Abstract
Although many studies have examined effects of metal mine effluents (MMEs) on receiving environments, few have compared the roles of individual and mixed metals relative to whole effluents. The objective of the present set of studies was to examine whether Cu, Ni, or Se, alone or in a mixture, causes comparable effects to those observed in fathead minnows (Pimephales promelas) exposed to an environmentally relevant MME (45 % process water effluent [PWE]). Metal bioaccumulation, fathead minnow (FHM) morphometrics, and egg production were compared between treatments over a 21-day exposure. FHMs were exposed to similar waterborne concentrations and species of metals in single and mixed metal treatments relative to 45 % PWE. FHMs were also exposed to similar concentrations of metals in single and mixed metal treatments relative to 45 % PWE through the diet (Chironomus dilutus — a representative prey species). However, only FHMs exposed to 45 % PWE had reduced egg production (60–80 % less than controls). Our findings indicate that Cu, Ni, and Se exposures and bioaccumulation did not contribute to decreased reproductive output in FHMs under the conditions that were examined. We also found no evidence to believe that these metals were responsible for decreased egg production in PWE. It is therefore reasonable to suggest that these metals have limited potential to cause reproductive effects in MMEs with similar composition and water chemistry conditions. Overall, this study highlights the importance of examining single and mixed metal exposures prior to suggesting that adverse effects in fish exposed to MMEs occur due to bioaccumulation of metal(s).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metal mine effluents (MMEs) can be a significant source of metals that have the ability to negatively impact organisms in receiving aquatic environments. Many studies have documented effects from exposure to MMEs, particularly to invertebrate and fish populations. For example, reduced benthic invertebrate densities (Hruska and Dubé 2004), and reproductive changes (e.g., altered egg production, larval deformities, egg sizes, growth) (Muscatello et al. 2006; Rickwood et al. 2006; Jezierska et al. 2009; Driessnack et al. 2011; Rozon-Ramilo et al. 2011a, b), altered gill functions and hormone levels (Levesque et al. 2003), cellular changes (Payne et al. 2001), as well as increased tissue metal burdens in several fish species (Dubé et al. 2005, 2006; Weber et al. 2008; Driedger et al. 2010) have been documented as a result of exposure to several different types of MMEs. Because effects can be harmful to the aquatic ecosystems, metal mining operations typically monitor their effluents closely to ensure safe release into the environment.
In Canada, the federal Fisheries Act, through the Environmental Effects Monitoring (EEM) program in the Metal Mine Effects Regulations (MMER), legislates subject metal mines to monitor their effluents and verify that they do not affect aquatic invertebrates, fish, or fisheries (Ribey et al. 2002). If effects are confirmed through the program, investigations of cause (IOC) approaches are subsequently followed. The IOC approaches consist of confirming and identifying the source, and identifying and characterizing the chemical(s) (Hewitt et al. 2003, 2005). Chemical characterization and identification, as the final tier, includes identification of specific causes of any confirmed observed effects. In cases when MMEs are found to cause effects, determining the causes is often complex. The aquatic environments that receive MMEs not only contain high concentrations of a variety of metals and metalloids, but are also often contaminated by organics or endocrine disrupting compounds from other sources (e.g., waste water treatment plants, urban run-off) (Dubé et al. 2006; Rickwood et al. 2006). In addition, there might be historical contamination from past mining operations or other industries, or multiple effluents from a single mining operation. The different type of MME exposures can lead to different types of effects (Rozon-Ramilo et al. 2011a) or potentially lead to cumulative effects due to the exposure to multiple stressors. Metals, organics, and/or historical contamination can each contribute to effects in the aquatic environment, confounding the central question of determining whether the cause of observed effects is the current effluent under regulation. Consequently, identifying causative chemicals or metals is a main objective if MMEs are found to cause effects in the environment.
