Abstract
Cadmium (Cd) is a toxic and nonessential element. Because of its toxicity, Cd soil contamination is a major environmental risk to living organisms. Several studies have reported on the successful use of biochar to immobilize Cd in soil as it reduces Cd accumulation in plant parts. This research reports on the contrasting effect of biochar on enhancing Cd uptake by plants. A cassava stem biochar produced through low-temperature pyrolysis was applied to natural Cd-contaminated soil that also had a high zinc (Zn) concentration. Vigna radiata L. (a green bean) was grown in treatments receiving three biochar rates, i.e., 5, 10, and 15 %, respectively. The results showed that the 10 % biochar-amended soil had a positive effect on promoting plant growth and seed yield. Unfortunately, 15 % biochar-amended soil caused an adverse effect to plant growth. Cadmium uptake by plants increased with increasing biochar application rate. Zinc uptake by plants tended to decrease with biochar application. Cadmium and Zn bioavailability in soil was significantly reduced with an increasing biochar application rate. The results also showed that the biochar-amended soil could be an alternative and cost-effective method to promote plant growth and decrease Cd mobility in soil. The ratio of Cd concentration in plant root to soil was higher than 1, while the translocation factor from root to shoot was less than 1. These results indicate that the cultivation of V. radiata L. coupled with biochar application is an appropriate method to enhance Cd phytostabilization efficiency of V. radiata L. in Cd-polluted sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cadmium (Cd) is a nonessential, toxic element for living organisms. It is naturally present in the atmosphere, water, and soil (Gallego et al. 2012). As Cd is beneficial in various industrial processes such as electroplating, alloy manufacturing, pigments, plastics, cadmium-nickel batteries, pesticides, mining, pigments and dyes, textile operations, and refining, its use in the industry has been increasing. However, it causes an adverse effect on people’s health (Rao et al. 2010). Cadmium soil contamination occurs from natural and industrial activities. Plants can easily uptake and accumulate Cd even at low concentrations in agricultural soil (Sterckeman et al. 2011). Several plants develop mechanisms to block or eliminate Cd, as it is a nonessential and toxic element to plants (Gallego et al. 2012; White and Brown 2010). The presence of Cd in consumable plants can affect human health (Sterckeman et al. 2011). The consumption of rice containing Cd levels over the long term is a significant health issue (Simmons et al. 2005). Zinc (Zn) at lower concentration is an essential micronutrient for plants (Yadav et al. 2009). However, excess Zn becomes toxic to plants as well (Kamal et al. 2004).
Biochar is a carbon-rich porous material produced by pyrolysis and burning of biomass under conditions of limited oxygen and relatively low temperature. Biochar amendment in soil helps to increase soil fertility and promote plant growth by retaining nutrients in the soil (Houben et al. 2013a; Park et al. 2011). The effect of biochar on plant growth varied with the type of biochar and the amount of application rates (Park et al. 2011). In general, the rate of biochar application ranged between 0.2 and 20 % (Mukherjee and Lal 2014). In addition, biochar can adsorb metal ions and thus is used for heavy metal immobilization (Li et al. 2013; Park et al. 2013; Uchimiya et al. 2010). Heavy metals in soil can be adsorbed on the biochar surface, leading to a decrease in heavy metal bioavailability in soil (Trakal et al. 2011). Biochar persists in soil, while soil organic amendments used to immobilize heavy metals are subject to biodegradation (Lehmann and Joseph 2009; Bolan et al. 2014). Therefore, biochar has received more attention to achieve heavy metal immobilization. The use of biochar as a soil amendment for any specific purposes must be studied case by case, depending on the biochar characteristics and soil properties (Bolan et al. 2014; Uchimiya et al. 2011).
Phytoremediation is a relatively low-cost technology that has the potential to decontaminate soil polluted by heavy metals (Prasad 2003). However, the efficiency of plants on metal remediation depends on plant-generated biomass and several growth limiting factors such as the phytotoxicity of the heavy metals (Prasad 2003; Upadhyay et al. 2007). Many researchers have focused on the application of biochar for heavy metal stabilization in soil (Houben et al. 2013b; Trakal et al. 2011; Yadav et al. 2009). Meanwhile, a number of studies have revealed that biochar can increase the bioavailability of some toxic metals to plants (Bolan et al. 2014). The application of biochar in heavy metal-contaminated soil for improving plant growth can also lead to an increase in heavy metal uptake by some plants. Some researches have also studied the effect of biochar on plant growth and phytoremediation efficiency (Paz-Ferreiro et al. 2014). Therefore, the study of the effect of biochar on the mobility or stabilization of heavy metals in contaminated soils should be performed (Bolan et al. 2014).
