. The composition of the prepared sample in weight percentage was analyzed and found to be 13.5% yttrium, 1.0% europium, 2.7% lanthanum, 1.4% cerium, 4.4% aluminum, 16.2% calcium, 2.2% barium, 0.4% sulfur, and balance oxygen. X-ray diffraction (XRD) analysis using an X’pert PRO PANalytical revealed that yttrium and europium in the red phosphors of the waste lamp were mainly present as oxide compounds. Despite the presence of 0.6% sulfur, the lack of sulfur compounds assigned to the XRD patterns was due to the very low sulfur content (below the detection level using XRD). Chemical reagents like hydrochloric acid (35% purity; Daejung), sulfuric acid (95% purity; Daejung), nitric acid (65% purity; RiedeldeHa¨en), and hydrogen peroxide (30% purity; Merck) were used without further purification.
First, 5 g of a stock sample was charged with 100 mL acid solution of a predetermined concentration at 5% pulp density into a 250 mL glass reactor fitted with a water bath. The slurry was stirred at a fixed agitation speed (200 rpm, optimized during batch leaching experiments) using a magnetic stirrer and paddle. All the experiments were performed in a closed system for 1 h at 55°C (until specified). After the completion of leaching time, solid–liquid separation was conducted, and the filtrate was collected to analyze its metal contents and the leach liquor using inductively coupled plasma spectroscopy (iCAP 7400 Duo; Thermo Scientific). The leaching efficiency of the metals was determined to be:
where MIS and MLL are the metal content in the input mass and the leach liquor’s output volume, respectively. The surface morphology of the solid samples was analyzed using scanning electron microscopy (SEM) and energy dispersive x-ray (EDX) images acquired by a Supra 40VP (Carl Zeiss, Germany).
Results and Discussion
Effect of Acid Media
The influence of the acid medium on leaching metals from the fluorescent powder was investigated in three different mineral acids (HCl, H2SO4, and HNO3) at two different concentrations (0.5 M and 2.0 M). The results in Fig. 1 show that the red phosphor rare earths (yttrium and europium) were able to leach in all types of the acid media; however, their efficiency significantly increased in high acid solutions. The maximum of 87.4% yttrium was leached by 2.0 M HCl solution while it was 84.8% and 82.5% using HNO3 and H2SO4, respectively, at the same concentration. The leaching efficiency of europium with 2.0 M HCl and 2.0 M HNO3 was unchanged (~ 87%), and was 81.1% with 2.0 M H2SO4 solution. The REM content of the green phosphor (cerium and lanthanum oxides) did not leach in any acid solution, showing the material's refractoriness.27 Approximately 45% barium and > 90% calcium could be dissolved in the HCl and HNO3 solutions of any concentration, albeit they did not leach in the H2SO4 solution. This behavior essentially showed the low solubility of the sulfate compounds (BaSO4 and CaSO4) that form in the H2SO4 solution. As the leaching efficiency of the red phosphor REMs was below the desirable level, an oxidant was added in order to examine the progress in metal leaching in the next set of experiments.
Effect of H2O2 Addition with Change of Acid Media
The maximum leaching efficiency of yttrium and europium was well below 90% using any mineral acid. Furthermore, the oxidant H2O2 was added to improve the leaching of the red phosphor from waste fluorescent lamp powder. Henceforth, the leaching process was optimized by adding a fixed dosage (5 vol.%) of H2O2 into different acid media solutions of two concentrations (0.5 M and 2.0 M). Figure 2 depicts significant progress in red phosphor REM leaching by introducing H2O2 into the leaching system. As can be seen, all the yttrium was efficiently leached in 2.0 M HCl solution along with ~ 90% europium, but they were below 40% and < 25%, respectively, with the 0.5 M HCl solution. The addition of H2O2 into the 2.0 M HNO3 system gave 96.4% yttrium and 91% europium, which was about 92% and 89% of the respective REMs using 2.0 M H2SO4. The enhanced leaching obtained by the addition of H2O2 can be corroborated by its ability to oxidize the sulfur compounds, albeit in low concentration and with interaction of oxygen atoms with surface metal cations.28 This has also been demonstrated by Diesen and Jonsson,29 while Lousada et al.30 reported the higher adsorption energy of H2O2 to the Y2O3 surface with the activation energy and enthalpy values of 44 kJ/mol. At the same time, the H2O2 also allows a vigorous reaction enhanced by the increased rate of the reaction through oxygen production during the peroxide decomposition.13
Effect of Pulp Density
The effect of pulp density on the metal leaching efficiency was examined at different pulp densities, varying from 3 wt.% to 9 wt.% of the sample in the 2.0 M HCl solution with 5 vol% H2O2 at 65°C for 1 h. The results in Fig. 3 show that the leaching efficiency of the metals, including the REMs, did not change with 3% and 5% pulp density. Further increases in the pulp density significantly decreased the efficiency of the red phosphor REMs and reached about 89% yttrium and 81% europium at 9% pulp density; however, other metals, like aluminum, calcium, and barium, did not show much change in their leaching efficiency. The phenomenon of declining REM leaching at higher pulp density can be ascribed to the solution's lower availability of surface area per unit volume.31 On the other hand, no change in other metal leaching can be corroborated to their possible leaching in low acid concentrations, as observed in acid variation experiments. By analyzing the experimental results, the pulp density of 5% was optimized.
