Abstract
Nearly half of freshwater wetlands have been lost due to human disturbance. In response, wetlands are being restored to retain their ecosystem services. A potentially adverse consequence of wetland function is the production of methylmercury (MeHg). We measured concentrations of mercury (Hg) species and ancillary parameters in groundwaters and surface waters from four natural and 16 restored wetlands in northern New York State, USA to investigate differences in concentrations of Hg species among wetlands. We found no obvious differences in concentrations of total mercury (THg) and methylmercury in pond waters between natural and restored wetlands. High values of %methylmercury were evident in both ground (38.8 ± 27.6%) and surface waters (43.4 ± 25.6%) suggesting these wetland complexes are highly efficient in converting ionic Hg to methylmercury, regardless if restored or natural. High methylation efficiency may be due to observed drying and rewetting cycles. Hg in pond waters is likely derived from direct atmospheric deposition or by mobilization from near-wetland shallow sediments, in addition to groundwater inflows. Water flow of groundwaters from the associated watershed into pond waters resulted in increases in concentrations of THg and methylmercury. Dissolved organic matter likely plays an important role in the supply of Hg to pond waters. Relationships between methylmercury and %methylmercury with sulfate and nitrate in groundwaters may suggest some chemical limitation on Hg methylation at higher concentrations of these anions. Because of the similarity in Hg dynamics for natural and restored wetlands, the most effective strategy to mitigate methylmercury production would be to decrease atmospheric Hg deposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands are generally areas with standing water for a considerable period. They have special soil conditions and organisms due to their persistent hydrologic condition of soil saturation, and represent important linkages between upland terrestrial and aquatic ecosystems (Mitsch and Gosselink 2007). Notably, wetlands typically experience wet-dry cycles which facilitate alternating redox conditions (Driscoll et al. 1998; Strickman and Mitchell 2017). Although wetlands occupy less than 9% of land area, they provide a disproportionate amount of ecosystem services, including water storage, retention of carbon and nutrients, and important habitat (Zedler and Kercher 2005). Wetland loss is a significant environmental issue (National Research Council 2001). It is estimated that nearly half of global wetland area has been lost over the last two centuries, with the remaining wetlands largely highly degraded (Zedler and Kercher 2005). Similarly in the United States, human activities such as agriculture, residential development, silviculture and other land use conversions have contributed to marked loss of wetlands (Dahl 2011; Zedler and Kercher 2005) Conservation programs including Public–Private Partnerships (PPP) are helping to conserve and restore wetlands.
Wetlands are important landscape features for transformations of mercury (Hg). They serve as net sinks for total mercury (THg), and are critical environments for methylmercury (MeHg) production and supply to downstream aquatic ecosystems (Galloway and Branfireun 2004; Driscoll et al. 2007; Selvendiran et al. 2008). Atmospheric deposition is the main source of Hg to most remote aquatic ecosystems (Benoit et al. 2003; Driscoll et al. 1998, 2007; Fitzgerald et al. 1998). Besides natural sources, Hg is emitted directly to the atmosphere from human activities associated with power plants, smelting and other industrial processes, as well as artisanal and small-scale gold mining (UNEP 2019). Moreover, Hg previously deposited to the Earth’s surface can be subsequently reduced and remitted back to the atmosphere as elemental Hg (Driscoll et al. 2013). Mercury is a global pollutant, because gaseous elemental Hg emitted to the atmosphere has a long residence time (0.5 to 1 year) before deposition to the Earth’s surface (Driscoll et al. 2013). However, recent measurements have shown that regional decreases in primary emissions have resulted in decreases in atmospheric Hg concentrations and deposition in the eastern U.S. (Zhang et al. 2016; Zhao et al. 2016; Gerson and Driscoll 2016).
The accumulation of organic matter and saturated soils in wetlands promote reducing conditions which facilitates the conversion of ionic Hg (Hg2+) to methylmercury through anaerobic microbes, including sulfate and iron reducing bacteria, methanogens and archaea (Benoit et al. 2003; Podar et al. 2015; Selvendiran et al. 2008). In contrast to THg, only a small amount of methylmercury is derived from atmospheric deposition; most is transformed from ionic Hg within ecosystems. In situ production of methylmercury in freshwater wetlands has been shown to be related to temperature, pH, microbial activity, sulfate, nitrate and dissolved organic carbon concentrations, and hydrologic conditions (Zillioux et al. 1993; Benoit et al. 2003; Selvendiran et al. 2008; Todorova et al. 2009). In northern regions, methylation is most prominent during summer, associated with higher temperature and low flow conditions (Selvendiran et al. 2008). Methylmercury strongly bioaccumulates and biomagnifies in the aquatic food chains, and generally drives human and wild life exposure (Driscoll et al. 2007; Lacerda and Fitzgerald 2001; Zillioux et al. 1993). Methylmercury is a neurotoxic substance, and exposure to this contaminant is especially problematic for young children and women of child-bearing age (Driscoll et al. 2013; Sunderland et al. 2016). Elevated exposure to methylmercury occurs primarily through consumption of fish (Driscoll et al. 2013; Sunderland et al. 2018). However, recent studies have shown rice consumption can be another important pathway of methylmercury exposure, especially in central China (Kwon et al. 2018; Zhang et al. 2010).