The cassava stem is an agricultural residue, and it is easy to carbonize to biochar in mass production. The aim of this study was to investigate the effect of cassava stem biochar on the efficiency of Cd and Zn phytoremediation by Vigna radiata L., a green bean. High levels of Cd- and Zn-contaminated soil were collected from Mae Tao subcatchment, Mae Sot District, Tak Province, Thailand. In addition, the capacity of biochar on Cd adsorption from an aqueous solution was investigated.
2 Materials and Methods
2.1 Preparation of Contaminated Soil
The contaminated soil was collected from an agricultural area at Mae Tao subcatchment. The sampling site location was N 16.673861, E 98.626007. Soil was collected at a depth of 0–20 cm, homogenized, air-dried, ground, and passed through a 2-mm sieve.
2.2 Analysis of Soil Characteristics
The physical and chemical properties of soil, including soil texture, soil pH, cation exchange capacity (CEC), % organic matter (OM), % total nitrogen (N), C/N ratio, available phosphorous (P), extractable potassium (K), extractable calcium (Ca), and extractable magnesium (Mg) were analyzed. To determine the total concentrations of Cd and Zn (acid-digested forms), soil was acid-digested using an open tube digestion method, according to standard US EPA method 3050B (1996). The digested sample was analyzed with a flame atomic adsorption spectrophotometer (FAAS, Perkin Elmer AAnalyst 800) at wavelengths 326.1 and 213.9 nm for Cd and Zn, respectively. To determine the concentrations of the bioavailable forms of Cd and Zn, soil was extracted by diethylene triamine pentaacetic acid (DTPA) at the soil extractant ratio of 1:2 and analyzed with FAAS (Quevauviller et al. 1998).
2.3 Biochar Preparation and Its Adsorption Efficiency
Cassava stem was collected and air-dried for 1 month before chopping to the approximate size of 10–15 cm. Mass carbonization was carried out in a 200-L char stove under anoxic condition. The pyrolysis stove temperature was maintained at 350 °C. Carbonization was conducted for 120 min followed by cooling overnight in the stove. The biochar product was then kept in an open container to absorb moisture for further use in the pot experiment. The cassava stem biochar had the following physical and chemical properties: surface area of 6.88 m2 g−1, CEC of 93.57 cmol kg−1, 66.7 ± 0.12 % carbon, 2.78 ± 0.16 % hydrogen, 0.22 ± 0.08 % nitrogen, and 30.29 ± 0.34 % oxygen (by difference). Cadmium was not detected in the biochar. The biochar was ground and sieved to particle sizes between 250 and 500 μm for batch static adsorption study.
The Langmuir isotherm is useful for grading the adsorption efficiency of adsorbents (Okeola and Odebunmi 2010), and the Langmuir adsorption isotherm was used to calculate Cd adsorption capacity of biochar in an aqueous solution (Kołodynska et al. 2012; Tangjuank et al. 2009; Trakal et al. 2014). A batch adsorption experiment was performed in triplicate, and Cd adsorption capacity of biochar was calculated by using an isotherm model. The batch static experiment was conducted in an aqueous solution of cadmium nitrate [Cd (NO3)2] serving as a Cd (II) ion source. The initial concentrations of Cd (NO3)2 were varied from 10 to 50 mg L−1. A 0.10-g biochar was accurately weighed, filled in a polyethylene (PE) filter bag, and then placed in 250-mL PE bottles containing 100 mL of Cd (NO3)2. The PE bottles then were shaken at 120 rpm for 60 min at room temperature. After that, the biochar bag was removed. The solution was acidified with concentrated HNO3 to produce a pH less than 2.0. The amounts of Cd (II) ion in acidified solutions were analyzed using FAAS. Cd (II) ion adsorbed on PE filter bags was studied as a control batch to deduct from the total adsorption on biochars.
2.4 Greenhouse Pot Experiment
To study the effect of biochar on plant growth and metal uptake, a pot experiment was conducted in a greenhouse. The local green bean (V. radiata L.) plant strain, Kamphaeng Saen 1 (KS1), was obtained from the Department of Agriculture, Thailand. The cassava stem biochar was ground and sieved to a particle size less than 2 mm which conforms to the present soil particle size. The contaminated soil was mixed with biochar at three rates: 5, 10, and 15 % (w/w). The control treatment without biochar application was also performed. The greenhouse study was performed in triplicate, using a completely randomized design. Biochar-amended soils were filled into 16-L plastic pots (19 cm in top diameter, 15 cm in bottom diameter, and 30 cm in height). Each pot was filled with biochar-amended soil to 90 % pot capacity. Each pot was randomly placed in the greenhouse for 2 weeks with daily watering at field capacity before planting. Four green bean seeds were planted in the soil at 1 cm depth. During the process, NPK fertilizer (15-15-15) was added to the soil at week 4 (the blossom stage) and week 6 (the seeding stage) (Garcia and John 1976).