Traditionally, laboratory metal toxicology studies have mostly involved assessing effects on fish with exposures to a single metal, often with only a single route of exposure (i.e., waterborne or dietary). Although these studies provide valuable information on metal toxicity and uptake routes, they might not be environmentally realistic in waterbodies exposed to metal mixtures such as MMEs. Multiple metals can cause different effects than single metals, through additive, less than additive, or more than additive relationships (Norwood et al. 2003). In order to assess effects of complex metal effluents, and to include both waterborne and dietary exposures, the use of mesocosms has recently been introduced into monitoring programs (Rickwood et al. 2006; Driessnack et al. 2011; Rozon-Ramilo et al. 2011a, b). Because dietary uptake is an important source of toxicity with certain metals (e.g., Cu and Se), the addition of a live food source also exposed to the contaminants increases the environmental relevance (Lemly 1997; Kamunde et al. 2002; Muscatello et al. 2006). Trophic transfer of metals and metal bioavailability are believed to play an important role in contributing to effects, including metal accumulation in fish tissues and altered reproduction in fathead minnows (FHM; Pimephales promelas) (Rickwood et al. 2006; Driessnack et al. 2011; Rozon-Ramilo et al. 2011b).
It is to be noted that the specific metals that contribute to the biological effects from MME exposures are still largely unknown. Metals such as Cu, Ni, and Se are known to cause reproductive impacts in fish under elevated exposure levels. Elevated Cu and Ni concentrations have each been shown to contribute to lower egg production in minnows (Mount 1968; Pickering 1974; Geckler et al. 1976; Horning and Neiheisel 1979). Se bioaccumulation has been reported to cause larval deformities and reproductive failure in fish when consumed through the diet (Lemly 1997; Holm et al. 2005). Furthermore, Cu and Se uptake through the diet in lakes exposed to MMEs is believed to contribute to decreased reproductive condition in fish (Pyle et al. 2005). Cu, Ni, and Se are commonly found at elevated concentrations in MMEs; however, it is necessary to explore whether they are responsible for toxic effects under specific conditions of the MMEs. Metal bioavailability and toxicity is highly influenced by water chemistry parameters, such as pH, alkalinity, and hardness, although each metal is not affected in the same way (Niyogi and Wood 2004). MMEs are often released into the environment under slightly acidic and high hardness conditions, which could influence metal toxicity to resident biota.
The objective of this research was to investigate possible causes of toxicity in adult FHMs exposed to a Canadian MME using an approach similar to that followed in IOC (i.e., to identify particular metals responsible for toxic effects). Specifically, we wanted to compare and contrast FHM response patterns (tissue-specific metal bioaccumulation, and fish morphometrics and reproductive performance) from exposure to an MME relative to effluent equivalent doses of Cu, Ni and Se, both singly as well as in mixture. We hypothesized that FHMs exposed through the water and diet to similar concentrations of these three metals to those found in 45 % PWE, under matching water chemistry conditions, would exhibit comparable tissue-specific metal bioaccumulation and alterations in reproductive performance. We examined a MME which has been shown to lead to bioaccumulation of a variety of metals in benthic invertebrate and fish tissues, as well as induce reproductive changes in breeding pairs of FHMs in a number of previous studies (Rickwood et al. 2006; Rozon-Ramilo et al. 2011a, b). This MME — Process Water Effuent (PWE) — is released at concentrations of approximately 51,000,000 m3/year (2009) into the Junction Creek Watershed, near Sudbury, Ontario, Canada. The environmental concentration of PWE has been estimated at 45 % in the receiving environment based on discharge and stream conditions (Rickwood et al. 2006).