Freshwater wetlands are particularly sensitive to Hg pollution (Driscoll et al. 2007). Understanding the biogeochemical processing and cycling of Hg in wetlands is critical to an assessment of the risk of Hg contamination in polluted areas (Lacerda and Fitzgerald 2001). Research has demonstrated the function of restored wetlands for a variety of diverse ecosystem services, such as water storage, carbon sequestration and improving water quality (Hogan et al. 2004; Zedler and Kercher 2005); however, fewer studies have examined Hg cycling and methylmercury production in restored wetlands. Our hypothesis for this study is that there is no difference in the processing of Hg between restored and natural wetlands. To test this hypothesis, we measured the chemical and hydrological characteristics of surface and groundwaters from 16 restored wetlands and four natural wetlands near St. Lawrence River in New York State. We compared concentrations of THg and methylmercury from surface and groundwaters in natural and restored wetlands over a growing season and examined temporal patterns in groundwater and lake stage.
Materials and methods
Site description
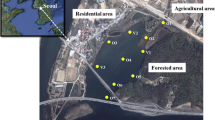
Twenty wetland sites along St. Lawrence River in New York State were selected for this study. The St. Lawrence River is one of the largest rivers in the world, and an important water resource for both the United States and Canada. Among these wetland sites, four are natural wetlands and the remaining 16 are restored wetlands, of varying age and biophysical characteristics (Table S-1). The latitude and longitude of the study wetlands range from 44.1 to 45.0°N and 74.5 to 76.0°W, respectively (Fig. 1). The restored wetlands were reconditioned as a PPP within the St. Lawrence River watershed. The most common soil texture in the study area is silt clay, followed by silt clay loam and silt loam. The dominant land covers are agricultural crops, pasture, and forest. Restored wetlands were generally in watersheds dominated by agricultural lands, while natural wetlands were generally in watersheds dominated by forested lands. Based on a hydrogeomorphic classification, the study wetlands encompass three classes: riverine, slope and depressional wetlands (Table S-1). Wetland vegetation includes hardstem bulrush (Scirpus Spp.), reed canary grass (Phalaris arundinacea), cattail (Typha Spp.) and meadow willow (Salix petiolaris) (Hwang 2018).
Historical meteorological data were obtained for five regional sites from Climate Data Online (CDO) of the National Oceanic and Atmospheric Administration (NOAA) National Centers for Environmental Information website (http://www.ncdc.noaa.gov/cdo-web/) for the period 1989–2010. The historical data suggest that the temperature of the study region is cool, with abundant precipitation. During the study period from May to October 2015, the average temperature increased from May, reached the maximum value in August (19.3 ± 0.4 °C), then decreased successively in September and October (8.2 ± 0.7 °C). Annual average precipitation for the five nearby weather stations is 961 ± 79 m, with the highest quantity occurring in fall. Monthly precipitation was relatively uniform over the study period, the lowest was in May and the highest was in September and October, with a gradual increase over this interval (see Results). Peak snowmelt occurred in March and April.
Wetland restoration involved placement of a berm for stage control and increased area. Wetlands were also “enhanced” by reversing agricultural drainage “improvements” such as drainage ditches. Berms were generally constructed from excavated material. The impoundments typically have a control structure for the outlet. Most of the study wetlands are located within reaches of first and second order streams. The wetlands discharged to nearby streams/rivers (Hwang 2018). All wetland ponds are shallow (1.15 ± 0.59 m) with a small surface area (2.39 ± 1.61 ha) (Table S-1). From water column temperature observations, these wetlands were well-mixed throughout the year (Hwang 2018).
Waters sampling
Water stage and quality were conducted for both surface and groundwaters from the 20 wetland sites. Groundwater wells made of 5 cm diameter PVC pipes were installed in the upland adjacent to the wetlands. Wells were used to evaluate ground water contributions to the wetlands, characterize change in groundwater stage and sample for associated wetland water chemistry. The average well depth from the top soil was 1.11 ± 0.35 m; the depths for natural and restored wetlands ranged from 1.08 to 2.01 and from 0.38 to 1.40, respectively (Hwang 2018). Ground and surface water levels were measured hourly with pressure sensors (U20 HOBO data loggers, Onset Computer Corporation, Bourne, MA, USA) placed at bottom of the wells and ponds, respectively. Atmospheric pressure reference sensors were deployed at each site in the above-ground section of the well casings.
Each wetland and associated groundwater was sampled for Hg species and other water quality measurements on five dates from May to October in 2015. Teflon bottles were used to collect water samples, which were double bagged and contained 0.4% hydrochloric acid (HCl) prior to collection. The “clean hands/dirty hands” technique was used for sample collection (EPA 2002, Method 1631, Version E). Before collecting pond waters, Teflon bottles were rinsed three times with the water to be sampled. Pond sample sites were located near the wetland outlet.
Groundwater samples were collected by pumping water from the groundwater wells. The collection flask and tubing were rinsed with about 10 mL of the groundwater three times before sample collection. On occasion the quantity of groundwater was limited at some sites, especially during the dry season (from July to September). During these periods, Milli-Q water was used to rinse the flask and tubing twice before collection, with the third and final rinse was conducted with sample water. Similar to surface water collections, the “clean hands/dirty hands” technique was used to sample groundwater. During the dry season from June to August, on some sampling dates and at some sites there was inadequate water in the groundwater wells to collect samples.