2.5 Plant and Soil Analysis
Plant samples in each treatment were collected at week 2 (the beginning of growth), week 4 (the blossom stage), week 6 (the seed-filling stage), and week 8 (the harvest stage). The harvested plants were thoroughly washed with tap water before rinsing with deionized water. For plant growth determination, root length and stem height were measured. Each plant was separated into shoot (leaf and stem) and root for weighting. Plants were oven-dried to measure dry weight. Plants were acid-digested with an open tube digestion method according to the method of US EPA 3050B (1996). Cadmium and zinc concentrations in plants were analyzed using FAAS. At 8 weeks after planting, the number of bean pods per pot was recorded to determine crop yield. Soil samples were collected every 2 weeks. The soil pH and concentrations of acid-digested (total metals) and bioavailable forms of Cd and Zn were analyzed. In addition, the soil’s chemical properties after 8 weeks of planting, including CEC, % OM, % N, available P, extractable K, extractable Ca, extractable Mg, and C/N ratio, were analyzed.
To evaluate plants’ metal accumulation, the biological concentration factor (BCF) and translocation factor (TF) were calculated. BCF is the metal concentration ratio of metals in plant root to metal concentration in soil as given in Eq. (1), and TF is described as the ratio of metal concentration in plant shoot to metal concentration in plant root as given in Eq. (2) (Bech et al. 2012; Malik et al. 2010; Yoon et al. 2006).
2.6 Statistical Analysis
Data of all treatments were analyzed using one-way analysis of variance at 95 % confidence interval. Duncan’s multiple range test was used to detect significant differences at p < 0.05 using the SPSS 17 program.
3 Results and Discussion
3.1 Adsorption Efficiency of Cd Ion onto Biochar
Figure 1 shows the linear adsorption isotherm of Cd (II) ion on biochar. It shows that the Cd (II) ion adsorption characteristic on the biochar surface fits well with the Langmuir isotherm. The maximum adsorption capacity of biochar from the Langmuir isotherm was 20.53 mg g−1. In this study, cassava stem biochar had a good Cd adsorption efficiency. A comparison of Cd adsorption capacities by various types of biochar produced from different biomaterials, pyrolysis conditions, and activation methods is presented in Table 1.
3.2 Promoting Plant Growth in Contaminated Soil by Biochar Application
The physical and chemical characteristics of soil collected from a contaminated site before planting are presented in Table 2. After directed seeding of V. radiata L. in contaminated soil, the percentages of seed germination of all treatments were 100 %. Table 3 shows plant growth in contaminated soil under different biochar application rates. During 2 to 6 weeks after planting, plant growth increased by increasing biochar rates. At week 8, 10 % biochar promoted plant height and fresh weights of shoot and root. In contrast, 15 % biochar caused retardation of plant growth compared to the control treatment without biochar application. In addition, plants showed early mortality symptoms with yellow leaves. It might be postulated that a high biochar rate causes an imbalance of nutrients in the soil. The numbers of total bean pods and ripe bean pods 8 weeks after planting are presented in Fig. 2. The treatment of 10 % biochar application rate had the highest number of bean pods. Meanwhile, the amounts of bean pods in the treatment of 0, 5, and 15 % biochar application showed no significant difference at p < 0.05. These findings indicate that the 10 % application rate of biochar in contaminated soil significantly promoted plant growth and bean pod production.
A high rate of biochar could thus improve soil pH in acidic soil, but over application of biochar could cause high soil alkalinity, resulting in deficiency of plant micronutrients. Zheng et al. (2013) reported that 5 % biochar was the optimum concentration for promoting the growth of maize. A 15 % biochar derived from green waste promoted the highest growth of Indian mustard (Park et al. 2011). On the other hand, Upadhyay et al. (2014) reported that the optimum rate of green waste biochar to lettuce growth was 30 ton ha−1; however, the application of 100 ton ha−1 green waste biochar decreased plant growth.