2 Materials and Methods
2.1 Study Site, Timeline, and Mesocosms
This research was performed at the University of Saskatchewan in Saskatoon, SK, Canada, and consisted of three separate studies. The first study (Cu vs. 45 % PWE) was performed from March to May 2009, the second study (Ni or Se vs. 45 % PWE) was performed in December 2009 to February 2010, and the third study (Cu, Ni, and Se mixture vs. 45 % PWE) was performed from January to March 2011. Each study involved the use of multi-trophic mesocosms with up to eight replicate, 10.3-l circular polyethylene artificial streams in order to expose breeding pairs of FHMs to MME, a single metal, or metal mixture. These mesocosms allow for isolated waterborne exposures, or simultaneous waterborne and dietary exposures over a period of 21 days, as a means of assessing chronic effects of MMEs on both invertebrates and fish. These multi-trophic mesocosms are an accepted component of Environment Canada’s EEM program, and have been described in details by Hruska and Dubé (2004) and Rickwood et al. (2006). These mesocosms have been found to be useful in evaluating reproductive effects in fish, and bioaccumulation of metals from MMEs to target organisms/tissues (e.g., Chironomus dilutus, FHM liver, gonad, gill, and carcass tissues) in Ontario (Hruska and Dubé 2004; Hruska and Dubé 2005; Dubé et al. 2006; Rickwood et al. 2006; Rozon-Ramilo et al. 2011a, b), New Brunswick (Dubé et al. 2005), Saskatchewan (Driessnack et al. 2011), and the Northwest Territories (Spencer et al. 2008). Animal use in the present studies was approved by the University of Saskatchewan Committee on Animal Care and Supply (UCACS) and Animal Research Ethics Board (AREB).
2.2 Reference Water, Process Water Effluent, and Metal Treatments
In our previous studies with MMEs, Vermillion River water had been the source of reference water (RW) for field-based mesocosms near Sudbury, Ontario, Canada (Rozon-Ramilo et al. 2011a). For the present laboratory study, synthetic RW with water chemistry parameters matched to Vermillion River water conditions was used. The same approach was also performed in a previous laboratory investigation with PWE (Rozon-Ramilo et al. 2011b). Therefore, RW in the present study consisted of a mixture of reverse osmosis (RO) water and dechlorinated laboratory water at concentrations of approximately 65 % RO and 35 % laboratory water in order to match the hardness, pH, alkalinity, and background metal concentrations of Vermillion River water (Rozon-Ramilo et al. 2011a). This mixture was also used as dilution water for the 45 % PWE. The exposure water in single metal and mixed metals treatments were also made up of the same ratio of RO and laboratory, and supplemented with calcium sulfate and sodium chloride under constant stirring, in order to match water hardness, salinity, pH, and alkalinity of the 45 % PWE.
Process water effluent (PWE) was shipped weekly from Sudbury, Ontario to the University of Saskatchewan. For these studies, PWE was chosen to be used at a concentration of 45 % dilution based on environmentally relevant concentrations in the receiving stream watershed. Concentrations of Cu, Ni, and Se in each of the respective single metal treatments were based on concentrations typically found in 45 % PWE. Historic ranges of Cu, Ni, and Se in 45 % PWE have been reported between 50 and 95, 53 and 114, and 7 and 10 μg/l, respectively (Rickwood et al. 2006; Rozon-Ramilo et al. 2011a). Therefore, the concentrations used for the present studies included Cu at 60 μg/l added as copper chloride dehydrate, Ni at 90 μg/l added as nickel (II) nitrate hexahydrate, and Se at 10 μg/l added as sodium selenate. Selenate was used because previous samples analyzed through ion chromatography-inductively coupled plasma-mass spectrometry indicated that selenate is the major species (~90 %) of Se present in the PWE (unpublished data). Due to changes in the mining process from a temporary metal production stoppage, compositions of metals in 45 % PWE differed from historic ranges. Thus, concentrations of metals used in the metal mixture treatment (Cu, Ni, and Se) were modified slightly (specifically, lower Cu – 30 μg/l, and higher Ni – 110 μg/l, compared to typical concentrations). Each metal treatment was produced every 1.5 to 2 days in 330-l holding tanks and stored for more than 24 h prior to use in the experimental exposures in order to achieve chemical equilibrium. This allowed for daily renewal of the metal treatments in the trophic transfer system.