Water samples were kept in coolers with ice after collection, then transported to the laboratory at Syracuse University for analysis. Half of the water sampled was filtered (after through mixing) within 48 h of collection, using the 0.45 um Millipore Express PLUS PES membrane filter for analysis of concentrations of THg and methylmercury. The filtered samples were placed into 250 mL Teflon bottles, and 0.4% HCl was added as a preservative. The unfiltered remaining sample was poured into polypropylene bottles for analysis of major solutes and nutrients. All samples were stored at 4 °C until analysis.
Chemical analyses
The methods used for chemical analysis of collected water samples are summarized in Supporting Information (Table S-2). Quality control was applied through duplicate sampling, instrument detection limits, initial and ongoing precision recovery, initial calibration verification (ICV), continuous calibration verification (CCV), initial calibration blank (CCB), method blanks (MB), matrix spike (MS), and matrix spike duplicates (MSD). Before analyzing samples, standard calibration curves were performed. All blanks were less than the method detection limits of 0.2 ng/L for THg and 0.002 ng/L for methylmercury, respectively. All recoveries of standards and spikes were in the range of acceptance criteria (Table S-4).
Data analyses
Wetlands were manually delineated on ArcGIS 10 (ESRI, Redlands, CA, USA) using aerial imageries, site pictures and multiple site observations. Drainage areas of wetlands were delineated on USGS StreamStats (https://water.usgs.gov/osw/streamstats/). A 30-m land use map was acquired from the National Land Cover Database 2011 provided by the Multi-Resolution Land Characteristics Consortium (http://www.mrlc.gov/nlcd2011.php). Subset of the downloaded land use map was clipped on ArcGIS to determine the composition of land use within the drainage areas.
All results are presented as a mean ± standard error. Data were log-transformed to meet the assumption of residual normality. Statistical comparisons of Hg variables among surface and groundwaters, wetland types, monthly change, well depths and wetland ages were made with a mixed—model analysis of variance (ANOVA). All statistically significant relationships and differences were determined at α ≤ 0.05. Relationships between Hg concentrations and ancillary variables were performed with linear regression. All statistical analyses were conducted using R, package version 2.0.6 (R Core Team 2017) and Mini Tab 17 (Minitab, Inc. 2014).
Results
Mercury species in natural and restored wetlands
The average concentrations of THg and methylmercury in pond waters (THg = 1.11 ± 0.77 ng/L; MeHg = 0.51 ± 0.50 ng/L) were approximately two and three times higher, respectively, than values in groundwaters (THg = 0.38 ± 0.35 ng/L; MeHg = 0.13 ± 0.19 ng/L). Although the variability in concentrations of Hg species was large across the study sites, the differences between surface and groundwaters were statistically significant for each sampling event (p < 0.001). The percent ratios of methylmercury/THg (%methylmercury) in the study wetlands were high in both surface (43.4 ± 25.6%) and groundwaters (38.8 ± 27.6%), with no differences between these types of waters (p > 0.1).
For surface waters, the mean concentrations of THg and methylmercury, as well as %methylmercury in restored wetlands were not significantly different from values in natural wetlands. In groundwaters, however, the average concentration of THg in restored wetlands (0.44 ± 0.38 ng/L) was higher than natural wetlands (0.18 ± 0.14 ng/L) (p = 0.003), with significant differences occurring each month during the study period. While the mean concentration of methylmercury in groundwaters of restored wetlands was around three times higher than in natural wetlands, this difference was just not statistically significant (p = 0.064). The mean values of %methylmercury in natural and restored wetlands were similarly high, without significant differences. As the wetlands exhibited high fractions of THg occurring as methylmercury (%methylmercury), there were relatively strong relationships between concentrations of methylmercury and THg, except for the groundwaters in natural wetlands (Fig. 2).
Relationships between concentrations of methylmercury(MeHg) and total mercury (THg) in a surface waters (SW) (NW: slope = 0.76, r2 = 0.68, p < 0.001; RW: slope = 0.42, r2 = 0.52, p < 0.001), and b ground waters (GW) (NW: slope = 0.09, r2 = 0.11, p > 0.1; RW: slope = 0.45, r2 = 0.40, p < 0.001) in study wetlands. Natural wetlands (NW) are represented by green dots, and restored wetlands (RW) are represented by orange triangles
Temporal patterns of THg and methylmercury were similar in both natural and restored wetlands. In surface waters, THg and methylmercury concentrations increased from May to June and then decreased during the low flow period in August. Concentrations increased again in September and then decreased from September to October. In groundwaters, THg concentrations increased from May to June and gradually decreased from June to October. Methylmercury concentrations in groundwaters exhibited no obvious monthly change other than a pattern similar to THg (Fig. 3).
