Abstract
A study was undertaken using urban soils in Detroit, MI and reference materials (cerussite, anglesite, pyromorphite, apatite, goethite, calcite, pyrolusite, and peat) to determine which geochemical forms of Pb measured by sequential extraction analysis are bioaccessible. The results suggest that the water soluble (Pb-fulvic acid complexes), exchangeable, and part of the carbonate-occluded fractions are bioaccessible. The Fe oxide-occluded, Mn oxide-occluded, and higher molecular weight component of the organically bound fraction are not bioaccessible. Sequential extraction predicts the presence of detectable levels of bioaccessible Pb in the rhizosphere when the summed total is ≥90 mg kg−1 and labile Pb is ≥30 mg kg−1. Cerussite (paint-Pb) and anglesite (auto-Pb), recovered mainly in the carbonate-occluded fraction, may cause an overestimation of calcite-Pb. Pyromorphite and apatite Pb (bone) may cause an overestimation of Fe oxide-occluded Pb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sequential extraction analysis (SE) is a method which has been used for many years to determine the geochemical partitioning of Pb (and other trace metals) using a selective dissolution approach (Laing 2010). SE has been applied widely in studies of Pb-contaminated soils in residential, industrial, and roadside urban settings (e.g., Harrison et al. 1981; Howard and Sova 1993; Ruiz-Cortes et al. 2005; Mielke et al. 2007; Madrid et al. 2008; Li et al. 2009; Malik et al. 2010; Mahanta and Bhattacharyya 2011). There is general agreement that the fractionated forms of Pb obtained by SE are operationally defined because extractants are not entirely selective, i.e., some dissolution on nontargeted phases is inevitable (Beckett 1989; Filgueiras et al. 2002). Inaccuracies are also introduced by resorption of Pb by undissolved phases during any given step although this can be minimized perhaps by adding a chelating agent to the extracting solution (Howard and Shu 1996; Raksasataya et al. 1997; Howard and Vandenbrink 1999). SE often involves a determination of a labile (water soluble and exchangeable) fraction, as defined here, which is generally regarded as the most mobile (leachable) and bioavailable, but bio-uptake will also certainly result from partial dissolution of other geochemical forms given the strongly acidic environment of the human stomach (pH 1.0–2.5). A different approach is the bioaccessibility (BA) method, which utilizes an extractant that mimics the human gastric system (Ruby et al. 1993, 1996, 1999; Rodriguez et al. 1999; Thums et al. 2008). Unfortunately, much of the previous work done with the BA method utilized mine wastes containing exceptionally high levels of Pb (e.g., Ruby et al. 1993, 1996; Hettiarachchi and Pierzynski 2004), in contrast to the comparatively low levels found in typical urban soils. Madrid et al. (2008) made a comparison between the SE and BA methods using urban soils, but the focus of their study was on particle size effects, and the labile fraction was not measured. It remains to be determined how SE may be fully utilized for risk assessment of urban soils.
The purpose of this study was to determine which geochemical forms measured by SE are extracted by the BA method. The two methods were tested and compared using urban soils, reference minerals, and peat. Previous work suggested that artifact weathering can have an ameliorating effect on Pb-polluted urban soils (Howard and Olszewska 2011). Thus, a chronological sequence of demolition site soils was studied to test the hypothesis that the geochemical forms and bioaccessibility of Pb will change over time as a function of humification, earthworm activity, artifact weathering, etc. Reference materials were studied to determine which geochemical forms of Pb identified by SE are bioaccessible. It has been suspected for many years that Pb mineral species may be important sinks for Pb, but identification of these phases in urban soils has been problematic (Cotter-Howells 1996; Hettiarachchi and Pierzynski 2004). Therefore, reference samples of those Pb minerals thought to be most common in urban soils were targeted for special study. There has been much recent interest in the bio-uptake of trace metals by earthworms (Sizmur and Hodson 2009; Sizmur et al. 2011; Fraser et al. 2011), hence, a preliminary assessment of the bioaccessibility of Pb in earthworm casts was also carried out at one site.
2 Materials and Methods
2.1 Field Work and Sampling
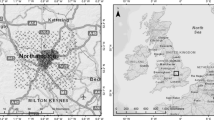
The chronological sequence of nine demolition site soils previously investigated (Howard et al. 2013) in downtown Detroit City (Fig. 1) was used for this study. Soils ranging in age from 3 to 92 years were described morphologically in hand-dug pits using standard USDA-NRCS methods (Soil Survey Staff 2010a, b) and the proposals of the International Committee on Anthropogenic Soils (Galbraith 2011). Thus, anthropogenic horizons developed in artificial fill (human-transported material) are designated by a “^” and those containing artifacts by “u.” At all sites studied, nineteenth century buildings of brick-and-mortar construction had been demolished. The ages of the soils were obtained from historic records obtained from the Burton Historic Collection at the Detroit Public Library and the City of Detroit Department of Dangerous Buildings and Demolition. Most sites are characterized by an artifact-rich profile comprised of an ^Au horizon, overlying a ^Cu or ^Cdku horizon, developed in a layer of fill less than 1 m thick resting on native soil. The soils are all formed on a nearly level landscape from mixed calcareous glacial sand and clayey diamicton parent materials (Howard 2010) under grass vegetation. Previous work shows that the soils are loamy, basic (pH 7.4–8.0), and enriched in organic carbon, carbonate, Fe oxides, and Mn oxides (Table 1), compared with native soils (Howard et al. 2012, 2013). Artifacts found in the soils (Table 2) are mainly brick, mortar, concrete, nails, glass, asphalt, lineoleum, wood, and ceramic materials (e.g., tile, pipe, electrical parts). Plaster was found only at site 9 below the ^Cu horizon. Carbonaceous artifacts in the form of bituminous waste (coal, asphalt, carbonaceous shale) are ubiquitous, except at site 3, where ferruginous waste is dominant. Soils were sampled in hand-dug pits. One kilogram samples of ^Au horizons and rhizosphere samples were collected in uncontaminated plastic bags. Soil samples were air-dried, sieved to obtain the less than 2 mm fraction, thoroughly mixed, and subsamples collected using the cone-and-quarter method for all analyses. Earthworms were collected from 1 m2 of ^Au horizon to a depth of 12 cm and transported to the lab in the soil. Worms were separated by wet sieving, washed, counted, and placed on a moist paper towel in the dark for 3 days to empty their guts by defecation. The paper was changed each day to prevent coprophagy. The worms were dried, weighed, frozen, and stored in an ice box until analysis. Worm casts were collected from worm burrows in the ^Cu horizon (site 9) by hand-picking under a binocular microscope and stored at 4 °C until analysis. Mineral reference samples of Pb-carbonate (cerussite), Pb-sulfate (anglesite), Pb-phosphate (pyromorphite), calcite, pyrolusite, goethite, and apatite were obtained from the Wayne State University Geology department. A commercially available sample of peat was also used.