Effect of Temperature
Although an elevated temperature often positively influences solid–liquid mass transfer, the effect of temperature was examined within a moderate range (35–65°C) due to the potential degradation of H2O2 at higher temperatures (~ 60°C).32 The results presented in Fig. 4 clearly show a positive impact of increased temperature (35°C to 65°C) as the yttrium leaching progressed from 44.6% to 100%, and the efficiency of europium leaching was enhanced from 42.8% to 95%. This behavior commonly reveals the exothermic nature of the leaching process, and can be ascribed to its shifting from diffusion control at low temperatures to chemical control at higher temperatures.33,34 Calcium was also leached efficiently, whereas ~ 44% of the barium was leached at all temperatures. The leaching of lanthanum and cerium was insignificant, while that of aluminum was only < 10%.
Effect of Time
The effect of time on the metal leaching from the waste fluorescent lamp powder was examined at 65°C up to 60 min of duration. The results in Fig. 5 reveal the progress in leaching efficiency of yttrium, europium, and calcium with respect to increasing time. It can be seen that only 8.4% yttrium, 15% europium, and 6.3% calcium were leached soon after the sample charging (within 1 min), which reached > 70% after 10 min of leaching. Within 30 min, almost all the yttrium was leached compared to 90% of the europium and calcium, which were finally analyzed to be ~ 95% after completing 60 min duration. The leaching trends show that the metal dissolution rate was initially slow; however, a prolonged contact allowed the attack and absorbance of H2O2 to the oxide surfaces leading to a smooth dissolution of the red phosphor.28,29,30
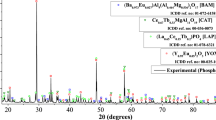
As expected, the leaching of other metals was negligible except for the 45% of barium measured in the final leach liquor. This was further confirmed by a comparative XRD study of the waste fluorescent powder and HCl leached residue. As shown in Fig. 6, the main diffraction peaks of the red phosphor (Y0.95Eu0.05)2O3 (ICDD ref. no.: 00-025-1011), green phosphor (Ce0.67Tb0.33MgAl11O19) (ICDD ref. no.: 01-036-0073), and (La0.65Ce0.15Tb0.2)PO4 (ICDD ref. no.: 01-078-6321), and blue phosphor (Ba0.973Eu0.027)Al9(Al0.464Mg0.536)2O17 (ICDD ref. no.: 01-072-6158) were observed through XRD characterization. The diffraction peaks assigned to the red phosphor were not observed in the leached residue which showed complete dissolution of the metals. This shows that the red phosphor REMs could be efficiently dissolved in the 2.0 M HCl solution and enhanced by the addition of H2O2. However, about 5% of europium that remained undissolved can be understood by the XRD analysis, indicating the undissolved europium as a part of the blue phosphor (Ba0.973Eu0.027)Al9(Al0.464Mg0.536)2O17 material. This was also supported by the SEM-EDX images of the raw sample and the leached residue (as shown in Fig. 7). It can be seen from Fig. 7a that the morphology of the fluorescent powder was closely associated with the bulk, indicating a higher occurrence of the yttrium and europium. On the other hand, the morphological distribution of the HCl leached residue in Fig. 7b is distant with detached particles. Moreover, the yttrium disappeared from the leached residue with only a trace presence of europium.