Boxplots showing seasonal patterns of (a) THg in surface waters, (b) THg in ground waters, (c) methylmercury (MeHg) in surface waters, and (d) methylmercury (MeHg) in ground waters in study wetlands. The median is represented by the middle line of each box, hinges represent the 0.25 and 0.75 quartiles, and whiskers represent the minimum and maximum values, the outliers represent the extremely higher values. Natural wetlands are shown by green, and restored wetlands are shown by orange
Relations with other parameters
Previous studies have demonstrated that DOC is an important carrier in the transport of Hg (e.g., Dittman et al. 2010; Mitchell et al. 2008). Ratios of THg: DOC and methylmercury: DOC were used to examine sources of THg and methylmercury relative to DOC inputs in natural and restored wetlands. The mean THg: DOC ratios in groundwaters (0.132 ± 0.103 μg-Hg/g-C) was significantly higher than in surface waters (0.104 ± 0.078 μg-Hg/g-C) (p = 0.04). In groundwaters, the THg:DOC ratios in restored wetlands (0.147 ± 0.109 μg-Hg/g-C) were significantly higher than natural wetlands (0.081 ± 0.061 μg-Hg/g-C) (p = 0.014), suggesting that the supply of THg per unit DOC was greater in groundwaters draining into restored wetlands than natural wetlands. The mean methylmercury: DOC ratio in the groundwaters of restored wetlands was more than two times higher than in natural wetlands, but not statistically different (p = 0.106).
Total Hg and methylmercury concentrations in pond waters showed positive relationship with SO42− (p < 0.05). In contrast, %methylmercury in groundwaters had a crude negative relationship with SO42− concentration, which was more evident for restored wetlands due to their greater range of SO42− concentrations (Fig. 4). Concentrations of methylmercury and %methylmercury values were variable in groundwaters with low SO42− (<10 mg S/L) and significantly decreased with increasing SO42−. Similar to the pattern for SO42−, the concentrations of methylmercury and %methylmercury were varied in groundwaters at low NO3− concentrations (≤2.0 mg N/L), and these values decreased with increases in NO3− concentration (>2.0 mg N/L) but these differences were not significant (p = 0.122).
Patterns of methylmercury (MeHg) and %methylmercury with SO42− and NO3− concentrations in ground water (GW) of study wetlands. a Methylmercury and SO42−; b %methylmercury and SO42−; c methylmercury and NO3−; d %methylmercury and NO3−. Green dots—natural wetlands (NW), orange triangles—restored wetlands (RW)
We found no relationships between THg, methylmercury and %methylmercury with wetland watershed area or land cover, or hydrogeomorphic class.
Hydrologic patterns
In general, groundwater well stage exceeded pond stage over the study period, suggesting overall drainage of water from the upslope watershed to the pond. Groundwater stage showed considerable variability in response to snowmelt and precipitation events. Higher groundwater stage was generally evident in early May associated with snowmelt, and later in June during a period of higher precipitation quantity. Groundwater and pond water stage generally decreased from July to early September, with more muted changes in pond level (Fig. 5a). During the autumnal drought, groundwater stage occasionally decreased below pond stage, suggesting limited or no subsurface inflow to the ponds under this condition (Fig. 5b). When groundwater stage was the lowest of year in August–September, some restored wetlands lost their surface water for groundwater recharge via subsurface flow. While this duration varied largely across the restored wetlands, natural wetlands hardly lost their surface water to groundwater. Groundwater and pond stage increased in late September and October associated with decreases in evapotranspiration in both restored and natural wetlands.
We examined relationships between THg, methylmercury and %methylmercury with the standard deviation of pond stage, and mean and standard deviation of groundwater stage across wetlands, finding a significant relationship between the standard deviation of groundwater stage and concentrations of methylmercury in pondwaters (p = 0.035) and nearly a significant relationship with %methylmercury (p = 0.056).
Discussion
Factors influencing mercury concentrations in natural and restored wetlands
Concentrations of THg, and methylmercury in the St. Lawrence wetlands were generally similar albeit on the low end of values reported for other wetland studies (Table S-3). In contrast, %methylmercury values appear high compared to many values reported for wetland studies in the literature.
In pond waters, there was no obvious difference in the Hg chemistry between natural and restored wetlands. THg and methylmercury were higher in surface waters than groundwaters. The hydrologic analysis of groundwater and pond stage indicates that through most of the study period the adjacent wetlands supplied groundwater to the ponds (Fig. 5). An exception to this pattern occurred during the low flow summer largely in August and early September, and this change was more pronounced for restored than natural wetlands. The inflow from groundwaters to surface waters, coupled with the increasing THg concentrations in surface waters would suggest that the groundwater flow is an important Hg sources to the surface waters. In addition to groundwater, the ponds also likely received THg from direct atmospheric deposition and/or mobilization from surface soils adjacent to the wetlands. The higher concentrations of THg in groundwaters of the restored wetlands may be evidence of the greater supply of THg due to the shallower soil depth, as the depth of groundwater collected for restored wetlands was less (at a mean depth of 1.03 m) than those for natural wetlands (1.39 m). Also, concentrations of Hg forms (Fig. 3) and SO42− and NO3− (Fig. 4) concentrations were lower and far less variable in the groundwaters draining natural wetlands, possibly because of less contamination by human activities (i.e., greater distance from roads, residences, agricultural activity). However, the robustness of this observation may be limited by small number of the natural wetlands investigated.