2.2 Soil Characterization
Standard methods were used to determine particle size distribution (pipette method), pH (1:1 soil/solution), organic matter content (loss on ignition method), cation exchange capacity, exchangeable bases, and percentage of base saturation (Singer and Janitzky 1986). Carbonate content was determined following Tessier et al. (1979) by measuring Ca using 1.0 g samples and 20 ml of 1 M NaOAc, shaken for 5 h on a wrist-action shaker (reported as CaCO3). Amorphous Fe oxide content was determined by analyzing Fe using 250 mg samples of finely ground soil and 50 ml of 0.2 M NH4-oxalate, shaken in the dark for 2 h on a wrist-action shaker (Hodges and Zelazny 1980). Free (total) Fe oxide content by analyzing Fe using 0.5 g of finely ground soil equilibrated with 40 ml of 0.3 M Na-citrate, 5 ml of 1 M NaHCO3, and 1 g of Na-dithionite by shaking overnight (modified DCB method of Mehra and Jackson 1960). Mn oxide content was determined by analyzing Mn using the extracts from step 4 in the sequential extraction procedure (described below). Mn oxide contents are reported as MnO2 and Fe oxide contents as Fe2O3.
2.3 Chemical Analyses
All chemical analyses were done using duplicate or triplicate samples of finely ground soil and supernatants acidified to pH < 2.0 with HNO3, stored at 4 ° C until analysis, and measured by flame atomic absorption spectrophotometry using a Perkin–Elmer AAnalyst 700 spectrometer equipped with a deuterium lamp background correction. Relative standard deviations of the analytical results were <10 %. Detection limits were 0.1 mg kg−1 (Pb), 0.1 mg kg−1 (Mn), 0.7 mg kg−1 (Fe), and 0.3 mg kg−1 (Ca). Linear regression analysis was carried out using the KaleidaGraph program, and the statistical significance of correlation coefficients (r) was tested using the standard t test method (Davis 1986).
2.3.1 Sequential Extraction Analysis of Pb
The sequential extraction procedure used is modified after Chao (1972), Tessier et al. (1979), and Miller et al. (1986). Samples were analyzed in duplicate using 2.5 g samples of finely ground soil, 20 ml of extracting solution, and a wrist-action shaker. The targeted forms, extracting solutions, and equilibration times, respectively, were as follows:
-
Step 1
(water soluble): distilled–deionized H2O, 16 h
-
Step 2
(exchangeable): 1 M MgCl2, 1 h
-
Step 3
(carbonate-occluded): 1 M NaOAc, 5 h
-
Step 4
(Mn oxide-occluded): 0.1 M NH2OH-HCl + 0.01 M HNO3, 30 min
-
Step 5
(organically bound): 0.1 M K4P2O7, 24 h
-
Step 6
(Fe oxide-occluded): 1 M NH2OH-HCl + 25 % (v/v) HOAc, 4 h
Each of the extracting solutions above also contained 400 mg kg−1 of nitrilotriacetic acid to prevent resorption (Howard and Shu 1996; Howard and Vandenbrink 1999). Sequential extraction of lead reference minerals was done using the same method as above.
2.3.2 Bioaccessibility Analysis
The bioaccessibility of Pb was determined using a physiologically based extraction test (Ruby et al. 1996; USEPA 2007a). Thus, a batch extraction yields an in vitro bioavailability level (termed bioaccessibility) which is used as a proxy for an in vivo test level (bioavailability). Studies have demonstrated a strong correlation (r 2 = 0.924) between bioaccessibility and bioavailability as well as high reproducibility (Drexler and Brattin 2007). A 1-g soil sample was placed in a 50-ml centrifuge tube containing 20 ml of 0.4 M glycine at pH 2.5. The tube was heated at 37 ºC in a water bath and periodically shaken for 1 h. The supernatant was removed by centrifugation, filtered to remove particulates, and analyzed for Pb. Worm casts were analyzed for bioaccessible Pb using the same method. Bioaccessibility analysis of cerussite, anglesite, and pyromorphite was done similarly using 4.0 g of sample and 40 ml of 0.4 M glycine/HCl (38 % v/v) solution placed in a 50-ml centrifuge tube and shaken by a wrist-action shaker at 100 rpm for 2 h at 25 °C. A pH of 2.5 ± 0.05 was maintained using trace metal grade HCl if necessary. The effect of glycine extraction on Pb sorbed by reference minerals was done using 1 g of mineral and 20 ml of spiking solution containing 20 mg kg−1 of Pb. Samples were shaken for 24 h and centrifuged, and the supernatants were collected for elemental analysis. The samples were then washed three times with distilled–deionized water, and the glycine extraction carried out as described above.