3.3 Effect of Biochar on Cd and Zn Uptake and Accumulation in V. radiata L.
Table 4 shows Cd and Zn contents accumulated in shoot and root biomass of V. radiata L. over a growing period. The results showed that Cd and Zn concentrations in roots were higher than those in shoots throughout the cultivation period. Cadmium and Zn accumulation in shoot and root biomass continued to increase with time. At week 4, Cd concentrations in shoot and root biomass were nearly 3- and 2-folds higher than those at week 2, respectively. At week 4, Cd concentration in roots with 5, 10, and 15 % biochar application increased significantly compared to the control treatment without biochar application. The highest Cd content in roots was found at week 4 with all rates of biochar application. At week 6, shoots in the treatment of 5 % biochar application had a maximum Cd accumulation. For all treatments at week 8, Cd accumulation in shoots tended to decrease by about 35 %, due to the completion of the production stage. Cadmium concentrations in bean seed and bean shell in treatments of 10 and 15 % biochar application were higher than those in 5 % biochar and without biochar applications. Cadmium concentrations in bean seed and shell of all treatments ranged between 5.6 and 8.5 mg kg−1, which was much higher than the Codex standard for polished rice (0.4 mg kg−1). Interestingly, Zn concentrations in the shoot and root decreased significantly with biochar application at weeks 8 and 4, respectively. However, no significant change for Zn accumulation in bean seed and bean shell in the presence of biochar was observed 8 weeks after planting.
Xian (1989) reported that Cd has greater uptake affinity than Zn. Cd does not have a known transfer function in plants and exhibits similar chemical properties to Zn (Pence et al. 2000; Tan et al. 2011). Our findings indicate that an increase of plant growth by biochar application promoted Cd uptake by plants. In contrast, several studies that reported on the application of biochar for heavy metal immobilization found that biochar stimulated Cd plant uptake, but it tended to reduce Zn uptake in shoots. Regarding the effect of biochar, it can be seen that the increase of metal uptake by plants depends on heterogeneity in response to soil and plant types (Paz-Ferreiro et al. 2014). Fellet et al. (2014) used three feedstock biochars, including orchards’ pruning residues, fir tree pellets, and manure pellets for soil amendment. They found that soil amended with fir tree pellet biochar increases Cd accumulation in three plants species.
To understand the role of biochar in soil, Cd and Zn adsorbed on biochar were analyzed. After harvesting plants, biochar was separated from the soil (only in the treatment of 15 % biochar application rate) and analyzed for Cd and Zn concentrations. The results showed that the concentrations of Cd and Zn adsorbed on biochar were 15.43 ± 0.06 and 173.33 ± 23.60 mg kg−1, respectively. The amount of Zn adsorption on biochar was 11-fold higher than that of Cd adsorption, indicating that biochar plays an important role in Zn adsorption in high Zn-contaminated soil. However, Zn concentration in soil (1220 mg kg−1) was 21-fold higher than Cd concentration in soil (58 mg kg−1), indicating that Cd has a higher adsorption affinity than Zn. The findings are similar to those of Rees et al. (2014), who reported that effective metal sorption by biochar affinity increases in the order of lead (Pb), copper (Cu), Cd, Zn, and nickel (Ni).
3.4 Efficiency of Biochar in Reducing Cd and Zn Bioavailability in Soil
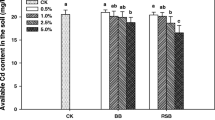
The results of the bioavailability of Cd and Zn extracted by DTPA are presented in Table 5. The results indicate that Cd and Zn bioavailability in soil was efficiently reduced by biochar application. Cd and Zn bioavailability decreased with the increase of biochar rates. Zinc bioavailability was approximately 2–3-folds higher than Cd bioavailability in all treatments. At week 8 of cultivation, Cd bioavailability in soil at all biochar rates was not significantly different, but it significantly decreased when compared to the control treatment without biochar application. Zinc bioavailability in soil with 15 % biochar application decreased significantly when compared to 5 and 10 % biochar applications. These results suggest that 5 % biochar application is a cost-effective biochar application rate to reduce Cd bioavailability.
The mechanism of heavy metal immobilization in contaminated soil depends on soil and amendment properties, the presence of organic matter, and other metals in soil that affect the bioavailability of metals (Romkens et al. 2009). Furthermore, soil OM increases the competitive adsorption mechanism of organic matter and metal ions onto a biochar surface (Chorom et al. 2013). The role of biochar in reducing the bioavailable forms of metals in soil mostly occurs from adsorption of metal ions onto biochar surfaces. Specific biochar properties perform specific adsorption efficiency, which is induced by different metal immobilization (Park et al. 2011; Uchimiya et al. 2010). Table 6 shows the increase of available P in soil, which is important for plant growth during the blossom and seed-filling stages. The increase of available P in soil stimulated the increase of Cd accumulation in V. radiata L., which is similar to the findings of Panwar et al. (1999), who reported that the increase of P concentration led to an increase of Cd concentration in plants.
