Abstract
The sulfate input and the occurrence of dryout and rewetting may promote the production of toxic methylmercury (MeHg) in a constructed wetland, Stormwater Treatment Area 2 (STA-2) in South Florida. Therefore, the aim of this study was to investigate the influences of inflow water quality, especially inflow sulfate, and the dryout and rewetting cycle on the mercury (Hg) methylation in three independent cells of STA-2 from 2000 to 2007. Because the majority of the total Hg (THg) bioaccumulated in fish is in MeHg form, THg concentration in mosquitofish was used to present the MeHg production in STA-2. Mosquitofish THg in Cells 1 and 2 (with median values of 0.101 and 0.02 mg/kg, respectively) were significantly higher than in Cell 3 and inflow (both with a median value of 0.01 mg/kg). The difference in mosquitofish THg among the three cells was likely a result of the drying and rewetting cycles occurred in Cells 1 and 2, which promoted the Hg methylation. Inflow sulfate, inorganic Hg, and chloride exhibited a significant correlation with mosquitofish THg in cells, suggesting that these inflow variables played important roles on the Hg methylation. The results indicate that inflow sulfate may likely stimulate sulfate-reducing bacteria and subsequently lead to produce MeHg in the three cells. Our findings in this study indicate that preventing the occurrence of dryout in wetland will help to decline the Hg methylation, and sulfate input is a key factor to influence the Hg methylation in wetland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to its characteristics, such as low melting and boiling points, mercury (Hg) is a globally spread pollutant and released from both natural and anthropogenic sources (Hylander and Meili 2003). As a result of centuries of anthropogenic activities, such as mining and fossil fuel burning, the global atmospheric Hg deposition rate is approximately three times greater than in preindustrial times, leading to increased concentrations in freshwater systems and biota even in remote areas that are free from direct anthropogenic impacts (Ullrich et al. 2001). Hg contamination generally only becomes a global environmental concern when the methylation of inorganic Hg (IHg) to methylmercury (MeHg) occurs. As a potent neurotoxin, MeHg is the most toxic form of Hg in the environment. Owing to its lipophilic and protein-binding properties, MeHg is also the only Hg compound that is readily bioaccumulated and biomagnified in the food web, which may also pose a threat to humans and other fish-eating animals (Ullrich et al. 2001; Hylander and Meili 2003).
In situ production of MeHg via microbial methylation is the primary source of MeHg to the most aquatic systems (Gilmour and Henry 1991; Gilmour et al. 1992). As far as we know, sulfate-reducing bacteria (SRB) are widely accepted as the primarily methylators of Hg methylation (Compeau and Bartha 1985; Gilmour et al. 1992; Ullrich et al. 2001). SRB thrive in oxygen-free and carbon-rich environments, degrade organic matter, and reduce sulfate to sulfide in both freshwater and estuarine sediments (Ullrich et al. 2001). The formation of MeHg produced in the environments depends on factors that control SRB population growth or metabolic function (Winfrey and Rudd 1990; Gilmour and Henry 1991; Ullrich et al. 2001) and on the bioavailability of Hg for SRB (Ullrich et al. 2001). The significant ecological factors include dissolved organic carbon (DOC) (Ullrich et al. 2001; Aiken et al. 2003), SO4 2−/S2− (Benoit et al. 1999, 2001; Ullrich et al. 2001), pH (Winfrey and Rudd 1990; Regnell 1994; Ullrich et al. 2001), and temperature (Bodaly et al. 1993; Ullrich et al. 2001), which put effect on the microbial growth. The bioavailability of Hg for SRB is likely influenced by complex ligands in environments, such as chlorides (Barkay et al. 1998; Ullrich et al. 2001), sulfides (Benoit et al. 1999, 2001; Ullrich et al. 2001), and organic compounds (Barkay et al. 1997; Ullrich et al. 2001; Aiken et al. 2003), which determine the ability of Hg to cross the microbial cell membranes. Wetlands particularly possess many environmental factors that promote Hg methylation and are recognized as “hot spots” for MeHg production (Gilmour et al. 1992; St. Louis et al. 1994; Branfireun et al. 1999). Besides, the dryout and rewetting cycle in wetland has been found to enhance Hg methylation and microbial sulfate reduction, thereby causing increased Hg methylation in the wetlands (Dmytriw et al. 1995; Gilmour et al. 2004b). Furthermore, Ackerman and Eagles-Smith (2010) reported that agricultural wetlands are potential hot spots for MeHg contamination due to their periodic flooding schedules.