2.3 Trophic-Transfer System
Chironomus dilutus were obtained from in-house laboratory cultures. Experimental C. dilutus cultures were raised in RW, 45 % PWE, or one of the metal treatments in order to ensure FHMs were exposed to potential contaminants through both the water and diet (see Tables 1–3 for composition). Egg sacs of C. dilutus were isolated from laboratory held brood stocks and distributed as two eggs sacs per stream (repeated every 7 days for 3 weeks) in order to provide adequate food (1 g/day) to the breeding pairs of FHMs for the duration of the 21-day exposure period (Rickwood et al. 2006). Egg sacs were placed directly into the multi-trophic mesocosms and water temperature was maintained at 23 °C (±2 °C). Water or treatment exchanges began after the first 4 days of C. dilutus culturing and were done at a rate of 1 turnover every 2 days. During the first 4 days of culturing all egg sacs were in water-only in order to reduce variability in culturing success between treatments. Each multi-trophic mesocosm was made up of a sediment layer (~2.54 cm of pre-cleaned silica sand), a feeding barrier to control the amount of C. dilutus available to FHMs, a spawning tile, and a mesh screen to prevent adult C. dilutus and FHMs from escaping the streams. Larval C. dilutus were fed a blend of Tetramin™ slurry (~1 g/egg sac) every second day throughout the culturing period and the exposure period.
2.4 Pre-exposure Period
The pre-exposure period was performed in order to assess baseline FHM reproduction in RW and ensure all breeding pairs met performance criteria for the exposure period. Six- to eight-month old FHMs were obtained from Osage Catfisheries Inc. (Osage Beach, MO, USA). Body weight, total length, and secondary sex characteristics (banding, nuptial tubercles, dorsal pad, fin dot, and ovipositor) were recorded and each fish was placed randomly into a stream until a male and female were present in each stream. Water exchanges were set at 1 turnover/day and temperature was maintained at 25 °C (±2 °C) with submersible aquarium heaters under conditions of a 16:8-h light/dark photoperiod. Breeding pairs were fed frozen bloodworms (C. riparius) twice daily at a feeding rate of approximately 1 g/day.
Egg production was monitored daily by removing the breeding tile from each stream, scraping the eggs from the tile onto a petri dish, and photographing the eggs. Breeding pairs for the exposure period were selected based on spawning at least once in the pre-exposure period and >80 % fertilization success. An egg was considered to be infertile if it was either opaque, had a visibly precipitated yolk, or contained no yolk (Ankley et al. 2001). In order to meet this criteria, the lengths of the pre-exposures varied between studies (14 days for the first study [April 1 to April 15, 2009], 10 days for the second study [January 5 to January 15, 2010] and 7 days for the third study [February 4 to February 11, 2011]). Eighteen, 28, and 18 pairs were chosen for the exposure period in the first, second, and third studies, respectively. Pairs were divided into their respective treatments (RW, 45 % PWE, single metal, or metal mixture [MM]) and statistical analyses using one-way analysis of variance (ANOVA) were performed on the pre-exposure egg production (total number of eggs, eggs/female/day, and breeding attempts) to verify that there were no significant differences between treatments (α = 0.05, n = 6 [first and third exposures] and n = 7 [second exposures]). Following this analysis, breeding pairs were randomly assigned to a stream within their treatment.
2.5 Exposure Period
FHMs were exposed to their assigned treatment for 21 days for each study. Water exchanges were again set at 1 turnover/day and temperature was maintained at 25 °C (±2 °C). Water samples from each treatment were taken on days 7, 14, and 21 in pre-labeled high density polyethylene sample bottles obtained from Testmark Laboratories in Sudbury, Ontario, Canada. Samples were taken directly from a single stream which was chosen randomly from each mesocosm treatment table, sealed in a ziplock bag, and sent for analysis in a cooler chilled with ice. Weekly water quality measurements were conducted by Testmark Laboratories, in accordance with the analytical methodology of the American Public Health Association (APHA) and US Environmental Protection Agency (EPA). These water samples were analyzed for total metals using inductively coupled plasma-mass spectrometry (ICP-MS) with digestion (concentrations of 41 elements were measured), Ca2+ and Mg2+ concentrations using ICP-MS without acidification [and converted to total water hardness expressed as the equivalent of calcium carbonate (CaCO3)], anions by ion chromatography, and dissolved organic carbon (DOC) and total organic carbon (TOC) with a Dohrman TOC Analyzer. TOC and DOC samples were analyzed using the APHA 5310 standard method. DOC samples were run through a 0.45-μm nylon filter. Daily water quality measurements were performed at the University of Saskatchewan. These measurements included temperature, dissolved oxygen (DO), and conductivity (YSI meter; Yellow Springs Instruments, Yellow Springs, OH, USA), ammonia (Rolf C. Hagen, Edmonton, AB, Canada), pH (Oakton pHTestr 3; Oakton Instruments, Vernon, IL, USA), and alkalinity (LaMotte Company, Chestertown, MD, USA).