2.3.3 Total Pb Analysis
Total Pb analysis was performed according to USEPA method 3051a (USEPA 2007b). Briefly, 0.5 g of soil and 10 mL of concentrated trace metal grade nitric acid (68 % v/v) were combined in a Teflon tube and heated to 175 °C in a CEM Mars Xpress microwave digestion unit. Each sample was then diluted to ∼40 mL, centrifuged, and the supernatant was analyzed for Pb by flame atomic absorption according to Standard Method 3111b (Clesceri et al. 1998). In addition to analyzing soil samples collected, a blank sample and a sample with a known concentration of lead, standard reference material (SRM), were also tested to ensure method reproducibility (NIST 2008). Sample blanks were low, less than 1 mg kg−1 (in most cases nondetectable), and the concentrations of SRMs were found to be within ±5 % of known values. After thawing, earthworms were heated overnight in an oven at 150 °C and then digested in 4 ml of HNO3 and 0.5 ml of 20 % H2O2. The digestion was brought to a final volume of 25 ml with distilled–deionized water and then analyzed for total Pb.
3 Results and Discussion
3.1 Sequential Extraction of Urban Soils
All of the ^Au horizons studied are contaminated with Pb (Table 3), averaging more than six times the local background level. These results are similar to those of previous work, indicating that Pb contamination is widespread in soils of downtown Detroit (Howard and Sova 1993; Detroit Free Press 2003; Franklin 2005; Marin 2007; Howard and Olszewska 2011). Most of the Pb is in organically bound (O) and Fe oxide-occluded (F) forms, but water soluble (W) and exchangeable (E) forms are elevated, with an average labile (L) Pb content of ∼22.3 % of the summed total. The average Mn oxide-occluded (M) level exceeds that of the carbonate-occluded (C) form. In relative order of average abundance, the predominant geochemical forms of Pb are O > L > F > M > C. An average of 50.9 % of summed total Pb is O + L.
All forms of Pb obtained by SE tend to show a positive correlation with soil age (Table 3) but only that of W-Pb is statistically significant (Table 4). Despite clear evidence in the field of progressive weathering of iron and calcareous artifacts (Howard et al. 2013), most of the soil properties measured show a poor correlation with soil age. Likewise, except for the statistically significant correlations between Fe oxide and Mn-oxide contents, and Mn oxide-occluded Pb, there is a poor correlation between soil component mass and the associated level of Pb measured by SE. This suggests that the random effects of the demolition process and variations in parent material are outweighing the effects of time on the geochemical partitioning of Pb. The poor correlations between organic matter content, organically bound Pb, and soil age are attributed to the presence of both soil organic matter and carbonaceous artifacts such as coal and soot, which are ubiquitous in the soils studied (total organic carbon was measured by the loss on ignition method). There is a highly statistically significant negative correlation between pH and soil age, which can be attributed to progressive weathering of calcareous artifacts in ^Au horizons, leaching and reprecipitation to form ^Cku horizons with increasing soil age (Howard et al. 2013). Weathered wrought-iron nails are also common in the soils. Amorphous Fe oxides have a greater specific adsorption affinity for Pb than their crystalline counterparts (Schwertmann and Taylor 1977), which accounts for the relatively high levels of F-Pb in the two youngest soils (∼40 % of summed total). The decrease in F-Pb levels with increasing soil age may be explained by the fact that goethite and crystalline ferrihydrite comprise corroded nails after only 3 years of weathering (Howard et al. 2013).
The highly significant decrease in pH with increasing in soil age (Tables 3 and 4) suggests that the corresponding increase in W-Pb may be ascribed simply to a decrease in pH-dependent cation exchange capacity. However, previous studies have shown that Pb is not soluble in elemental form under oxidizing conditions. Above pH 6–7, most Pb which is mobile and phytoavailable is complexed with low molecular weight humic substances (McBride et al. 1997; Antoniadis and Alloway 2002; Halim et al. 2003; Kim et al. 2010; Tack 2010). For example, Kaste et al. (2005) found water soluble Pb-organic complexes with molecular weights >5,000 Da, which is in the range of fulvic acid (Stevenson 1982). Hence, the increase in W-Pb is probably explained better by the fact that Pb-fulvic acid stability constants decrease with decreasing pH (Schnitzer and Skinner 1967; Stevenson 1982). Note that the youngest soil studied lacks an ^Au horizon, hence complexation with water soluble soil organic matter does not appear to account for the significant level of W-Pb there. Amrhein et al. (1992) suggested that when soils are dried for analysis, the death of microbes and plant roots can release significant water soluble organic carbon without the aromatic structure typical of soil humus. This may explain the presence of W-Pb in the two youngest soils studied, which have little or no E-Pb, and relatively low levels of O-Pb (Table 3). The further increase in W-Pb with time may reflect humification associated with progressive ^Au horizon development and, in the oldest soils, the presence of water soluble humic substances produced by weathering of coal and other carbonaceous artifacts (Howard et al. 2013).
3.2 Bioaccessible Pb in Urban Soils
The results obtained using the BA method show that bioaccessible Pb was found only in the most heavily contaminated soils, and when summed, total Pb is greater than about 90 mg kg−1 (Table 5). This fact, and the highly significant correlations between BA-Pb and summed total Pb (Table 4), suggests that total Pb is useful as a general indicator of soils in downtown Detroit containing detectable levels of mobile and bioavailable Pb. Our results are also consistent with the Canadian trigger level of ≥100 mg kg−1. Data from the Michigan Department of Community Health show that the annual average blood Pb level in young children has decreased over time since the Pb poisoning epidemic of the 1970s (Bickel 2010). However, there is still great concern over bioaccumulation in humans resulting from long-term exposure to low levels of particulate Pb derived from resuspended urban soil (Clark et al. 2006, 2008; Laidlaw and Filippelli 2008; Zahran et al. 2013). Given that total Pb levels in downtown Detroit are generally >400 mg kg−1 (Detroit Free 2003), our results agree with the proposed soil-to-air-to-child pathway of urban Pb exposure, i.e., resuspension of dry urban soil during the dry season may be contributing to ongoing elevated blood Pb levels observed in children of Detroit.