3.5 Changes of Chemical Properties in Biochar-Amended Soil During Cultivation
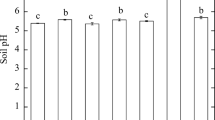
The changes of soil pH during plant growth are shown in Fig. 3a. The results indicate that soil pH increased after biochar application but then tended to decrease and stabilize after 2 weeks. At week 8, there was no significant difference of soil pH in all treatments. However, decreasing of pH in biochar-amended soil without plants was less than that in the presence of green beans (Fig. 3b). Hence, it can be indicated that plants do have an effect on soil pH. Fresh biochar application in soil usually affects soil pH. Thomson et al. (1993) reported that soil pH during cultivation of bean crops decreased due to acidification of the rhizosphere for N2 fixation. Nitrogen for plant uptake is found in three main forms: (i) NO3 − anion, (ii) NH4 + cation, and (iii) nitrogen fixation from N2 in the air. Uptake of NH4 + cation by plants and N2 fixation cause the release of H+ and lead to acidic soil surrounding the rhizosphere (Bolan et al. 1991). Biochar has oxidation and adsorption properties of organic acids as a result of soil pH shift (Houben et al. 2013b). In general, biochar can increase soil pH and maintain a stable soil pH level in the presence of biochar carbonate contents due to its chemical buffering capacity (Cao et al. 2009; Yuan et al. 2011). In this study, the presence of rich concentrations of Zn and Cd also presented an adsorption mechanism for metals to be adsorbed onto the carbonate, which correlates with the presence of hydroxyl, carboxylate, and carbonyl groups (Lee et al. 2010). Therefore, metal adsorption on these functional groups results in less alkaline biochar-amended soil and alkaline soil property.
Soil properties are important factors that affect metal uptake by plants (Tangahu et al. 2011). The production of biochar at a low pyrolysis temperature results in high biochar yields and low ash content, which also leads to lower alkaline pH (Singh et al. 2010; Song and Guo 2012; Hassan and Aarts 2011). Table 6 presents the soil characteristics of each treatment after 8 weeks of cultivation. The soil, a silty clay loam, had a moderately fine texture soil. The soil pH of biochar amendments of 5, 10, and 15 % biochar was slightly alkaline. It was observed that soil OM increased by increasing the biochar rate. In the soil environment, biochar mobilized and released OM, N, P, and soluble K (Ameloot et al. 2013; Mukherjee and Zimmerman 2013). Organic matter served as the nutrients for microbial growth and degradation (Mclatchey and Reddy 1998). This study found that the increase of other soil nutrients such as K, Ca, and Mg in the treatment with biochar application resulted from an increase of OM. Since CEC correlated with the increase of O and C contents, CEC in biochar-amended soil increased by increasing the biochar rate (Lee et al. 2010). Therefore, soil with biochar application had an increase in soil fertilities. These results are similar to the previous findings of Liang et al. (2006) and Houben et al. (2013a). The increase of microbial population resulted in the decomposition of organic matter by biological action; nevertheless, the increase of microbial did not result in a biotransformation process of valence change. Since Cd exists naturally only in a valence state of +2, microbial oxidation or reduction of Cd is improbable (Riser-Roberts 1998).
3.6 Performances of Biochar on Cd and Zn Accumulation and Translocation in V. radiata L.
Owing to the positive effects of biochar on promoting plant growth, stimulating Cd accumulation in plant root and limiting Cd accumulation in plant shoot, biochar can be used for Cd phytostabilization. Table 7 shows BCF values of Cd and Zn during plant cultivation in contaminated soil. The results showed that BCFCd increased with plant growth, except at week 8 when plant growth declined due to a declining growth phase. BCFCd values greater than 1 were observed in the treatments with biochar application at weeks 4 and 6. BCFCd values increased with an increase in the biochar rate. These findings suggest that biochar application increases BCFCd and Cd hyperaccumulation in V. radiata L. during the blossom and seed-filling stages. BCFZn was lower than BCFCd at all cultivation periods. However, biochar had no effect on BCFZn, except at week 4. BCFZn significantly decreased in the treatments with biochar application compared to the control treatment at week 4 after planting.
The TF value reflects plant’s ability to translocate metals from root to shoot. The results of TFCd and TFZn in V. radiata L. are presented in Table 6. The results show that TFCd increased with time of growth period. However, TFCd was less than 1 at all cultivation periods. At week 4, TFcd was reduced in the treatment with biochar application. In addition, TFCd was greater than TFZn at all cultivation periods. These results indicate that V. radiata L. has limited Cd and Zn translocation from root to shoot. However, the plant with high BCF and low TF is considered as a good phytostabilizing plant (Cui et al. 2007; Malik et al. 2010; Yoon et al. 2006). These findings suggest that V. radiata L. has the ability to retain Cd in its root and limit Cd translocation from root to shoot. Thus, V. radiata L. is suitable for Cd phytostabilization in Cd and Zn co-contaminated soil.