Sulfate at 60 to100 times that of background levels (≤1 mg/L) is delivered to the Everglades in the runoff from Everglades Agricultural Area (EAA) (Orem 2004). Stormwater Treatment Areas (STAs), which are a system of large treatment wetlands, shallow and freshwater marshes, are constructed primarily to remove total phosphorus (TP) from the EAA runoffs before it is discharged to the Everglades Protection Area (Chimney et al. 2000). About 70 % of TP loads was removed through STAs (Orem et al. 2011), but little has been done to reduce sulfate contamination of the ecosystem (i.e., only about 11 % sulfate reduction) (Orem 2004; Orem et al. 2011). Except for the sulfate input, the cycle of dryout and rewetting occurred during the operation in some of STAs, for example, the drought occurred in the early operation period of Stormwater Treatment Area 2 (STA-2). The objective of this work was to investigate the Hg issue in STAs; STA-2 taken as the example for this study. The work included two parts: (1) the effect of dryout and rewetting on the production of MeHg in STA-2; and (2) the relationship between inflow water quality variables and the MeHg production in STA-2, especially the impact of inflow sulfate load on the Hg methylation. This study can obtain the insight into the factors influencing on the Hg methylation and help to better manage and resolve the MeHg issue in the constructed wetlands.
2 Materials and Methods
2.1 Study Area
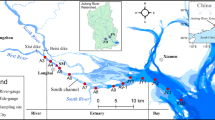
STA-2 is located in Western Palm Beach County, Florida, immediately west of Water Conservation Area 2 (WCA-2) (Fig. 1), and is divided into four parallel north-south treatment cells with a total surface area of approximately 8,000 ac before 2012 (Cell 4 is constructed in 2007 with about 2,000 ac). Water from flow structures Station 6 (S-6) and Gate 328 (G-328) enter the supply canal and are conveyed southward to the inflow canal, which extends across the northern perimeter of the STA-2. A series of inflow culverts convey water from the inflow canal to the respective treatment cells. Water then flows southward through the treatment cells and eventually discharges into the discharge canal via culverts or gated spillways. The outflow pump station Gate 335 (G-335) conveys water to WCA-2.
2.2 Data Collection and Analytical Methods
As a condition of its operating permits, the South Florida Water Management District (District) is required to monitor Hg in surface water quality at various locations throughout the Everglades Protection Area. The unfiltered water samples of inflow S-6, G-328, and G-328B (located in the supply canal) were collected biweekly for water quality analysis, including total Hg (THg) and MeHg analyses. The inflow fish was semiannual sampled in the supply canal. The fish sample in every cell was collected monthly if the fish was available. The fish sampling points for the three cells were showed in Fig. 1; four sampling points (A, AA, BB, and CC, respectively) in Cell 1 and three sampling points (A, B, and C, respectively) both in Cells 2 and 3.
Since only Cells 1, 2, and 3 in STA-2 operated during the study period (from 2000 to 2007), Cell 4 was not included in this study. The following inflow water variables, including sulfate, pH, DOC, temperature, chloride, and IHg (THg minus MeHg), were selected to discuss the influence of inflow water quality on the Hg methylation in the constructed wetland, and the inflow sulfate was the focus of the water quality variables in the present study (Table 1).
It is found that greater than 95 % of THg in fish tissue is in the MeHg form (Celo et al. 2006; Grieb et al. 1990). Furthermore, the analysis of fish tissue for THg, which is a more straightforward and less costly procedure than the analysis for MeHg, can be interpreted as being equivalent to the analysis of MeHg (Fink et al. 2005). Therefore, the fish THg in cells was to represent the in situ MeHg production in the constructed wetland in the present study. The three species of fish, mosquitofish (Gambusia holbrooki), sunfish (Lepomis spp.), and largemouth bass (Micropterus salmoides), were sampled for determining THg bioaccumulation in fish. Among the three species of fish, the mosquitofish is at the low trophic level, which is the prey fish for sunfish and largemouth bass; hence, the THg biomagnifications in sunfish and largemouth bass are greater than in mosquitofish, making the THg data in sunfish and largemouth bass more complicated than in mosquitofish. Moreover, widespread occurrence in the Everglades, relatively small home range, rapid population turnover, and short life span (average life span only 4 to 5 months) make mosquitofish adjust to new condition more rapidly than the populations of sunfish and bass that are longer-lived (Fink et al. 2005; Rumbold and Fink 2006). These characteristics make the mosquitofish a potentially excellent representative indicator of short-term, localized changes in MeHg through time (Fink et al. 2005; Rumbold and Fink 2006). Therefore, in the present study, mosquitofish THg was treated as the MeHg bioindicator. At each sampling site, between 75 and 250 mosquitofish were collected; the individual fish was stored on ice, refrigerated for not more than 48 h, and then composited and homogenized using a Polytron® apparatus. Thereafter, the homogenate was frozen prior to shipment on blue ice or double-bagged wet ice to the Florida Department of Environmental Protection (FDEP) mercury clean laboratory.