There is a poor correlation between BA-Pb and soil age (Table 4), suggesting that the distribution of BA-Pb is governed more by the random effects of building demolition than duration of weathering. Thus, soil age does not appear to be a useful predictor of BA-Pb. There is a highly significant positive correlation between L-Pb and BA-Pb (Table 4). However, L-Pb is generally significantly higher than BA-Pb (Table 5). L-Pb may be overestimated because nitrilotriacetic acid, used to inhibit resorption during SE, solubilized some Pb from nontargeted phases. Alternatively, BA-Pb may be underestimated because of pyromorphite precipitation during BA extraction. Farm animal bones are common at some sites, especially site 9, but the nature and extent of soil phosphate minerals are unknown. L-Pb is a better indicator of the presence of BA-Pb in the rhizosphere than in the bulk ^Au horizon (Table 5), but the reason for this is unknown. SE predicts the presence of detectable levels of BA-Pb in the rhizosphere when W-Pb is ≥20 mg kg−1 and L-Pb is ≥30 mg kg−1 (Table 5).
At site 9 (Fig. 1), an extensive system of earthworm burrows is present in anthrosols formed by 92 years of pedogenesis under an artificial irrigation system that operated from 1918 to 1988. A count showed a population density of approximately 100–133 worms m−2, about 75 % of which were probably mature animals. This is comparable to the results of worm counts of forest soils (Lee 1985). A sample of Lumbricus terrestris there contained ∼7 mg kg−1 Pb. This level is within the range (2.2–10.4 mg kg−1) reported previously by Franklin (2005) for earthworms in roadside soils of Detroit. The amount of BA-Pb (5.6 mg kg−1) measured in earthworm casts from the burrows is much lower than that in the corresponding bulk samples of ^Au horizon and rhizosphere (Table 5). Although it is reasonable to expect lower levels of bioaccessible Pb in soil as a consequence of earthworm uptake, these results contrast with those of previous studies which have found the opposite relationship (Udovic and Lestan 2007; Sizmur et al. 2011; Ruiz et al. 2011). It is well established that earthworms bioaccumulate Pb in their tissues (Morgan and Morgan 1988; Corp and Morgan 1991; Nahmani et al. 2007) although the amount of uptake varies with species (Ernst et al. 2008). Perhaps the discrepancy is the result of differences in the earthworm species being compared or to the fact that the casts in the burrows are older and more degraded than those at the surface.
3.3 Sequential Extraction and Bioaccessibility of Lead Mineral Species
In terms of bulk chemical composition, cerussite, pyromorphite, and anglesite reference samples contain 51.0, 35.4, and 19.1 % Pb, respectively. However, the relative magnitude of summed total Pb recovered by SE (Table 6) is in the order: cerussite (Ksp = 10−4.7) > anglesite (Ksp = 10−7.8) > pyromorphite (Ksp = 10−84.4). These summed total concentrations are in accordance with mineral solubility (Lindsay 1979; Ruby et al. 1994) rather than bulk total Pb content. Very little Pb was soluble in water and the relative amounts extracted correspond to total Pb contents. Little Pb was extracted by MgCl2, with anglesite producing the greatest amount of E-Pb. K4P2O5 generally recovered about 1/3 of the Pb extracted from reference minerals. Otherwise, cerussite and anglesite were mostly extracted by NaOAc during the operationally defined carbonate-occluded step, whereas pyromorphite was mainly solubilized along with Fe oxides during the harshest acid extraction. These results differ somewhat from those of Harrison et al. (1981) who found that PbSO4 was extracted primarily by MgCl2. However, this may be due to weathering of crystalline anglesite in the soil environment to form a more readily solubilized noncrystalline PbSO4 (Chaney et al. 1988). The BA method extracted the most Pb from cerussite (Table 5), ∼52 % of the summed total, much less from anglesite (∼11 %), and almost none from pyromorphite (∼1 %). The Pb concentrations recovered by BA correspond to differences in mineral solubility.
Lead carbonate extracted by NaOAc during SE is not necessarily present as a discrete cerussite phase because trace metals may be sorbed or precipitated onto the surface of calcite (McBride 1979; Bailey et al. 2005). In the urban soils studied, therefore, the carbonate-occluded fraction (Table 3) very likely includes Pb associated with pedogenic calcite produced by the weathering of calcareous artifacts. The results in Table 6 suggest that the carbonate-occluded fraction may also include Pb derived from weathered cerussite and anglesite. Cerussite was the main component of Pb-based house paint (“white lead”), and anglesite (or noncrystalline PbSO4) may have accumulated in urban soils as a result of airborne deposition of auto-Pb (Olsen and Skogerboe 1975; Harrison et al. 1981) although it is thought to be too soluble to persist in soil for long time periods (Lindsay 1979). The solubilities of gypsum and cerussite are very similar (Table 2); hence, pedogenic anglesite could form hypothetically by dissolution and reaction of plaster (or drywall) and paint as follows:
However, the pH range of the soils studied is above the anglesite stability field (Adriano 2001). The soil pH values are within the “recrystallization window” (between pH 7.6 and 8.1) wherein bone apatite dissolves and reprecipitates as a more insoluble form of hydroxylapatite (Berna et al. 2004). Previous work (Ma et al. 1993, 1995) and the results of this study (described below) suggest that anthropogenic Pb can be sorbed or precipitated onto the surface of apatite crystals. Although the soils studied are calcareous, previous work shows that the pH of microsites surrounding plant roots can be lower than that of the bulk soil by several pH units (Maier et al. 2000). Under those conditions pyromorphite could form in the rhizosphere (Chaney et al. 2010). If so, our results suggest that pyromorphite and bone-Pb were recovered in the Fe oxide-occluded fraction during SE.