FHM egg production, egg size, and larval deformities, as well as C. dilutus emergence were monitored daily throughout the exposure period. Egg production and fertilization success were determined in the same manner as the pre-exposure period. Egg sizes were determined by randomly selecting 10 eggs per brood and analyzing them with Image Pro Plus 6.1 (Media Cybernetics Inc., Maryland, USA). After photographing the eggs, they were placed into egg cups, returned to the respective treatment, and aerated. Eggs were monitored in the egg cups until all were hatched or killed by fungal infection. Infected eggs were counted and removed daily. After complete hatching, larvae were moved into petri dishes and photographed again with the microscope. Larval deformities were determined by analyzing photographs for edema or spinal deformities.
At the end of the exposure period, FHM were anesthetized with methane tricainesulfonate (MS-222), assessed for secondary sex characteristics, total body weight, total length, and dissected to obtain livers, gills, gonads, and the carcass. Weights of these individual tissues were also obtained. Densities of C. dilutus were measured on day 21 by sampling three 9-cm2 cores per stream. Biofilm and C. dilutus were collected from each stream and these, along with the female fish tissue samples, were frozen in a cooler of dry ice and sent to Testmark Laboratories for metal analysis. Only female tissues were analyzed for metal concentrations in the present study because past studies have shown that resident female fish exposed to MMEs in the Junction Creek watershed accumulated greater concentrations of metals relative to the male fish (Weber et al. 2008). We were unable to collect sufficient quantities of biofilm for metal analysis in the final study due to low biofilm production on the streams. Metal analysis in the fish tissues and C. dilutus were done by ICPMS with Microwave Digestion. Testmark Laboratories used method blanks, positive controls, blank spikes and laboratory duplicates with water samples, as well as method blanks and DOLT-3 dogfish (Squalus acanthias) liver reference materials (obtained from the National Research Council of Canada) for the biological samples as their methods of quality control. Percentage recovery for selected elements (Cu, Ni, Se) was within the range of 96.9–113.0 % in study 1, 93.5–122.6 % in study 2, and 89.7–111.0 % in study 3. Percent recovery rates were similar to previously reported values for these types of tissues (Rozon-Ramilo et al. 2011a, b).
2.6 Metal Speciation
Speciation modeling was performed using Visual MINTEQ, version 3.0 (KTH, Department of Land and Water Resources Engineering, Stockholm, Sweden). To consider metal complexation with organic matter, we assumed that 40 % of active DOC was fulvic acid and 60 % was humic acid. Model input data were taken directly from the mean weekly water chemistry analyses and therefore included all elements, metals, and ions that were determined to be present in the various treatments.
2.7 Exposure Analysis
Data were analyzed and graphed using PASW Statistics 18.0.0 (SPSS, Chicago, IL) and Sigmaplot® Version 11 (San Jose, CA, USA). Water chemistry and metal burdens in C. dilutus and fish tissues were analyzed using one-way ANOVAs. Cumulative eggs/female and cumulative total spawning events were analyzed by Kolmogorov–Smirnov tests, while mean adult survival, condition factor, relative egg size, liver somatic index (LSI [%]), gonadosomatic index (GSI [%]), mean total deformities (%), mean fertilization success (%), and mean C. dilutus densities were also analyzed using one-way ANOVAs. The Shapiro–Wilk test was used to test parametric assumptions for normality and Levine’s test was used to test for homogeneity of variance prior to the one-way ANOVAs. Data that failed these assumptions were either transformed (arcsin (%) or log10) or analyzed using the non-parametric Kruskal–Wallis test. If a significant difference was detected by one-way ANOVA (p ≤ 0.05), Tukey’s HSD post-hoc test was then used to determine if differences were present between the treatment and the references, and/or among the treatments.