All data of the inflow water quality variables and THg in mosquitofish tissue used for this work were obtained from the District’s DBHYDRO database (www.sfwmd.gov/dbhydro). Data were generated by the District and the FDEP, both of which are certified by the Florida Department of Health under the National Environmental Laboratory Accreditation Program. For analytes other than THg and MeHg, surface water analyses were conducted by the District’s analytical chemistry laboratory on field-preserved samples using standard methods. THg determination in surface water was carried out using US Environmental Protection Agency (USEPA) Method 1631 (Mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry) or a modification of Method 1631. Moreover, THg determination in fish tissue followed either the USEPA Method 245.6 (Determination of mercury in tissues by cold vapor atomic absorption spectrometry) or, if at low level, a modification of Method 1631. MeHg analysis in surface water used modified USEPA Draft Method 1630 (Methylmercury in water and tissues by distillation, extraction, aqueous phase ethylation, purge and trap, isothermal GC separation, and cold vapor atomic fluorescence spectrometry).
Quality assurance (QA) measures were incorporated during the sample collection and laboratory analysis to evaluate the quality of the data. All of the above methods use performance-based standards employing the appropriate levels of QA/quality control (QC) required by the National Environmental Laboratory Accreditation Conference, the specific reference method, and the Protocol. Laboratory QC samples included method blanks, lab-fortified blanks, matrix spikes, standard reference materials, and laboratory duplicates. Field QC samples included trip blanks, field blanks, equipment blanks, both of pre-cleaned equipment at the start of sampling and field-cleaned equipment at the end of sampling, container and processing equipment blanks, and field duplicates (Rumbold and Fink 2006).
2.3 Statistical Analysis
All statistical analyses were conducted using PASW® Statistics 18.0 (SPSS Inc., Chicago, USA). Before correlation analysis, the data of inflow water quality and mosquitofish THg concentration were log-transformed to ensure normal distribution. The correlated relationship between individual inflow water quality variable (sulfate, pH, temperature, DOC, chloride, and IHg, respectively) and mosquitofish THg in the individual cell of STA-2 was evaluated using Pearson moment correlation analysis.
3 Results and Discussion
3.1 The Effect of Dryout on the Hg Methylation in STA-2
During the monitoring period at STA-2 from 2000 to 2007, mosquitofish THg concentration in inflow varied from 0.027 to 0.003 mg/kg with a medium value of 0.01 mg/kg, while mosquitofish THg concentration ranged from 0.321 to 0.0078 mg/kg with a medium value of 0.101 mg/kg in Cell 1, varied from 0.103 to 0.003 mg/kg with a medium value of 0.02 mg/kg in Cell 2, and ranged from 0.0335 to 0.0042 mg/kg with a medium value of 0.01 mg/kg in Cell 3. Overall, during the monitoring period, there was no difference in mosquitofish THg concentration between Cell 3 and inflow (ANOVA, p > 0.05). Nevertheless, THg concentrations of mosquitofish in Cells 1 and 2 from 2000 to 2007 were significantly greater than in inflow and Cell 3 (ANOVA, p < 0.001 for both Cell 1 and Cell 2 compared with inflow and Cell 3). The discriminations of THg levels in mosquitofish among the three cells and inflow were pronounced (Fig. 2), the greatest in Cell 1, median in Cell 2, and the lowest in Cell 3 and inflow.
The differences in mosquitofish THg between the three cells and inflow are likely attributed to the severe drought during 2000 and 2001, which occurred in both Cells 1 and 2. Cell 1 went periods of dryout in the fall of 2000 and 2001, and Cell 2 went period dry in the summer of 2001 (Fink et al. 2005). During the severe drought of 2000 through 2001 in Cells 1 and 2, supplemental water deliveries were made to Cell 3 to prevent dryout. The process of drying and rewetting has been found to provide fuel for Hg methylation and microbial sulfate reduction, thereby causing increased Hg methylation in the wetland (Dmytriw et al. 1995; Gilmour et al. 2004b). As soil dryout leads to organic matter, iron(II), and sulfide in the surficial soil oxidized to labile organic matter, iron(III), and sulfate, respectively (Dmytriw et al. 1995; Gilmour et al. 2004b), the rewetting of soils after dryout is accompanied by a flush release of sulfate, iron, and labile organic matter (Gilmour et al. 2004b). Following the flush release of sulfate, nutrients, and anoxic conditions, the metabolic activity of SRB is likely to be stimulated and additionally produce MeHg. Thus, the dryout and rewetting cycle made the Hg methylation in Cells 1 and 2 significantly greater than in Cell 3 and inflow, since no dryout occurred in Cell 3 and inflow.