4 Conclusions
Cassava stem biochar improved soil fertilities by increasing organic matter and soil nutrients. The optimum rate of biochar application in soil to increase plant growth and bean pod yield was 10 %. However, 15 % biochar had an adverse effect on plant growth. Cd accumulated in V. radiata L. root was higher than that in its shoot. Any rate of biochar application can promote Cd accumulation in plant roots. In addition, V. radiata L. had less Cd translocation from root to shoot, and biochar application reduced Cd translocation from root to shoot. These findings recommend the application of biochar for soil amendment during planting to enhance Cd phytostabilization. However, bean seed contained high levels of Cd. Therefore, V. radiata L. seed is not suitable for consumption. Zinc was accumulated more in the root than in the shoot. In addition, the accumulation of Zn on biochar was 11 times higher than that of Cd. These findings suggest that the application of biochar to immobilize Cd in contaminated soil with high Zn levels should be considered case by case.
References
Ameloot, N., Sleutel, S., Das, K. C., Kanagaratnam, J., & De Neve, S. (2013). Biochar amendment to soils with contrasting organic matter level: effects on N mineralization and biological soil properties. GCB Bioenergy. doi:10.1111/gcbb.12119.
Bech, J., Duran, P., Roca, N., Poma, W., Sanchez, I., Roca-Perez, L., Boluda, R., Barcelo, J., & Poschenrieder, C. (2012). Accumulation of Pb and Zn in Bidens triplinervia and Senecio sp. spontaneous species from mine spoils in Peru and their potential use in phytoremediation. Journal of Geochemical Exploration, 123, 109–113.
Bolan, N. S., Hedley, M. J., & White, R. E. (1991). Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. Journal of Plant and Soil, 134, 53–63.
Bolan, N., Kunhikrishnan, A., Thangarajan, R., Kumpiene, J., Park, J., Makino, T., Kirkham, M. B., & Scheckel, K. (2014). Review remediation of heavy metal(loid)s contaminated soils: to mobilize or to immobilize? Journal of Hazardous Materials, 266, 141–166.
Cao, X. D., Ma, L. N., Gao, B., & Harris, W. (2009). Dairy-manure derived biochar effectively sorbs lead and atrazine. Environmental Science and Technology, 43, 3285–3291.
Chorom, M., Karkaragh, R. M., Kaviani, B., & Kalkhajeh, Y. K. (2013). Monometal and competitive adsorption of Cd, Ni, and Zn in soil treated with different contents of cow manure. Applied and Environmental Soil Science. doi:10.1155/2013/510278. Article ID 510278.
Cui, S., Zhou, Q., & Chao, L. (2007). Potential hyper-accumulation of Pb, Zn, Cu and Cd in endurant plants distributed in an old smeltery, northeast China. Environmental Geology, 51, 1043–1048.
Fellet, G., Marmiroli, M., & Marchiol, L. (2014). Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Science of the Total Environment, 468–469, 598–608.
Garcia, L., & John, J. H. (1976). Foliar fertilization of soybeans during the seed-filling period. Agronomy Journal, 68(4), 653–657.
Gallego, S. M., Pena, L. B., Barcia, R. A., Azpilicueta, C. E., Iannone, M. F., Rosales, E. P., Zawoznik, M. S., Groppa, M. D., & Benavides, M. P. (2012). Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environmental and Experimental Botany, 83, 33–46.
Hassan, Z., & Aarts, M. G. M. (2011). Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants. Environmental and Experimental Botany, 72, 53–63.
Houben, D., Evrard, L., & Sonnet, P. (2013a). Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Biomass and Energy, 57, 196–204.
Houben, D., Evrard, L., & Sonnet, P. (2013b). Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere, 92, 1450–1457.
Kamal, M., Ghaly, A. E., Mahmoud, N., & Cote, R. (2004). Phytoaccumulation of heavy metals by aquatic plants. Environment International, 29, 1029–1039.
Kołodynska, D., Wnetrzak, R., Leahy, J. J., Hayes, M. H. B., Kwapinski, W., & Hubicki, Z. (2012). Kinetic and adsorptive characterization of biochar in metal ions removal. Chemical Engineering Journal, 197, 295–305.
Lee, J. W., Kidder, M., Evans, B. R., Paik, S., Buchanan, A. C., Garten, C. T., & Brown, R. C. (2010). Characterization of biochars produced from cornstovers for soil amendment. Environmental Science and Technology, 44, 7970–7974.
Lehmann, J., & Joseph, S. (2009). Biochar for environmental management (p. 416). London: Earth Scan Publishers.