Based on the relative mineral solubilities of principal constituents (Table 2) and field observations, the artifact weathering stability sequence in the urban soils studied is Glass = Cinders = Glazed brick > Unglazed Brick > Nails > Bone > Concrete > Mortar > Plaster or Drywall = Paint. The above results, indicating that cerussite is extracted primarily by NaOAc, and the data in Table 3 showing that no Pb was detected in the MgCl2 and NaOAc extractions of the two youngest soils, may indicate that significant weathering of house paint (cerussite) requires more than ∼24 years in the basic soils studied. This inference is consistent with both thermodynamic calculations, indicating that the solubility of cerussite (or hydrocerussite) increases rapidly below pH 7.0 (Scheetz 2004), and the results of a laboratory weathering experiment (Dubay 2012), showing that the equivalent of 30 years of leaching, was required to dissolve away gypsum (drywall) in a simulated urban soil. It is also consistent with petrographic data, suggesting that significant dissolution of portlandite in mortar artifacts required more than 39 years of weathering when buried in soil (Howard et al. 2013). The presence of plaster or drywall can apparently inhibit the dissolution of calcite comprising calcareous artifacts through the common ion effect (Dubay 2012).
3.4 Bioaccessibility Tests of Spiked Reference Materials
Calcite, pyrolusite, and peat sorbed almost all (∼95 %) of the Pb spike (Table 7), whereas apatite sorbed ∼42 % and goethite only sorbed ∼11 %. This is consistent with previous work, indicating that calcite, Mn oxides, and peat have a very strong affinity for Pb (e.g., Howard and Vandenbrink 1999). The amount of Pb sorbed by pyrolusite that was subsequently recovered by the BA method was below detection limits and very little was also recovered from peat and apatite. In contrast, calcite yielded about half, and goethite nearly all, of the Pb sorbed although the “shock loadings” used in this study may be overestimating how much is bioaccessible under natural conditions. Thus, the results of this study suggest that little or no Pb associated with Mn oxide, peat, and apatite is bioaccessible, whereas significant amounts of Pb associated with calcite will dissolve in the human stomach. It is interesting that despite the fact that Fe oxides are well-known for their specific adsorption affinity for Pb, little fixation of Pb by goethite occurred. Instead, Pb was apparently sorbed by exchange sites on the mineral surface. Thus, exchangeable Pb on the goethite surface is bioaccessible but that occluded inside the mineral is not. The data imply that it is primarily freshly precipitated amorphous ferrihydrite that immobilizes Pb by specific adsorption and that occlusion of Pb in goethite must happen early during the chemical weathering process. It is well established that Mn oxide has an extremely high affinity for Pb, and our data suggest that it can continue immobilizing Pb even as chemical weathering proceeds over time. These laboratory results agree with our field data (Table 3), indicating that F-Pb is abundant only in the two youngest soils, whereas M-Pb is significant throughout the chronological sequence of demolition site soils studied.
4 Conclusions
Despite clear evidence in the field of humification, artifact weathering and earthworm activity, the only statistically significant trends with increasing soil age are a positive correlation with water soluble Pb and a negative correlation with pH. Generally speaking, there is no statistically defined evolution in either the geochemical partitioning or bioaccessibility of Pb with increasing soil age. Instead, the random effects of the demolition process on variations in parent material outweighed the effects of time in the urban soils studied. There is a reasonable correlation between labile Pb measured by the sequential extraction and bioaccessibility methods. However, the results of both techniques should perhaps be regarded as semiquantitative because of uncertain artificial effects inherent in the extraction procedures themselves. Sequential extraction predicts operationally the presence of detectable levels of bioaccessible Pb in the rhizosphere when the summed total is ≥90 mg kg−1 and labile Pb is >30 mg kg−1. The bioaccessible fraction corresponds to the water soluble, exchangeable, and part of the carbonate-occluded fraction. The water soluble fraction is inferred to be comprised of Pb complexed with lower molecular weight humic substances and possibly other organic compounds comprising soil microbes. The Fe oxide-occluded, Mn oxide-occluded, and higher molecular weight component of the organically bound fraction are not bioaccessible. Pb occlusion by Fe oxides occurs early (<3 years) during the chemical weathering process, whereas specific adsorption by Mn oxides is ongoing. Inaccuracies introduced in SE by selectivity and resorption problems are further compounded by the presence of artifacts in urban soils. Cerussite comprising house paint and anglesite derived from auto-Pb or pedogenic reactions between plaster and paint can contribute significantly to the carbonate-occluded fraction, thereby resulting in an overestimation of Pb presumably associated with pedogenic calcite. Pyromorphite of rhizospheric origin and bone-Pb can contribute to the Fe oxide-occluded fraction leading to an overestimation of Pb specifically adsorbed by ferrihydrite and goethite. The preliminary analysis of the effect of earthworm activity on Pb bioaccessibility was inconclusive.
References
Adriano, D. C. (2001). Trace elements in terrestrial environments. Berlin: Springer.
Al-Barrak, K., & Rowell, D. L. (2006). The solubility of gypsum in calcareous soils. Geoderma, 136, 830–837.
Amrhein, C., Strong, J. E., & Mosher, P. A. (1992). Effect of deicing salts on metal and organic matter mobilization in roadside soils. Environmental Science and Technology, 26, 703–709.
Antoniadis, V., & Alloway, B. J. (2002). The role of dissolved organic carbon in the mobility of Cd, Ni and Zn in sewage sludge-amended soils. Environmental Pollution, 117, 515–521.