From Fig. 3, it is seen that no clear trend existed in mosquitofish THg concentration in inflow; however, the levels of mosquitofish THg concentrations in all the three cells were decreasing until the beginning of 2004, and kept stabilized from 2004 to 2007. The trends of mosquitofish THg for the three cells indicate that the Hg methylation in all the three cells of STA-2 mainly occurred from 2000 to 2003. Similarly, the mosquitofish THg concentrations in all the three cells along with the concentrations of inflow sulfate (Fig. 4) and IHg (Fig. 5) again clearly demonstrated that the Hg methylation in all the three cells mainly occurred before 2004. Since, the buildup of sulfide (the reduction product of sulfate) and/or depletion of labile organic matter may likely end the pulse of MeHg production that follows the rewetting. The buildup of sulfide in sediment porewater appears to inhibit the Hg methylation through forming insoluble HgS, which decreases the Hg availability to SRB (Gilmour et al. 1992; Benoit et al. 1999; Orem et al. 2011). The labile organic matter provides carbon source for the metabolic activity of SRB (Gilmour et al. 1992; Ullrich et al. 2001). It comes to the conclusion that the conditions in all three cells from 2004 to 2007 were no longer fit for the Hg methylation.
3.2 The Influence of Inflow Water Quality Variables on the Hg Methylation
Since the conditions in all the three cells from 2004 to 2007 were no longer fit for the Hg methylation, accordingly, the correlated relationships between inflow water quality variables and mosquitofish THg in all the three cells were assessed until the beginning of 2004 (Table 2).
Inflow sulfate loading was negatively, significantly correlated with mosquitofish THg in all the three cells (Fig. 6), which accounted for 42, 58.5, and 50.5 % of the variations in mosquitofish THg concentrations in Cells 1, 2, and 3, respectively (Table 2). Among the inflow water quality variables, sulfate loading to STA-2 was of the most concern. The significant correlations between inflow sulfate load and the levels of mosquitofish THg in all the three cells indicate that sulfate input to STA-2 likely stimulated the activity of SRB and played a crucial role in the Hg methylation in all the three cells of the STA-2. The dual effect of sulfur on the Hg methylation, that is, the stimulation effect of sulfate and the inhibition effect of sulfide, produces the so-called “Goldilocks effect”, where the levels of sulfate and sulfide are just right for the Hg methylation and the MeHg producing rate is maximum (Orem 2007). This conceptual model for the role of sulfur in the MeHg production has been verified for the Everglades by field, laboratory, and mesocosm experiments (Orem 2007). In the Everglades, Orem (2004) reported that the areas of the highest MeHg production occur where sulfate contamination is moderate (2–10 mg/L), and sulfide levels are low enough to avoid the inhibition of the MeHg formation. Gilmour and Henry (1991) proposed an optimal sulfate concentration range of 19 to 48 mg/L (optimum concentration was ~29 mg/L) for the Hg methylation by SRB in sediments, above which the methylation is inhibited and below which sulfate becomes limiting for the Hg methylation and sulfate-reduction processes. Gilmour et al. (1992) found that the MeHg production increases with sulfate concentrations up to 10 mg/L and declines when porewater sulfide exceeds 0.6 mg/L. Weber (1993) demonstrated that the Hg methylation completely stops at a sulfate concentration exceeding about 480 mg/L. King et al. (1999) observed active MeHg formation in the presence of 2.88 g/L sulfate and millimolar concentrations of dissolved sulfide. In the present study, the negatively correlated relationships between inflow sulfate and mosquitofish THg in all the three cells indicate that the sulfate concentration in inflow was much greater than the optimal concentration for the MeHg production in all three cells of STA-2. Similarly, Selvendiran et al. (2008) also found a significantly negative correlation between MeHg and sulfate concentration (6.33 ± 0.9 mg/L) during the growing season in a forested wetland, along with an increase in MeHg concentration concomitant with the decrease in sulfate concentration in wetland stream waters.