Note that DOC concentrations were elevated in pond water (11.38 ± 3.61 mg C/L) relative to groundwaters (3.33 ± 2.89 mg C/L) (p < 0.001), suggesting the mobilization of DOC from surface deposits in the wetlands to surface waters. This supply of DOC could facilitate the transport of higher THg concentrations in pond waters (Dittman et al. 2010). Also, there was no difference in the THg:DOC ratio in pond waters between restored and natural wetlands, suggesting that pond restoration does not strongly influence the supply of DOC relative to the supply of THg. The differences in THg:DOC in groundwaters were again likely due the differences in depth groundwaters were collected; a shallower soil depth for the collection of groundwater in restored wetlands was coincident with higher DOC than for natural wetlands. Generally concentrations of organic carbon in soil and soil solutions are highest at the soil surface and decrease with soil depth (Dittman et al. 2007; Smith et al. 2014).
Similar to THg, there was no obvious difference in methylmercury concentrations between natural and restored wetlands in surface or groundwaters. The most distinctive feature of Hg dynamics from this study is the relatively high %methylmercury values in wetlands, suggesting a highly efficient conversion of ionic Hg to methylmercury (Fig. 2 and Table S-3). It is not clear why these wetlands are so effective in the production of methylmercury. One plausible explanation is the cyclical wetting of wetland sediments associated with precipitation events and the subsequent drying during the intervening interval (Fig. 5). Drying of wetlands or sediments allows for the mineralization of organic matter and release of associated ionic Hg (Chen et al. 2012). Re-wetting of wetland sediments following precipitation events allows for the development of reducing conditions and promotes methylation of the mineralized ionic Hg. Successive wetting and drying cycles in wetlands over the summer season (Fig. 5) allows for the efficient production of methylmercury (Eckley et al. 2017; Wasik et al. 2015). We found a significant relationship between variation in groundwater stage and methylmercury concentrations in wetlands, which seems to be consistent with this mechanism of methylmercury production (p = 0.035). The high %methylmercury values could also be explained by strong binding between Hg and DOC leading to a greater partitioning of Hg in the aqueous phase and increases in Hg methylation (Liu et al. 2008).
Values of %methylmercury were similar for both natural and restored wetlands, which suggests the function of methylmercury production in restored wetlands was similar to natural wetlands (Strickman and Mitchell 2017). The close positive relationship between THg and methylmercury (Fig. 2) indicates that there are not major additional factors controlling the conversion of ionic Hg to methylmercury in these ecosystems; the main control on methylmercury production in the study wetlands would appear to be supply of ionic Hg (Dennis et al. 2005). Concentrations of SO42− and possibly NO3− may have had some influence on methylmercury production in groundwaters. Sulfate has a complex relationship with methylmercury production (Benoit et al. 2003; Gilmour and Henry 1991; Glimour 2011). Sulfate reducing bacteria are important in the production of methylmercury (Podar et al. 2015). Under low SO42− concentrations methylation may be SO42− limited; increases in SO42− concentrations can stimulate production of methylmercury. At high SO42− concentrations under reducing conditions, the production of sulfide can form charged aqueous complexes or precipitate ionic Hg limiting its availability for methylation. The result is an optimum concentration of SO42− for the production of methylmercury, whose value varies with environmental conditions such as DOC and iron concentrations (Glimour 2011). The patterns of methylmercury and %methylmercury in groundwater are suggestive of this relationship with SO42−. Concentrations of SO42− are elevated in some of groundwater in restored wetlands. The source of this SO42− is likely due to interaction of saline geologic deposits and groundwater during excavation of the wetlands (Franzi et al. 2000), resulting in elevated concentrations of SO42− in some of the groundwaters in the restored wetlands. We observed lower %methylmercury values in these high SO42− (>10 mg S/L) groundwaters and overall %methylmercury decreases with increasing SO42−. Lower SO42− groundwaters had higher and more variable methylmercury concentrations and %methylmercury values (Fig. 4).
The source of elevated NO3− in wetland groundwaters is likely runoff from agricultural lands adjacent to the restored wetlands. It has been observed that elevated NO3− can limit methylmercury production (Matthews et al. 2013; Shih et al. 2011; Todorova et al. 2009). The mechanism for this effect is not clear. Nitrate is a strong oxidant. In its presence, sediment iron will oxidize and ferric oxide can effectively adsorb Hg limiting methylation and transport in water. Alternatively, NO3− can limit the activity of SO42− reducing bacteria and methylmercury production from this pathway (Matthews et al. 2013; Strickman and Mitchell 2018; Todorova et al. 2009). Like for SO42−, we observed a curvilinear, but not significant, relationship between concentrations of methylmercury and NO3− in groundwaters. At low concentrations of NO3−, methylmercury and %methylmercury values were variable; and methylmercury concentrations and %methylmercury decreased with increases in NO3− at concentrations above 2.0 mg N/L (Fig. 4). Note while these methylmercury patterns with SO42− and NO3− in the groundwaters of restored wetlands are interesting and suggestive, they did not seem to have any influence on the high methylmercury production efficiency (%methylmercury) in pond waters.