Bailey, E. H., Mosselmans, J. F. W., & Young, S. D. (2005). Time-dependent surface reactivity of Cd sorbed on calcite, hydroxylapatite and humic acid. Mining Magazine, 69, 563–575.
Beckett, P. H. T. (1989). The use of extractants in studies on trace metals in soils, sewage sludges, and sludge-treated soils. In B. A. Stewart (Ed.), Advances in soil science (Vol. 9, pp. 144–176). Berlin: Springer.
Berna, F., Matthews, A., & Weiner, S. (2004). Solubilities of bone mineral from archaeological sites: the recrystallization window. Journal of Archaeological Science, 31, 867–882.
Bickel, M. J. (2010). Spatial and temporal relationships between blood lead and soil lead concentrations in Detroit, Michigan. M. S (p. 67). Detroit, Michigan: Thesis, Dept. of Civil and Environ. Eng., Wayne State Univ.
Chaney, R. L., Mielke, H. W., & Sterrett, S. B. (1988). Speciation, mobility and bioavailability of soil lead. In B. E. Davies & B. G. Wixsom (Eds.), Lead in soils: issues and guidelines (p. 315). Kent, England: Science Reviews, Ltd.
Chaney, R. L., Broadhurst, C. L., & Centofanti, T. (2010). Phytoremediation of soil trace elements. In P. S. Hooda (Ed.), Trace elements in soils (pp. 312–352). West Sussex: Wiley.
Chao, T. T. (1972). Selective dissolution of manganese oxides from soils and sediments with acidified hydroxlamine hydrochloride. Soil Science Sociey of America Proceedings, 36, 764–768.
Clark, H. F., Brabander, D. J., & Erdil, R. M. (2006). Sources, sinks, and exposure pathways of lead in urban garden soil. Journal of Environmental Quality, 35, 2066–2074.
Clark, H. F., Hausladen, D. M., & Brabander, D. J. (2008). Urban gardens: lead exposure, recontamination mechanisms, and implications for remedial design. Environmental Research, 107, 312–319.
Clesceri, L. S., Greenberg, A. E., & Eaton, A. D. (1998). Method 3111—metals By Flame Atomic Absorption Spectrometry. 20th ed. Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association, American Water Works Association, and Water Environment Federation.
Corp, N., & Morgan, A. J. (1991). Accumulation of metals from polluted soils by the earthworm Lumbricus rubellus: can laboratory exposure of control worms reduce biomonitoring problems? Environmental Pollution, 74, 39–52.
Cotter-Howells, J. (1996). Lead phosphate formation in soils. Environmental Pollution, 93, 9–16.
Davis, J. C. (1986). Statistics and data analysis in geology (p. 646). New York: Wiley.
Detroit Free Press (2003). Free Press soil study: samples offer a cross-section of contamination. Detroit Free Press, January 23, 2003.
Drexler, J. W., & Brattin, W. J. (2007). An in vitro procedure for estimation of lead relative bioavailability: with validation. Human Ecological Risk Assessment, 13, 383–401.
Dubay, B. R. (2012). Urban soil genesis, weathering of waste building materials, and bioavailability of lead in a chronosequence at former demolition sites, Detroit, Michigan. M.S. thesis (p. 107). Detroit, MI: Dept. of Geology, Wayne State University.
Duchesne, J., & Reardon, E. J. (1995). Measurement and prediction of portlandite solubility in alkali solutions. Cement and Concrete Research, 25, 1043–1053.
Ernst, G., Zimmermann, S., Christie, P., & Frey, B. (2008). Mercury, cadmium and lead in different ecophysiological groups of earthworms in forest soils. Environmental Pollution, 156, 1304–1313.
Filgueiras, A. V., Lavilla, I., & Bendicho, C. (2002). Chemical sequential extraction for metal partitioning in environmental solid samples. Journal Environmental Monthly, 4, 823–857.
Franklin, L. (2005). Organic fractionation and chemical characterization of organometallic forms of lead in contaminated Michigan soils. PhD dissertation, Dept. of Civil and Environmental Engineering (p. 230). Detroit, MI: Wayne State University.
Fraser, A., Lambkin, D. C., Lee, M. R., Schofield, P. F., Mosselmans, J. F. W., & Hodson, M. E. (2011). Incorporation of lead into calcium carbonate granules secreted by earthworms living in lead contaminated soils. Geochimica et Cosmochimica Acta, 75, 2544–2556.
Galbraith, J. M. (2011). Proposed revisions to the future 12th edition of Keys to Soil Taxonomy. International Committee for Anthropogenic Soils Circular Letter 7, 6 pp.
Halim, M., Conte, P., & Piccolo, A. (2003). Potential availability of heavy metals to phytoextraction from contaminated soils induced by exogenous humic substances. Chemosphere, 52, 265–275.
Harrison, R. M., Laxen, D. P. H., & Wilson, S. J. (1981). Chemical associations of lead, cadmium, copper and zinc in street dusts and roadside soils. Environmental Science and Technology, 15, 1378–1383.
Hettiarachchi, G. M., & Pierzynski, G. M. (2004). Soil lead bioavailability and in situ remediation of lead-contaminated soils: a review. Environmental Progress, 23, 78–93.
Hodges, S. C., & Zelazny, L. W. (1980). Determination of noncrystalline soil components by weight difference after selective dissolution. Clays and Clay Minerals, 28, 35–42.
Howard, J. L. (2010). Late Pleistocene glaciolacustrine sedimentation and paleogeography of southeastern Michigan, USA. Sedimentary Geology, 223, 126–142.
Howard, J. L., & Sova, J. (1993). Sequential extraction analysis of lead in Michigan roadside soils: mobilization in the vadose zone by deicing salts. Journal of Soil Contamination, 2, 361–378.