Plenty of studies have recognized that sulfate loading is an important factor in causing the increased Hg methylation in the Everglades (Orem 2004; Gilmour et al. 2007b; Corrales et al. 2011; Orem et al. 2011). Sulfur used in the agricultural application and sulfur released by the oxidation of organic EAA soils (including legacy agricultural applications and natural sulfur) are the primary sources of sulfate enrichment in the EAA canals (Orem 2004; Corrales et al. 2011; Orem et al. 2011). In general, sulfate concentration decreases from north to south in the Everglades ecosystem (Orem 2004; Orem et al. 2011). The dual effect of sulfur on the MeHg production and the north-to-south gradient in sulfate concentrations in the Everglades provide geographic context to the MeHg distributions (Orem et al. 2011). Unenriched areas of the ecosystem with sulfate concentration less than 1 mg/L exhibit low levels of MeHg due to sulfate limitation on the Hg methylation (Orem et al. 2011). In sulfate-enriched areas (concentrations more than 20 mg/L), buildup of sulfide inhibits the MeHg production (Orem et al. 2011). Areas with intermediate concentrations of sulfate (1–20 mg/L) have sulfate and sulfide levels that promote the maximum MeHg production (Gilmour et al. 2007a), where the porewater sulfide concentrations are moderate (5–150 μg/L) (Gilmour et al. 1998; Orem et al. 2011). Several researchers have proposed the sulfate level in surface water to reduce the formation of MeHg in the ecosystem as much as possible. Corrales et al. (2011) recommended 1 mg/L as a sulfate threshold to control the MeHg formation in the Everglades and emphasized that above this level, particularly above 2 mg/L, the ecological risk to the ecosystem increases because at intermediate levels of sulfate, the Hg methylation is optimized. Jeremiason et al. (2006) also demonstrated that sulfate concentrations below 1 mg/L would not favor the Hg methylation process, eventually helping to depress the MeHg levels within the ecosystem as well. Likewise, Orem (2007) stated that a desirable goal for sulfate concentration in the Everglades would be approaching the background level (≤1 mg/L).
Inflow IHg was positively, significantly correlated with the mosquitofish THg in Cells 1 and 2, but not in Cell 3 (Fig. 7), and explained 48 and 51.3 % of the mosquitofish THg variations in Cells 1 and 2, respectively (Table 2). Fink (2002) reported that MeHg is likely to be synthesized primarily from the new IHg being supplied by runoff and wet and dry atmospheric deposition, not from soil release, even following a dryout event. Gilmour et al. (2004a) found that the increase in MeHg in surface sediments and in fish shows a linear response to the Hg addition and suggested that the newly deposited Hg is much more available for methylation and bioaccumulation than is existing Hg in surface soils. In this study, the good correlations between inflow IHg and mosquitofish THg in Cells 1 and 2 are consistent with the findings of Fink (2002) and Gilmour et al. (2004a), indicating that the newly supplied IHg from inflow for STA-2 was the Hg source for the methylation. No significant correlation between inflow IHg and mosquitofish THg in Cell 3 demonstrates that together, too high sulfate concentration in inflow and no dryout occurrence made the capability of Hg methylation limited in Cell 3, which caused that the Hg methylation in Cell 3 likely could not respond well to the change in inflow IHg loading.
Inflow chloride was positively, significantly correlated with the mosquitofish THg in Cells 1 and 2, but not in Cell 3, and accounted for 44.2 and 51.1 % of the mosquitofish THg variations in Cells 1 and 2, respectively (Table 2). The influence of chloride on the Hg methylation is likely contributable to the competition of chloride for binding Hg, forming chloride-mercury complexes, which endow negatively charged forms (e.g., HgCl3 −, HgCl4 2−). Comparatively, the neutral form (HgCl2) is more bioavailable for microbes than negatively charged forms, because its uptake by microbes is likely a passive diffusion process (Barkay et al. 1997; Ullrich et al. 2001). Barkay et al. (1997) reported that when chloride concentrations were above 1 mM, a decreased bioavailability of Hg(II) to the bioindicator was attributed to an increased proportion of negatively charged chlorine-mercury complexes. Hence, the inversely correlated relationships between inflow chloride and the mosquitofish THg in Cells 1 and 2 indicate that the concentration of chloride in inflow favored the formation of negatively charged chlorine-mercury complexes. No significant correlation between inflow chloride and mosquitofish THg in Cell 3 again suggests that the limited capability of Hg methylation in Cell 3 likely could not respond well to the change in inflow chloride loading.
No correlation was found between inflow pH and mosquitofish THg in Cells 1 and 2, but inflow pH positively, significantly affected the mosquitofish THg in Cell 3, and accounted for 47.8 % of the variations in mosquitofish THg concentration in Cell 3 (Table 2). Generally, the Hg methylation in acidic environment is prone to be enhanced in comparison with alkaline surrounding, and elevated Hg levels in fish are commonly found in acidified lakes (Gilmour and Henry 1991; Ullrich et al. 2001). It is uncertain whether the stimulation of methylation in lake water is a direct effect of low pH on the methylation process, or whether it is related to other factors that are influenced by pH, such as the loss of volatile Hg species from water surfaces, or changes in Hg solubility and partitioning (Ullrich et al. 2001). The positive effect of inflow pH on the mosquitofish THg in Cell 3 was unexpected to occur, which maybe attributable to the unclear effect of pH on Hg methylation. In the present study, the inflow water condition was slightly alkaline, the pH value in inflow was chiefly within the scope of 7 and 8; therefore, insignificant correlation between inflow pH and the mosquitofish THg in Cells 1 and 2 may indicate that the range of inflow pH was possibly too narrow either to influence the availability of Hg to the methylating microorganisms or to impose any effect on the microbial community.