Temporal variation of mercury concentrations
The temporal variation of THg in both surface and groundwaters may be related to meteorological conditions and water table depth. In the absence of local industrial activity, atmospheric deposition is likely the dominant source of THg to the study area (Driscoll et al. 2007; Yu et al. 2014). Temperature is likely an important factor driving monthly variation in THg. Concentrations of THg typically increase during the low flow summer period due to the release of THg from mineralization of organic matter coupled with water losses to the atmosphere associated with increases in evapotranspiration (Selvendiran et al. 2008). As a result, the overall monthly variation of lower THg and methylmercury during the late spring and fall and generally higher concentrations during summer were expected. Superimposed on this pattern was lower concentrations in August. Two factors may have contributed to the lower THg and methylmercury during this period. First, we observed a marked decrease in groundwater stage in late summer (Fig. 5) suggesting a decrease in groundwater flow to the ponds and possibly a decrease in the mobilization of THg inputs from near pond sediments. Second, the longer hydrologic residence time associated with lower inflows to the ponds and higher water temperatures may have allowed for greater photoreduction of THg and loss by evasion from the pond surface. Evasion has been shown to be an important loss mechanism for THg in lakes (Denkenberger et al. 2012; Selvendiran et al. 2009; Ullrich et al. 2001).
High inputs of dissolved organic matter to wetlands is likely due to plant production coupled with reduced decomposition of organic matter associated with saturated soil conditions (Lacerda and Fitzgerald 2001; Mitsch and Gosselink 2007; Mitchell et al. 2008). Inputs of dissolved organic carbon coupled with modest SO42− concentrations at the study sites could promote the production methylmercury (Driscoll et al. 1998; Gilmour et al. 1998; Ullrich et al. 2001). A more likely contributing factor for the high methylmercury production efficiency is the wetting and drying cycles occurring in wetland sediments throughout the summer season which continuously promotes methylmercury formation. (Driscoll et al. 2007; Strickman and Mitchell 2017). At a coarse macroscale, evidence for the importance of the drying and rewetting of sediments may be the marked increase in THg and methylmercury in pond waters from the dry low flow period in August to the higher stage conditions in September, driven by three relatively large precipitation events (Figs. 3 and 5). Note that the highest %methylmercury was observed in September, a month which is the transition between the dry summer and wet fall season, but is characterized by warmer temperatures that allow for methylmercury formation.
Although these wetlands exhibit high %methylmercury values, we see little difference in the ability of the restored and natural wetlands to process Hg. Investigators have indicated concern for methylmercury production in different stages of constructed wetlands. Sinclair et al. (2012) observed elevated concentrations of methylmercury sediments and invertebrates in newly created wetlands for stormwater management compared to natural control wetlands, but concentrations decreased with wetland age. Strickman and Mitchell (2017) found that recently created wetlands for stormwater management were low in organic matter and had low rates on methylmercury production and low sediment methylmercury concentrations, while production and accumulation increased with wetland age. In contrast, the variability we observed across the restored wetlands likely masked any effect of wetland age on THg, methylmercury or %methylmercury. Note, the youngest restored pond studied was seven years old, so the fact that we did not have the opportunity to investigate a recently restored pond likely limits an evaluation of pond age on Hg dynamics.
Conclusions
In this study, we found no obvious differences in concentrations of Hg species in surface waters between natural and restored wetlands. Monthly variation of THg and methylmercury concentrations were similar in both natural and restored wetlands, with higher concentrations under warmer conditions in late spring and early fall, and lower values under cooler conditions in early spring and late fall. Lower concentrations were also observed during the low flow summer condition, likely due to decreases in water inflows and increases in evasion losses associated with increased hydraulic residence time. Concentrations of THg and methylmercury were higher in pond waters than groundwater inflows. This pattern suggests that in addition to groundwater inputs, surface water Hg was supplied by atmospheric deposition and the mobilization of Hg from near-surface sediments. We observed relatively high methylmercury to THg ratios (%methylmercury) in the study wetlands indicating that these systems are effective in converting ionic Hg to methylmercury, in both restored and natural wetlands. Drying and rewetting cycles, which occurred throughout the summer in response to precipitation events likely, contribute to the high methylation efficiency. While methylation efficiency was high in the study wetlands, there was some evidence that methylation may have been limited in groundwaters of restored wetlands due to high concentrations of SO42− (>10 mg S/L) and/or NO3− (>2.0 mg N/L). Relationships between DOC and THg and methylmercury were weak across sites, but high concentrations of dissolved organic matter likely was important in the transport of Hg to surface waters.
Wetlands provide valuable services. The study wetlands were restored to improve habitat for fisheries and wildlife. Wetlands are critical zones of the landscape for the production of methylmercury and its transport to downstream aquatic ecosystems. Indeed, these wetlands were very effective in converting of ionic Hg to methylmercury, irrespective if restored and natural. These results suggest that management of methylmercury production in restored wetlands should focus on the ultimate source of Hg inputs, atmospheric deposition.