Howard, J. L., & Shu, J. (1996). Sequential extraction analysis of heavy metals using a chelating agent (NTA) to counteract resorption. Environmental Pollution, 91, 89–96.
Howard, J. L., & Vandenbrink, W. J. (1999). Sequential extraction analysis of heavy metals in sediments of variable composition using nitrilotriacetic acid to counteract resorption. Environmental Pollution, 106, 285–292.
Howard, J. L., & Olszewska, D. (2011). Pedogenesis, geochemical forms of heavy metals, and artifact weathering in an urban soil chronosequence, Detroit, Michigan. Environmental Pollution, 159, 754–761.
Howard, J. L., Clawson, C. R., & Daniels, W. L. (2012). A comparison of mineralogical techniques and potassium adsorption isotherm analysis for relative dating and correlation of late Quaternary soil chronosequences. Geoderma, 179–180, 81–95.
Howard, J. L., Dubay, B. R., & Daniels, W. L. (2013). Artifact weathering, anthropogenic microparticles, and lead contamination in urban soils at former demolition sites, Detroit, Michigan. Environmental Pollution, 179, 1–12.
Kaplan, M. F., & Mendel, J. E. (1982). Ancient glass and the safe disposal of nuclear waste. Archaeology, 35(4), 22–29.
Kaste, J. M., Friedland, A. J., & Miller, E. K. (2005). Potentially mobile lead fractions in montane organic-rich soil horizons. Water, Air, and Soil Pollution, 167, 139–154.
Kessler-Arnold, K. A., & O’Hearn, M. (1989). Background concentrations of metals and cyanide in lower Michigan soils. In 44 th Purdue Ind. Waste Conf. Proc (pp. 33–47). MI, Lewis: Chelsea.
Kim, K. R., Owens, G., & Naidu, R. (2010). Effect of root-induced changes on dynamics and plant uptake of heavy metals in rhizosphere soil. Pedosphere, 20, 494–504.
Laidlaw, M. A. S., & Filippelli, G. M. (2008). Resuspension of urban soils as a persistent source of lead poisoning in children: a review and new directions. Applied Geochemistry, 23, 2021–2039.
Laing, G. D. (2010). Analysis and fractionation of trace elements in soils. In P. S. Hooda (Ed.), Trace elements in soils (pp. 53–80). New York: Wiley.
Lee, M. K. (1985). Earthworms. Their ecology, and relationships with soils and land use. New York: Academic.
Li, F., Fan, Z., Xiao, P., Oh, K., Ma, X., & Hou, W. (2009). Contamination, chemical speciation and vertical distribution of heavy metals in soils of an old and large industrial zone in northeast China. Environmental Geology, 57, 1815–1823.
Lindsay, W. L. (1979). Chemical equilibria in soils. New York: Wiley.
Ma, Q. Y., Traina, S. J., Logan, T. J., & Ryan, J. A. (1993). In situ lead immobilization by apatite. Environmental Science and Technology, 27, 1803–1810.
Ma, Q. Y., Logan, T. J., & Traina, S. J. (1995). Lead immobilization from aqueous solutions and contaminated soils using phosphate rock. Environmental Science and Technology, 29, 1118–1126.
Madrid, F., Diaz-Barrientos, E., & Madrid, L. (2008). Availability and bio-accessibility of metals in the clay fraction of urban soils of Sevilla. Environmental Pollution, 156, 605–610.
Mahanta, M. J., & Bhattacharyya, K. G. (2011). Total concentrations, fractionation and mobility of heavy metals in soils of urban area of Guwahati, India. Environmental Monitoring and Assessment, 173, 221–240.
Maier, R. M., Pepper, I. L., & Gerba, C. P. (2000). Environmental microbiology (p. 585). New York: Academic.
Malik, R. N., Jadoon, W. A., & Husain, S. Z. (2010). Metal contamination of surface soils of industrial city Sialkot, Pakistan: a multivariate and GIS approach. Environmental Geochemistry and Health, 32, 179–191.
Marin, M. S. (2007). Geochemical forms of lead and bio-uptake by earthworms in contaminated urban soils (p. 102). Detroit, MI: PhD dissertation, Dept. of Civil and Environmental Engineering, Wayne State University.
McBride, M. B. (1979). Chemisorption and precipitation of Mn2+ at CaCO3 surfaces. Soil Science Society of America Journal, 43, 693–698.
McBride, M., Sauve, S., & Hendershot, W. (1997). Solubility control of Cu, Zn, Cd, and Pb in contaminated soils. European Journal of Soil Science, 48, 337–346.
Mehra, O. P., & Jackson, M. L. (1960). Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clays and Clay Minerals, 7, 317–327.
Mielke, H. W., Gonzales, C. R., Powell, E., Jartun, M., & Mielke, P. W. (2007). Nonlinear association between soil lead and blood lead of children in metropolitan New Orleans, Louisiana: 2000–2005. Science of the Total Environment, 388, 43–53.
Miller, W. P., Martens, D. C., & Zelazny, L. W. (1986). Effect of sequence in extraction of trace metals from soils. Soil Science Society American Journal, 50, 598–601.
Morgan, J. E., & Morgan, A. J. (1988). Earthworms as biological monitors of cadmium, copper, lead and zinc in metalliferous soils. Environmental Pollution, 54, 123–138.
Nahmani, J., Hodson, M. E., & Black, S. (2007). A review of studies performed to assess metal uptake by earthworms. Environmental Pollution, 145, 163–179.
NIST. (2008). Standard Reference Material 2585: trace elements in soil containing lead from paint. Gaithersburg: National Institute of Standards & Technology.
Olsen, K. W., & Skogerboe, R. K. (1975). Identification of soil lead compounds from automotive sources. Environmental Science and Technology, 9, 227–230.