Leaching Kinetics and Mechanism
In addition to the parametric optimization, the leaching kinetics for the yttrium and europium dissolution from the time scale data was generated at temperatures between 35°C and 65°C. The data were examined to fit with heterogeneous solid–liquid mass transfer onto the surface of the assumed small particles. At first, the commonly applied shrinking core models with the chemical- and diffusion-controlled mechanism were evaluated using Eqs. 2 and 3, respectively:32,33,34
where x is the leaching fraction at the time t (in min), and kc and kd are the rate constants for the chemical- and diffusion-controlled reactions, respectively. The analysis of data fitting did not show linearity (graphs not shown here for the sake of brevity); thus, another model using the logarithmic rate law was tested using:32,34
where kl is a logarithmic rate constant. The plots of − ln(1 − x) versus t showed linear fittings for yttrium and europium dissolution, as shown in Fig. 8a and b, respectively). Good fitting of data was evident, and also with the values of the regression coefficients (i.e., R2 > 0.98). The logarithmic model fits revealed that the yttrium and europium dissolution of the red phosphor essentially relies on the real-time observations and experimental conditions, and certainly not upon the hypothetical model for particles shrinking towards the core.35,36
Furthermore, the slope values of the line equations were used for determining the apparent activation energy (Ea) of yttrium and europium leaching through the Arrhenius equation:33,34
where kr is a rate constant, A Arrhenius constant, Ea the apparent activation energy, R the universal gas constant (8.314 kJ/mol), and T the absolute temperature (in K). Using the slope values (from the linear plots of Fig. 8a and b) as the rate constants at a particular temperature, the linear graphs of lnkr versus 1/T (as shown in Fig. 8c) were plotted. Then, the slope values were used to calculate the apparent activation energy of yttrium and europium that were determined to be Ea(Y) = 85.5 kJ/mol and Ea(Eu) = 55.1 kJ/mol, respectively. The obtained values of Ea indicated that a higher activation energy is required to overcome the energy barrier for dissolving yttrium and europium, which is chemically controlling the overall leaching process by following the logarithmic rate law.32 Based on the experimental findings, the stepwise leaching mechanism can be understood as follows: (1) a larger portion of yttrium and europium dissolution from the waste fluorescent powder through the chemical reaction between (Y0.95Eu0.05)2O3 and HCl, (2) H2O2 sufficiently increases the activation energy of the system which initially took time to be adsorbed to the oxide surface of the phosphors, (3) a simultaneous oxidation of sulfur compounds, which were present in small quantities, and (4) soon after the H2O2 decomposition into water and oxygen atoms, the rapid reaction rate chemically-drives the leaching reactions to give the maximum soluble products of yttrium and europium in the leach liquor.
Conclusion
The accessibility and affordability of critical raw materials, including REMs, are significant for economic and sustainable growth. Hence, the present study has demonstrated the extraction of REMs from an unconventional source of waste fluorescent lamps; however, the study was limited to the selective leaching of red phosphor REMs (yttrium and europium). Leaching with different mineral acids of different concentrations, showed a higher efficiency in 2.0 M HCl while it was least with 0.5 M H2SO4, which additionally rejected calcium and barium in the leach residue. Leaching was found to increase up to 99% yttrium and ~ 90% europium by adding 5 vol.% H2O2 in 2.0 M HCl, which was identical with HNO3 (96% yttrium and 91% europium) but not adopted due to the associated gaseous hazards with NOx emission. A temperature increase up to 65°C could further enhance the leachability, reaching 100% yttrium and > 95% europium, along with 95% calcium and 44% barium in the leach liquor. Leaching conducted at varied temperatures (35–65°C) and times (1–60 min) gave kinetics data that fit well with the logarithmic model. The values of apparent activation energy calculated for yttrium (Ea(Y), 87.8 kJ/mol) and europium (Ea(Eu), 54.1 kJ/mol) indicated that leaching of red phosphor REMs followed a chemically controlled mechanism. This study offers two main advantages: securing a secondary supply of REMs from unconventional resources, and providing an environmentally sustainable route for waste treatment.
References
S. Ilyas, H. Kim, and R.R. Srivastava, Sep. Purif. Technol. 254, 117634 (2021).
F. Habashi, Extractive metallurgy of rare earths. Can. Metall. Quart. 52, 224 (2013).
S. Ilyas, H. Kim, R.R. Srivastava, and S. Choi, J. Clean. Prod. 278, 123435 (2021).
G. Prameswara, I. Trisnawati, P. Mulyono, A. Prasetya, and H.T.B.M. Petrus, JOM 73, 988 (2021).
G. Hearty, “Rare earths: next element in the trade war?” (Center for Strategic and International Studies, 2019). https://www.csis.org/analysis/rare-earths-next-element-trade-war#:%7e:text%3dWith%20the%20trade%20war%20having%2cnext%20salvo%20in%20the%20conflict.%26text%3dA1%253A%20Rare%252Dearth%20elements%20are%2cwhich%20possess%20similar%20chemical%20properties. Accessed 29 Sept 2021
S. Ilyas, H. Kim, and R.R. Srivastava, Sustainable Urban Mining of Precious Metals (CRC, Boca Raton, 2021).
J.-H. Lee, and J. Woo, Sustainability 12, 10191 (2020).