Both inflow DOC and temperature showed insignificant correlation on the mosquitofish THg in all the three cells (Table 2). The effect of DOC on the Hg methylation is complicated. DOC can both decrease and enhance the bioavailability of Hg for microbes (Gorski 2004). The Hg bioavailability can be reduced by DOC, enhancing photochemical reduction of Hg(II) to Hg0 (Ravichandran 2004), and can be enhanced by the mixed complexes that contain both DOC and reduced sulfur groups (DOC-Hg-SH) (Hsu-Kim et al. 2005; Miller et al. 2007). DOC also can act as energy source for microbial activity to stimulate the Hg methylation (Ullrich et al. 2001). In the areas of Florida Everglades, DOC concentration is quite high in the Everglades marshes due to the high natural production of organic carbon in the peat soils and wetlands (Liu et al. 2008; Aiken et al. 2011), and DOC has been found to be strongly correlated to THg and MeHg in this region (Liu et al. 2008). However, in the present study, inflow DOC displayed no effect on the Hg methylation in all the three cells of STA-2, suggesting that the effect of inflow DOC on the Hg methylation in STA-2 was likely hard to explain because of DOC bearing the complicated property on the Hg methylation. Moderately high temperatures have been found a stimulating effect on the Hg methylation, which is most likely for the sake of the enhanced microbial activity by the increase in temperature (Bodaly et al. 1993; Ullrich et al. 2001), but in this study, no correlated relationship between inflow temperature and mosquitofish THg was observed in Pearson correlation analysis. Inflow temperature mainly ranged from 20 to 30 °C, kept relatively high and almost stable, so the narrow seasonal change in water temperature may be difficult to impact on the microbial activity noticeably.
The results indicate that inflow sulfate, IHg, and chloride were critical inflow water quality parameters influencing MeHg production in STA-2. Some studies also have evidenced the correlation between MeHg production and surface water quality. MeHg production in a temperate lake is found to be related to the Hg(II), sulfate, and DOC input to the lake (Watras and Morrison 2008).
4 Conclusion
Results from this study indicate that the drying and rewetting cycles in Cells 1 and 2 promoted the Hg methylation in STA-2, leading to the MeHg production greater than in Cell 3 and inflow, where no drying occurred. Pearson correlation results demonstrate that inflow sulfate, IHg, and chloride showed significantly correlated relationships with mosquitofish THg in cells, suggesting that these inflow variables played important roles on the Hg methylation in the cells of STA-2. The significant correlations between inflow sulfate and mosquitofish THg in all the three cells suggest that SRB were likely stimulated by the sulfate input to STA-2 and subsequently produced MeHg. And what is more, the negative impacts of inflow sulfate on the Hg methylation in all the three cells indicate that inflow sulfate concentration was much greater than the optimal concentration for the Hg methylation. Our findings in this study indicate that preventing the occurrence of dryout from wetlands will help to abate the Hg methylation, and sulfate input to wetlands is a key factor to affect the Hg methylation.
References
Ackerman, J. T., & Eagles-Smith, C. A. (2010). Agricultural wetlands as potential hotspots for mercury bioaccumulation: experimental evidence using caged fish. Environmental Science and Technology, 44, 1451–1457.
Aiken, G. R., Haitzer, M., Ryan, J. N., & Nagy, K. (2003). Interactions between dissolved organic matter and mercury in the Florida Everglades. Journal de Physique IV, 107, 29–32.
Aiken, G. R., Gilmour, C. C., Krabbenhoft, D. P., & Orem, W. (2011). Dissolved organic matter in the Florida Everglades: implications for ecosystem restoration. Critical Reviews in Environmental Science and Technology, 41, 217–248.
Barkay, T., Gillman, M., & Turner, R. R. (1997). Effects of dissolved organic carbon and salinity on bioavailability of mercury. Applied and Environmental Microbiology, 63, 4267–4271.
Barkay, T., Turner, R. R., Rasmussen, L. D., Kelly, C. A., & Rudd, J. W. M. (1998). Bioluminescence Methods and Protocols. In R. A. LaRossa (Ed.), Luminescence facilitated detection of bioavailable mercury in natural waters (pp. 231–246). Totowa, NJ: Humana Press.