Raksasataya, M., Langdon, A. G., & Kim, N. D. (1997). Inhibition of Pb redistribution by two complexing agents (cryptand and NTA) during sequential extraction analysis. Analytica Chimica Acta, 347, 313–323.
Reesman, A. L. (1974). Aqueous dissolution studies of illite under ambient conditions. Clays and Clay Minerals, 22, 443–454.
Rodriguez, R. R., Basta, N. T., Casteel, S. W., Armstrong, F. P., & Ward, D. C. (1999). An in vitro method to estimate bioavailable arsenic in contaminated soils and solid media. Environmental Science and Technology, 33, 642–649.
Ruby, M. V., Davis, A., Link, T. E., et al. (1993). Development of an in vitro screening test to evaluate the in vivo bioaccessibility of ingested mine-waste lead. Environmental Science and Technology, 27, 2870–2877.
Ruby, M. V., Davis, A., & Nicholson, A. (1994). In situ formation of Pb phosphates in soils as a method to immobilize Pb. Environmental Science and Technology, 28, 646–654.
Ruby, M. V., Davis, A., Schoof, R., Eberle, S., & Sellstone, C. M. (1996). Estimation of bioavailability using a physiologically based extraction test. Environmental Science and Technology, 30, 422–430.
Ruby, M. V., Schoof, R., Brattin, W., Goldade, M., Post, G., Harnois, M., et al. (1999). Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environmental Science and Technology, 33, 3697–3705.
Ruiz, E., Alonso-Azcarate, J., & Rodriguez, L. (2011). Lumbricus terrestris L. activity increases the bioavailability of metals and their accumulation in maize and barley. Environmental Pollution, 159, 722–728.
Ruiz-Cortes, E., Reinoso, R., Diaz-Barrientos, E., & Madrid, L. (2005). Concentrations of potentially toxic metals in urban soils of Seville: relationship with different lands uses. Environmental Geochemistry and Health, 27, 465–474.
Scheetz, C. D. (2004). Distribution, transport, and fate of lead on shooting ranges (p. 47). Blacksburg, Va: M.S. thesis, Dept. of Geological Sciences, Virginia Polytechnic Institute and State University.
Schnitzer, M., & Skinner, S. I. M. (1967). Organo-metallic interactions in soils. 5. Stability constants of Pb, Ni, Mn, Co, Ca, and Mg fulvic acid complexes. Soil Science, 103, 247–252.
Schwertmann, U., & Cornell, R. M. (2000). Iron oxides in the laboratory: preparation and characterization (2nd ed.). Weinheim: Wiley-VCH VerlagGmbH.
Schwertmann, U., & Taylor, R. M. (1977). Iron oxides. In J. B. Dixon & S. B. Reed (Eds.), Minerals in soil environments (pp. 145–180). Madison: Soil Science Society of America.
Singer, M. J., and Janitzky, P. (1986) Field and laboratory procedures used in a soil chronosequence study. U.S. Geological Survey Bull. 1648, 49 pp.
Sizmur, T., & Hodson, M. E. (2009). Do earthworms impact metal mobility and availability in soil?—a review. Environmental Pollution, 157, 1981–1989.
Sizmur, T., Palumbo-Roe, B., Watts, M. J., & Hodson, M. E. (2011). Impact of earthworm Lumbricus terrestris (L.) on As, Cu, Pb and Zn mobility and speciation in contaminated soils. Environmental Pollution, 159, 742–748.
Soil Survey Staff. (2010a). Soil survey manual. Washington, DC: USDA-NRCS, U.S. Govt. Print. Office.
Soil Survey Staff. (2010b). Keys to soil taxonomy (11th ed.). Washington, DC: USDA-NRCS, U.S. Govt. Print. Office.
Stevenson, F. J. (1982). Humus chemistry: genesis, composition, reactions. New York: Wiley.
Tack, F. M. G. (2010). Trace elements: general chemistry, principles and processes. In P. S. Hooda (Ed.), Trace elements in soils (pp. 9–37). West Sussex: Wiley.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of trace metals. Analytical Chemistry, 51, 844–851.
Thums, C. R., Farago, M. E., & Thornton, I. (2008). Bioavailability of trace metals in brownfield soils in an urban area in the UK. Environmental Geochemistry and Health, 30, 549–563.
Udovic, M., & Lestan, D. (2007). The effect of earthworms on the fractionation and bioavailability of heavy metals before and after soil remediation. Environmental Pollution, 148, 663–668.
USEPA (2007a) Estimation of relative bioavailability of lead in soil and soil-like materials using in vivo and in vitro methods. OSWER 9285.7-77, 23 pp.
USEPA. (2007b). Method 3051A—microwave assisted acid digestion of sediments, sludges, soils, and oils. Washington, D.C.: U.S. Environmental Protection Agency.
Zahran, S., Laidlaw, M. A. S., McElmurry, S. P., Filippelli, G. M., & Taylor, M. (2013). Linking source and effect: resuspended soil lead, air lead, and children’s blood lead levels in Detroit, Michigan. Environmental Science and Technology, 47, 2839–2845.
Acknowledgments
Thanks to Amy Benchich, Ryan Thomas, and Sharla Wood (Wayne State University); Joe Calus and Eric Gano (USDA-NRCS); and John Galbraith and Julie Burger (VPI&SU) for their assistance during this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
The bioaccessible fraction corresponds to the water soluble, exchangeable, and part of the carbonate-occluded fraction.
Rights and permissions
About this article
Cite this article
Howard, J.L., Dubay, B.R., McElmurry, S.P. et al. Comparison of Sequential Extraction and Bioaccessibility Analyses of Lead Using Urban Soils and Reference Materials. Water Air Soil Pollut 224, 1678 (2013). https://doi.org/10.1007/s11270-013-1678-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1678-y