References
Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller C (2003) Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. In: Chai Y (ed) ACS Symp. Ser. 835, OC Braids, pp 262–297. Washington, DC: Am. Chem. Soc. https://doi.org/10.1021/bk-2003-0835.ch019
Chen CY, Driscoll CT, Kamman NC (2012) Mercury hotspots in freshwater ecosystems: drivers, processes, and patterns. In: Bank MS (ed) Mercury in the environment: pattern and process. University of California Press, Berkeley, CA, p 352
Dahl TE (2011) Status and trends of wetlands in the conterminous United States 2004 to 2009. U.S. Department of the Interior, U.S. Fish and Wildlife Service, Fisheries and Habitat Conservation, Washington, DC, p 107
Denkenberger JS, Driscoll CT, Branfireun BA, Eckley CS, Cohen M, Selvendiran P (2012) A synthesis of rates and controls on elemental mercury evasion in the Great Lakes Basin. Environ Pollut 161:291–298
Dennis IF et al. (2005) Distribution patterns of mercury in lakes and rivers of northeastern North America. Ecotoxicology 14:113–123. https://doi.org/10.1007/s10646-004-6263-0
Dittman JA, Driscoll CT, Groffman PM, Fahey TJ (2007) Dynamics of nitrogen and dissolved organic carbon at the Hubbard Brook Experimental Forest. Ecology 88(5):1153–1166. https://doi.org/10.1890/06-0834
Dittman JA, Shanley JB, Driscoll CT, Aiken GR, Chalmers AT, Towse JE, Selvendiran P (2010) Mercury dynamics in relation to dissolved organic carbon concentration and quality during high flow events in three northeastern U.S. streams. Water Resour Res 46(7):1–15. https://doi.org/10.1029/2009WR008351
Driscoll CT et al. (2007) Mercury Matters: Linking Mercury Science with Public Policy in the Northeastern United States. Hubbard Brook Research Foundation, Science Links Publication. Vol. 1, no. 3
Driscoll CT, Holsapple J, Schofield CL, Munson R (1998) The chemistry and transport of mercury in a small wetland in the Adirondack region of New York, USA. Biogeochemistry 40:10
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: Sources, pathways, and effects. Environ Sci Technol 47:4967–4983
Eckley CS, Luxton TP, Goetz J, McKernan J (2017) Water-level fluctuations influence sediment porewater chemistry and methylmercury production in a flood-control reservoir. Environ Pollut 222:32–41. https://doi.org/10.1016/j.envpol.2017.01.010
Fitzgerald WF, Engstrom DR, Mason RP, Nater EA (1998) The case for atmospheric mercury contamination in remote areas. Environ Sci Technol 32:1–7. https://doi.org/10.1021/es970284w
Franzi DA, Ridge JC, Pair DL, Desimone D, Rayburn JA, Barclay DJ (2000) Post-valley heads deglaciation of the Adirondack Mountains and Adjacent Lowlands Adirondack. J Environ Stud 21:119–146
Galloway ME, Branfireun BA (2004) Mercury dynamics of a temperate forested wetland. Sci Total Environ 325:239–254. https://doi.org/10.1016/j.scitotenv.2003.11.010
Gerson JR, Driscoll CT (2016) Is mercury in remote forested watershed of the Adirondack Mountains responding to recent decreases in emissions? Environ Sci Technol 50:10943–10950. https://doi.org/10.1021/acs.est.6b02127
Gilmour CC, Henry EA (1991) Mercury methylation in aquatic systems affected by acid deposition. Environ Pollut 71:131–169. https://doi.org/10.1016/0269-7491(91)90031-Q
Gilmour CC, Riedel GS, Ederington MC, Bell JT, Gill GA, Stordal MC (1998) Methylmercury concentrations and production rates across a trophic gradient in the northern Everglades. Biogeochemistry 40:327–345. https://doi.org/10.1023/a:1005972708616
Glimour CC (2011) A review of the literature on the impact of sulfate on methylmercury in sediments and soils. Smithsonian Environmental Research Center, Edgewater, MD
Hogan DM, Jordan TE, Walbridge MR (2004) Phosphorus retention and soil organic carbon in restored and natural freshwater wetlands. Wetlands 24:573–585. https://doi.org/10.1672/0277-5212(2004)024[0573:PRASOC]2.0.CO
Hwang K (2018) Ecohydrology of natural and restored wetlands in a glacial plain. Dissertations – ALL. 990. https://surface.syr.edu/etd/990
Kwon SY, Selin NE, Giang A, Karplus VJ, Zhang D (2018) Present and future mercury concentrations in Chinese rice: insights from modeling. Glob Biogeochem Cycles 32:437–462. https://doi.org/10.1002/2017GB005824
Lacerda LD, Fitzgerald WF (2001) Biogeochemistry of mercury in wetlands. Wetl Ecol Manag 9:291–293. https://doi.org/10.1023/a:1011851432573
Liu G et al. (2008) Distribution of total and methylmercury in different ecosystem compartments in the Everglades: implications for mercury bioaccumulation. Environ Pollut 153:257–265. https://doi.org/10.1016/j.envpol.2007.08.030
Matthews DA et al. (2013) Whole-lake nitrate addition for control of methylmercury in mercury-contaminated Onondaga Lake, NY. Environ Res 125:52–60. https://doi.org/10.1016/j.envres.2013.03.011
Minitab I (2014) Minitab Statistical Software, Release 17 for Windows, State College, Pennsylvania Minitab® is a registered trademark of Minitab, Inc. http://www.minitab.com
Mitsch WJ, Gosselink JG (2007) Wetlands, 4th edn. John Wiley & Sons, New York, NY
Mitchell CPJ, Branfireun BA, Kolka RK (2008) Total mercury and methylmercury dynamics in upland–peatland watersheds during snowmelt. Biogeochemistry 90:225–241. https://doi.org/10.1007/s10533-008-9246-z