The EU Green Deal – a roadmap to sustainable economie (Swich2green), https://www.switchtogreen.eu/the-eu-green-deal-promoting-a-green-notable-circular-economy/. Accessed 29 Sept 2021
R. Galvin, and N. Healy, Energy Res. Soc. Sci. 67, 101529 (2020).
K. Binnemans, P.T. Jones, B. Blanpain, T. Van Gerven, Y. Yang, A. Walton, and M. Buchert, J. Clean. Prod. 51, 1 (2013).
Memoria anual 2016 (Ambilamp, 2017), https://ambilamp.es/sites/default/files/memoria-actividad/files/memoria2016-ambilamp.pdf. Accessed 29 Sept 2021
L. Yurramendi, L. Gijsemans, F. Forte, J.L. Aldana, C. del Río, and K. Binnemans, Hydrometallurgy 187, 38 (2019).
A. Miskufova, A. Kochmanova, T. Havlik, H. Horvathova, and P. Kuruc, Hydrometallurgy 176, 216 (2018).
N. Shukla, and N. Dhawan, JOM 73, 1090 (2021).
Q. Tan, J. Li, and X. Zeng, Crit. Rev. Environ. Sci. Technol. 45, 749 (2015).
M. Tanaka, K. Koyama, H. Narita, and T. Oishi, Recycling valuable metals via hydrometallurgical routes, in Design for Innovative Value Towards a Sustainable Society. ed. by M. Matsumoto, Y. Umeda, K. Masui, and S. Fukushige (Springer, Amsterdam, 2012), p. 507.
A. Tuncuk, V. Stazi, A. Akcil, E.Y. Yazici, and H. Deveci, Miner. Eng. 25, 28 (2012).
C.H. Yan, J.T. Jia, C.S. Liao, S. Wu, and G.X. Xu, Tsinghua. Sci. Technol. 11, 241 (2006).
S. Zielinski, and A. Szczepanik, Hydrometallurgy 33, 219 (1993).
R. Shimizu, K. Sawada, Y. Enokida, and I. Yamamoto, J. Supercrit. Fluids 33, 235 (2005).
Z. Hubicki, and M. Olszak, J. Chromatogr. A 955, 257 (2002).
H.Y. Liu, J. Chen, and D.Q. Li, Sep. Sci. Technol. 47, 223 (2012).
B.J. Beaudry, and K.A. Gschneidner Jr., Chapter 2, preparation and basic properties of the rare earth metals, in Handbook on the Physics and Chemistry of Rare Earths. ed. by K.A. Gschneidner Jr., and L. Eyring (Elsevier, New York, 1978), p. 173.
C.H. Huang, W. Wang, Y.J. Liu, and J.G. Wu, Inorganic Chemistry Series Volume VII: Scandium, Rare Earth Elements (Science Press, Beijing, 1992).
B.B. Mishra, N. Devi, and K. Sarangi, Miner. Eng. 136, 43 (2019).
L.V. Resende, and C.A. Morais, Miner. Eng. 70, 217 (2015).
S. Ilyas, H. Kim, and R.R. Srivastava, JOM 73, 19 (2021).
C.M. Lousada, M. Yang, K. Nilsson, and M. Jonsson, J. Mol. Catal. A-Chem. 379, 178 (2013).
V. Diesen, and M. Jonsson, J. Adv. Oxid. Technol. 16, 16 (2013).
C.M. Lousada, A.J. Johansson, T. Brinck, and M. Jonsson, J. Phys. Chem. C 116, 9533 (2012).
R. Sattar, S. Ilyas, S. Kousar, A. Khalid, M. Sajid, and S.I. Bhukhari, Environ. Eng. Res. 25, 88 (2020).
S. Ilyas, R.R. Srivastava, H. Kim, and H.A. Cheema, Sep. Purif. Technol. 248, 117029 (2020).
F. Habashi, Principles of Extractive Metallurgy: Hydrometallurgy, Gordon and Breach, vol I. (Science Publishers, New York, 1969).
O. Levenspiel, Chemical Reaction Engineering, 3rd edn. (Wiley, New York, 1999).
K. Chabhadiya, R.R. Srivastava, and P. Pathak, J. Environ. Chem. Eng. 9, 105232 (2021).
H. Munir, R.R. Srivastava, H. Kim, S. Ilyas, M.K. Khosa, and B. Yameen, J. Chem. Technol. Biotechnol. 95, 2286 (2020).
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Project Nos. 2020R1A6A3A13073210 and 2020R1I1A1A01074249) and by the Brain Pool Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2019H1D3A2A02101993). The authors are thankful to Dr. Dipti Tanna for the language editing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, S., Ilyas, S. & Kim, H. Intensive Leaching of Red Phosphor Rare Earth Metals from Waste Fluorescent Lamp: Parametric Optimization and Kinetic Studies. JOM 74, 1054–1060 (2022). https://doi.org/10.1007/s11837-021-05112-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-05112-z