Li, M., Liu, Q., Guo, L., Zhang, Y., Lou, Z., Wang, Y., & Qian, G. (2013). Cu(II) removal from aqueous solution by Spartina alterniflora derived biochar. Bioresource Technology, 141, 83–88.
Liang, B., Lehmann, J., Solomon, D., Kinyangi, J., Grossman, J., O’Neill, B., Skjemstad, J. O., Thies, J., Luizão, F. J., Petersen, J., & Neves, E. G. (2006). Black carbon increases cation exchange capacity in soils. Soil Science Society of America Journal, 70, 1719–1730.
Malik, R. N., Husain, S. Z., & Nazir, I. (2010). Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pakistan Journal of Botany, 42(1), 291–301.
Mclatchey, G. P., & Reddy, K. R. (1998). Regulation of organic matter decomposition and nutrient release in a wet soil. Journal of Environmental Quality, 27, 1268–1274.
Mukherjee, A., & Lal, R. (2014). The biochar dilemma. Soil Research, 52(3), 217–230.
Mukherjee, A., & Zimmerman, A. R. (2013). Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma, 193–194, 122–130.
Okeola, F. O., & Odebunmi, E. O. (2010). Comparison of Freundlich and Langmuir isotherms for adsorption of methylene blue by agrowaste derived activated carbon. Advances in Environmental Biology, 4(3), 329–335.
Panwar, B. S., Singh, J. P., & Laura, R. D. (1999). Cadmium uptake by cowpea and mungbean as affected by Cd and P application. Water, Air, and Soil Pollution, 112, 163–169.
Park, J. H., Choppala, G., Bolan, N., Chung, J. W., & Chuasavathi, T. (2011). Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant and Soil, 348, 439–451.
Park, J. H., Choppala, G., Lee, S. J., Bolan, N., Chung, J. W., & Edraki, M. (2013). Comparative sorption of Pb and Cd by biochars and its implication for metal immobilization in soils. Water, Air, and Soil Pollution, 224, 1711. doi:10.1007/s11270-013-1711-1.
Paz-Ferreiro, J., Lu, H., Fu, S., Mendez, A., & Gasco, G. (2014). Use of phytoremediation and biochar to remediate heavy metal polluted soils: a review. Solid Earth, 5, 65–75.
Pence, N. S., Larsen, P. B., Ebbs, S. D., Letham, D. L. D., Lasat, M. M., Garvin, D. F., Eide, D., & Kochian, L. V. (2000). The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proceedings of the National Academy of Sciences of the United States of America, 97, 4956–4960.
Prasad, M. N. V. (2003). Phytoremediation of metal-polluted ecosystems: hype for commercialization. Russian Journal of Plant Physiology, 50, 686–700.
Quevauviller, P., Lachica, M., Barahona, E., Gomez, A., Rauret, G., Ure, A., & Muntau, H. (1998). Certified reference material for the quality control of EDTA- and DTPA-extractable trace metal contents in calcareous soil (CRM 600). Fresenius Journal of Analytical Chemistry, 360, 505–511.
Rao, K. S., Mohapatra, M., Anand, S., & Venkateswarlu, P. (2010). Review on cadmium removal from aqueous solutions. International Journal of Engineering, Science and Technology, 2, 81–103.
Rees, F., Simonnot, M. O., & Morela, J. L. (2014). Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase. European Journal of Soil Science, 65, 149–161.
Regmi, P., Moscoso, J. L. G., Kumar, S., Cao, X., Mao, J., & Schafran, G. (2012). Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. Journal of Environmental Management, 109, 61–69.
Riser-Roberts, E. (1998). Remediation of petroleum contaminated soils: biological, physical, and chemical processes (p. 576). Florida: CRC.
Romkens, P. F. A. M., Guo, H. Y., Chu, C. L., Liu, T. S., Chiang, C. F., & Koopmans, G. F. (2009). Prediction of cadmium uptake by brown rice and derivation of soil–plant transfer models to improve soil protection guidelines. Environmental Pollution, 157, 2435–2444.
Simmons, R. W., Pongsakul, P., Saiyasitpanich, D., & Klinphoklap, S. (2005). Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: implications for public health. Environmental Geochemistry and Health, 27, 501–511.
Singh, B., Singh, B. P., & Cowie, A. L. (2010). Characterisation and evaluation of biochars for their application as a soil amendment. Soil Research, 48(7), 516–525.
Song, W., & Guo, M. (2012). Quality variations of poultry litter biochar generated at different pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, 94(3), 138–145.
Sterckeman, T., Redjala, T., & Morel, J. L. (2011). Influence of exposure solution composition and of plant cadmium content on root cadmium short-term uptake. Environmental and Experimental Botany, 74, 131–139.