Benoit, J. M., Gilmour, C. C., Mason, R. P., & Heyes, A. (1999). Sulfide controls on mercury speciation and bioavailability in sediment pore waters. Environmental Science and Technology, 33, 951–957.
Benoit, J. M., Gilmour, C. C., & Mason, R. P. (2001). The influence of sulfide on solid-phase mercury bioavailability for methylation by pure cultures of Desulfobulbus propionicus (1pr3). Environmental Science and Technology, 35, 127–132.
Bodaly, R. A., Rudd, J. W. M., Fudge, R. J. P., & Kelly, C. A. (1993). Mercury concentrations in fish related to size of remote Canadian Shield lakes. Canadian Journal of Fishery and Aquatic Science, 50, 980–987.
Branfireun, B. A., Roulet, N. T., Kelly, C. A., & Rudd, J. W. M. (1999). In situ sulphate stimulation of mercury methylation in a boreal peatland: toward a link between acid rain and methylmercury contamination in remote environments. Global Biogeochemical Cycles, 13, 743–750.
Celo, V., Lean, D. R. S., & Scott, S. L. (2006). Abiotic methylation of mercury in the aquatic environment. Science of the Total Environment, 368, 126–137.
Chimney, M. J., Nungesser, M., Newman, J., Pietro, K., Germain, G., Lynch, T., Goforth, G., Moustafa, M. Z. (2000). 2000 Everglades Consolidated Report. Chapter 6: stormwater treatment areas—status of research and monitoring to optimize effectiveness of nutrient removal and annual report on operational compliance. West Palm Beach, FL: South Florida Water Management District.
Compeau, G. C., & Bartha, R. (1985). Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Applied and Environmental Microbiology, 50, 498–502.
Corrales, J., Naja, G. M., Dziuba, C., Rivero, R. G., & Orem, W. (2011). Sulfate threshold target to control methylmercury levels in wetland ecosystems. Science of the Total Environment, 409, 2156–2162.
Dmytriw, R., Mucci, A., Lucotte, M., & Pichet, P. (1995). The partitioning of mercury in the solid components of dry and flooded forest soils and sediments from a hydroelectric reservoir, Quebec (Canada). Water, Air, and Soil Pollution, 80, 1099–1103.
Fink, L. E. (2002). 2002 Everglades Consolidated Report. Appendix 2B-2: Status report on the effect of water quantity and quality on methylmercury production. West Palm Beach, FL: South Florida Water Management District.
Fink, L. E., King, J., Adak, P., Matson, F. (2005). 2005 South Florida Environmental Report. Appendix 2B-2: STA-2 Mercury Special Studies Project Report. West Palm Beach, FL: South Florida Water Management District.
Gilmour, C. C., & Henry, E. A. (1991). Mercury methylation in aquatic systems affected by acid deposition. Environmental Pollution, 71, 131–169.
Gilmour, C. G., Henry, E. A., & Mitchell, R. (1992). Sulfate stimulation of mercury methylation in freshwater sediments. Environmental Science and Technology, 26, 2281–2287.
Gilmour, C. C., Riedel, G. S., Ederington, M. C., Bell, J. T., Benoit, J. M., Gill, G. A., et al. (1998). Methylmercury concentrations and production rates across a trophic gradient in the Northern Everglades. Biogeochemistry, 40, 327–345.
Gilmour, C. C., Krabbenhoft, D. P., Orem, W. O. (2004a). 2004 Everglades Consolidated Report. Appendix 2B-3: Mesocosm studies to quantify how methylmercury in the Everglades responds to changes in mercury, sulfur, and nutrient loading. West Palm Beach, FL: South Florida Water Management District.
Gilmour, C. C., Krabbenhoft, D. P., Orem, W. O., Aiken, G. (2004b). 2004 Everglades Consolidated Report. Appendix 2B-1: Influence of drying and rewetting on mercury and sulfur cycling in Everglades and STA soils. West Palm Beach, FL: South Florida Water Management District and Florida Department of Environmental Protection.
Gilmour, C. C., Orem, W. H., Krabbenhoft, D. P., Mendelssohn, I. (2007a). 2007 South Florida Environmental Report. Appendix 3B-3: Preliminary assessment of sulfur sources, trends and effects in the Everglades (p. 83). West Palm Beach, FL: South Florida Water Management District.
Gilmour, C. C., Krabbenhoft, D. P., Orem, W. O., Aiken, G., Roden, E. (2007b). 2007 South Florida Environmental Report. Appendix 3B-2: Status report on ACME studies on the control of Hg methylation and bioaccumulation in the Everglades. West Palm Beach, FL: South Florida Water Management District.
Gorski, P. R. (2004). An assessment of bioavailability and bioaccumulation of mercury species in freshwater food chains. Madison, WI: Limnology and Marine Science. University of Wisconsin - Madison.