National Research Council (2001) Executive summary. In: Compensating for Wetland Losses Under the Clean Water Act. National Academies Press, Washington, DC, p 1–10
Podar M et al. (2015) Global prevalence and distribution of genes and microorganisms involved in mercury methylation. Sci Adv 1:e1500675. https://doi.org/10.1126/sciadv.1500675
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Selvendiran P, Driscoll CT, Bushey JT, Montesdeoca MR (2008) Wetland influence on mercury fate and transport in a temperate forested watershed. Environ Pollut 154:46–55. https://doi.org/10.1016/j.envpol.2007.12.005
Selvendiran P, Driscoll CT, Montesdeoca MR, Choi HD, Holsen TM (2009) Mercury dynamics and transport in two Adirondack lakes. Limnol Oceanogr 54(2):413–427. https://doi.org/10.4319/lo.2009.54.2.0413
Shih R, Robertson WD, Schiff SL, Rudolph DL (2011) Nitrate controls methylmercury production in a streambed bioreactor. J Environ Qual 40:1586–1592. https://doi.org/10.2134/jeq2011.0072
Sinclair KA, Xie Q, Mitchell CPJ (2012) Methylmercury in water, sediment, and invertebrates in created wetlands of Rouge Park, Toronto, Canada. Environ Pollut 171:207–215. https://doi.org/10.1016/j.envpol.2012.07.043
Smith DB, Cannon WF, Woodruff LG, Solano F, Ellefsen KJ (2014) Geochemical and mineralogical maps for soils of the conterminous United States. Open-File Report, (2014–1082). https://doi.org/10.3133/ofr20141082
Strickman RJ, Mitchell CPJ (2017) Methylmercury production and accumulation in urban stormwater ponds and habitat wetlands. Environ Pollut 221:326–334. https://doi.org/10.1016/j.envpol.2016.11.082
Strickman RJ, Mitchell CPJ (2018) Multifunctional wetlands: pollution abatement and other ecological services from natural and constructed Wetlands. In: Environmental contamination remediation and management. Springer, Cham. https://doi.org/10.1007/978-3-319-67416-2
Sunderland EM, Driscoll CT, Hammitt JK, Grandjean P, Evans JS, Blum JD, Jacobs W (2016) Benefits of Regulating Hazardous Air Pollutants from Coal and Oil-Fired Utilities in the United States. Environ Sci Technol 50:2117–2120. https://doi.org/10.1021/acs.est.6b00239
Sunderland EM, Li M, Bullard K (2018) Decadal changes in the edible supply of seafood and methylmercury exposure in the United States. Environ Health Perspect 126:017006. https://doi.org/10.1289/EHP2644
Todorova SG, Driscoll CT, Matthews DA, Effler SW, Hines ME, Henry EA (2009) Evidence for regulation of monomethyl mercury by nitrate in a seasonally stratified, eutrophic lake. Environ Sci Technol 43:6572–6578. https://doi.org/10.1021/es900887b
US EPA (2002) Method 1631, Revision E: mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. U.S. Environmental Protection Agency, Washington, DC
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293. https://doi.org/10.1080/20016491089226
UNEP (2019) Global mercury assessment 2018. UN Environment Programme, Chemicals and Heava, Switzerland
Wasik JKC, Engstrom DR, Mitchell CPJ, Swain EB, Monson BA, Balogh SJ, Almendinger JE (2015) The effects of hydrologic fluctuation and sulfate regeneration on mercury cycling in an experimental peatland. J Geophys Res Biogeosci Res 120:1697–1715. https://doi.org/10.1002/2015JG002993
Yu X, Driscoll CT, Warby RAF, Montesdeoca M, Johnson CE (2014) Soil mercury and its response to atmospheric mercury deposition across the northeastern United States. Ecol Appl 24:812–822
Zedler JB, Kercher S (2005) Wetland resources: status, trends, ecosystem services, and restorability. Annu Rev Environ Resour 30:39–74. https://doi.org/10.1146/annurev.energy.30.050504.144248
Zhang H, Feng X, Larssen T, Qiu G, Vogt RD (2010) In Inland China, rice, rather than fish, is the major pathway for methylmercury exposure. Environ Health Perspect 118:1183–1188
Zhang Y et al. (2016) Observed decrease in atmospheric mercury explained by global decline in anthropogenic emissions. Proc Natl Acad Sci 113:526–531. https://doi.org/10.1073/pnas.1516312113
Zhao L et al. (2016) Mercury methylation in rice paddies and its possible controlling factors in the Hg mining area, Guizhou province, Southwest China. Environ Pollut 215:1–9. https://doi.org/10.1016/j.envpol.2016.05.001
Zillioux E, Porcella DB, Benoit JM (1993) Freshwater wetland ecosystems. Environ Toxicol Chem 12:2245–2264. https://doi.org/10.1897/1552-8618
Acknowledgements
This study was funded by the University of Michigan Water Center. We thank personal from Clarkson University for their support of this study; Laura Lautz and Zeng Teng for valuable advice editing the paper; Nicholas Glick and Mariah Shannon Taylor for lab training; and Jacqueline Gerson, Geoffrey Dean Millard, Linghui Meng, Weiyao Gu and Habibollah Fakhraei for useful suggestions on data analysis and paper editing.
Funding
This study was funded by the University of Michigan Water Center. Contract # N017025.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, T., Driscoll, C.T., Hwang, K. et al. Total and methylmercury concentrations in ground and surface waters in natural and restored freshwater wetlands in northern New York. Ecotoxicology 29, 1602–1613 (2020). https://doi.org/10.1007/s10646-019-02155-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-019-02155-6