Tangahu, B. V., Abdullah, S. R. S., Basri, H., Idris, M., Anuar, N., & Mukhlisin, M. (2011). A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. International Journal of Chemical Engineering. doi:10.1155/2011/939161. Article ID 939161.
Tangjuank, S., Insuk, N., Tontrakoon, J., & Udeye, V. (2009). Adsorption of lead(II) and cadmium(II) ions from aqueous solutions by adsorption on activated carbon prepared from cashew nut shells. World Academy of Science, Engineering and Technology, 52, 110–116.
Tajar, A. F., Kaghazchi, T., & Soleimani, M. (2009). Adsorption of cadmium from aqueous solutions on sulfurized activated carbon prepared from nut shells. Journal of Hazardous Materials, 165(1–3), 1159–1164.
Thomson, C. J., Marschner, H., & Romheld, V. (1993). Effect of nitrogen fertilizer form on pH of the bulk soil and rhizosphere, and on the growth, phosphorus, and micronutrient uptake of bean. Journal of Plant Nutrition, 16(30), 493–506.
Trakal, L., Komarek, M., Szakova, J., Zemanova, V., & Tlustos, P. (2011). Biochar application to metal-contaminated soil: evaluating of Cd, Cu, Pb and Zn sorption behavior using single- and multi-element sorption experiment. Plant, Soil and Environment, 57, 372–380.
Trakal, L. T., Sigut, R., Sillerova, H., Faturikova, D., & Komarek, M. (2014). Copper removal from aqueous solution using biochar: effect of chemical activation. Arabian Journal of Chemistry, 7, 43–52.
Tan, C., Shanb, X., Xuc, G., Lina, Y., & Chena, Z. (2011). Phytoaccumulation of cadmium through Azolla from aqueous solution. Ecological Engineering, 37, 1942–1946.
Uchimiya, M., Lima, I. M., Klasson, K. T., Chang, S., Wartelle, L. H., & Rodgers, J. E. (2010). Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. Journal of Agricultural and Food Chemistry, 58, 5538–5544.
Uchimiya, M., Wartelle, L. H., Klasson, K. T., Fortier, C. A., & Lima, I. M. (2011). Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. Journal of Agricultural and Food Chemistry, 59, 2501–2510.
Upadhyay, K. P., George, D., Swift, R. S., & Galea, V. (2014). The influence of biochar on growth of lettuce and potato. Journal of Integrative Agriculture, 13(3), 541–546.
Upadhyay, A. R., Mishraa, V. K., Pandeya, S. K., & Tripathi, B. D. (2007). Biofiltration of secondary treated municipal wastewater in a tropical city. Ecological Engineering, 30, 9–15.
US Environmental Protection Agency (US EPA). (1996). Method 3050B acid digestion of sediments, sludges, and soils: revision 2. [Online] Available from: http://www.epa.gov/osw//hazard/testmethods/sw846/pdfs/3050b.pdf [2012, August 5]
White, P. J., & Brown, P. H. (2010). Plant nutrition for sustainable development and global health. Annals of Botany, 105, 1073–1080.
Xian, X. (1989). Effect of chemical forms of cadmium, zinc, and lead in polluted soil on their uptake by cabbage plants. Journal of Plant and Soil, 113, 257–264.
Xu, X., Cai, X., Zhao, L., Wang, H., Yu, H., & Gao, B. (2013). Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environmental Science and Pollution Research, 20(1), 358–368.
Yadav, S. K., Juwarkar, A. A., Kumar, G. P., Thawale, P. R., Singh, S. K., & Chakrabarti, T. (2009). Bioaccumulation and phyto-translocation of arsenic, chromium and zinc by Jatropha curcas L.: impact of dairy sludge and biofertilizer. Bioresource Technology, 100, 4616–4622.
Yoon, J., Cao, X., Zhou, Q., & Ma, L. Q. (2006). Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. The Science of the Total Environment, 368, 456–464.
Yuan, J., Xu, R., & Zhang, H. (2011). The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresource Technology, 102, 3488–3497.
Zheng, H., Wang, Z., Deng, X., Herbert, S., & Xing, B. (2013). Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma, 206, 32–39.
Acknowledgments
This research was supported by grants awarded through the 90th Year Chulalongkorn Scholarship, Graduate School, Chulalongkorn University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prapagdee, S., Piyatiratitivorakul, S., Petsom, A. et al. Application of Biochar for Enhancing Cadmium and Zinc Phytostabilization in Vigna radiata L. Cultivation. Water Air Soil Pollut 225, 2233 (2014). https://doi.org/10.1007/s11270-014-2233-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2233-1