Grieb, T. M., Driscoll, C. T., Gloss, S. P., Schofield, C. L., Bowie, G. L., & Porcella, D. B. (1990). Factors affecting mercury accumulation in fish in the upper Michigan peninsula. Environmental Toxicology and Chemistry, 9, 919–930.
Hsu-Kim, H., & Sedlak, D. L. (2005). Similarities between inorganic sulfide and the strong Hg(II)-complexing ligands in municipal wastewater effluent. Environmental Science and Technology, 39, 4035–4041.
Hylander, L. D., & Meili, M. (2003). 500 years of mercury production: global annual inventory by region until 2000 and associated emission. Science of the Total Environment, 304, 13–27.
Jeremiason, J. D., Engstrom, D. R., Swain, E. B., Nater, E. A., Johnson, B. M., Almendinger, J. E., et al. (2006). Sulfate addition increases methylmercury production in an experimental wetland. Environmental Science and Technology, 40, 3800–3806.
King, J. K., Saunders, F. M., Lee, R. F., & Jahnke, R. A. (1999). Coupling mercury methylation rates to sulfate reduction rates in marine sediments. Environmental Toxicology and Chemistry, 18, 1362–1369.
Liu, G. L., Cai, Y., Philippi, T., Kalla, P., Scheidt, D., Richards, J., et al. (2008). Distribution of total and methylmercury in the different ecosystem compartments in the Everglades: Implications for mercury bioaccumulation. Environmental Pollution, 153, 257–265.
Miller, C. L., Mason, R. P., Gilmour, C. C., & Heyes, A. (2007). Influence of dissolved organic matter on the complexation of mercury under sulfidic conditions. Environmental Toxicology and Chemistry, 26, 624–633.
Orem, W. H. (2004). Impacts of sulfate contamination on the Florida Everglades Ecosystem. United States Geological Survey.
Orem, W. H. (2007). Sulfur contamination in the Florida Everglades: initial examination of mitigation strategies. United States Geological Survey Open-file Report 2007–1374.
Orem, W. H., Gilmour, C. C., Axelrad, D., Krabbenhoft, D., Scheidt, D., Kalla, P., et al. (2011). Sulfur in the south Florida ecosystem: distribution, sources, biogeochemistry, impacts, and management for restoration. Critical Reviews in Environmental Science and Technology, 41, 249–288.
Ravichandran, M. (2004). Interactions between mercury and dissolved organic matter – a review. Chemosphere, 55, 319–331.
Regnell, O. (1994). The effect of pH and dissolved oxygen levels on methylation and partitioning of mercury in freshwater model systems. Environmental Pollution, 84, 7–13.
Rumbold, D. G., & Fink, L. E. (2006). Extreme spatial variability and unprecedented methylmercury concentrations within a constructed wetland. Environmental Monitoring and Assessment, 112, 115–135.
Selvendiran, P., Driscoll, C. T., Bushey, J. T., & Montesdeoca, M. R. (2008). Wetland influence on mercury fate and transport in a temperate forested watershed. Environmental Pollution, 154, 46–55.
St. Louis, V. L., Rudd, J. W. M., Kelly, C. A., Beaty, K. G., Bloom, N. S., & Flett, R. J. (1994). Importance of wetlands as sources of methyl mercury to boreal forest ecosystems. Canadian Journal of Fishery and Aquatic Science, 51, 1065–1076.
Ullrich, S. M., Tanton, T. W., & Abdrashitova, S. A. (2001). Mercury in the aquatic environment: a review of factors affecting methylation. Critical Reviews in Environmental Science and Technology, 31, 241–293.
Watras, C. J., & Morrison, K. A. (2008). The response of two remote, temperate lakes to changes in atmospheric mercury deposition, sulfate, and the water cycle. Canadian Journal of Fisheries and Aquatic Sciences, 65, 100–116.
Weber, J. H. (1993). Review of possible paths for abiotic methylation of mercury in the aquatic environment. Chemosphere, 26, 2063–2077.
Winfrey, M. R., & Rudd, J. W. M. (1990). Environmental factors affecting the formation of methylmercury in low pH lakes. Environmental Toxicology and Chemistry, 9, 853–869.
Acknowledgments
Authors wish to thank Southwest Petroleum University, China, Weifang University, China, IFAS/TREC, University of Florida, and the South Florida Water Management District for the support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, S., Ai, Z., Zheng, S. et al. Effects of Dryout and Inflow Water Quality on Mercury Methylation in a Constructed Wetland. Water Air Soil Pollut 225, 1929 (2014). https://doi.org/10.1007/s11270-014-1929-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-1929-6