3 Results
3.1 Water Chemistry and Metals
The water chemistry parameters and elevated metal concentrations for each of the three studies are presented in Tables 1 – 3. These tables include all water chemistry parameters that were measured during the studies; however, only metal concentrations that were statistically different between treatments are presented (with the exception of Cu in study 2). Overall, concentrations of over forty metals and elements were measured, including some of the common toxic metals such as Cd, Hg, Pb and Zn. However, these metals were either below detection limits or found not to be elevated in the 45 % PWE relative to RW in any of the studies (data not shown). Single metal, mixed metals, and 45 % PWE treatments were similar in water chemistry parameters in all three studies. Alkalinity, pH, conductivity, hardness, chloride, sulfate, and calcium levels were not significantly different between the single metal, mixed metals, or 45 % PWE treatments within each of the studies. Nitrate, magnesium, barium, boron, cobalt, lithium, and rubidium were typically elevated in the 45 % PWE relative to the other treatments within each study.
3.2 Waterborne Single Metal and Mixed Metal Treatments
In the first study, concentrations of total Cu were significantly increased in the Cu-only and 45 % PWE treatments relative to the RW and were not significantly different from each other (Table 1). In the second study, concentrations of total Ni and total Se were each significantly elevated in their respective single metal treatments and the 45 % PWE relative to the RW (Table 2). Concentrations of total Ni were not significantly different between the Ni-only treatment and the 45 % PWE treatment; however, concentrations of total Se were significantly different in the Se-only treatment compared to the 45 % PWE treatment. This was due to lower concentrations of Se in the 45 % PWE than what is typically observed. Concentrations of total Cu, Ni, and Se in the mixed metals study (study 3) were significantly different between the mixed metal and 45 % PWE treatments (Table 3) but were similar to levels from the first two studies.
3.3 Copper, Nickel, and Selenium Speciation in Exposure Water
Metal speciation modeling results suggest similar speciation patterns between 45 % PWE, Cu, Ni, Se, and the mixed metals treatments. The free ion Cu2+ was present at 5.44 % (study1), 1.00 % (study 2), and 2.24 % (study 3) in the 45 % PWE treatments. In the Cu treatment from study 1, Cu2+ was present at 0.92 %, while during the MM treatment it was present at 0.65 %. Ni2+ was present at 53.95 % (study1), 55.80 % (study 2), and 53.74 % (study 3) in the 45 % PWE treatments. In the Ni treatment from study 2, Ni2+ was present at 56.56 %, while during the MM treatment it was present at 51.74 %. Selenium was primarily in the selenate form, as found during the preliminary trials (unpublished data).
3.4 Metal Concentrations in Biofilm, C. dilutus, and Fish Tissues
3.4.1 Copper
Concentrations of Cu were significantly increased in the biofilm and C. dilutus tissues in each treatment with elevated waterborne Cu (Cu, 45 % PWE, and MM) relative to the RW treatments (Fig. 1a and b). In study 1, there was accumulation of Cu in the liver tissues of FHMs in both the Cu-only and 45 % PWE treatments. This pattern, however, was not observed in the mixed metal exposure. Small increases in concentrations of Cu were observed in the ovaries and gills of the MM treatment relative to the reference, but not in the gills of the 45 % PWE treatment relative to the MM treatment or the 45 % PWE treatment relative to the RW treatment in study 3.
3.4.2 Nickel
Concentrations of Ni were also significantly increased in the biofilm and C. dilutus of each treatment with elevated waterborne Ni (Ni, 45 % PWE, and MM) relative to the RW treatments (Fig. 2a and b). Bioaccumulation of Ni in FHM tissues was generally low. Small increases in concentrations of Ni were observed in the ovaries of 45 % PWE and Ni-only treatments relative to the Se treatment in study 2, and the ovaries of the MM treatment relative to RW and 45 % PWE treatments in study 3. There were also elevated concentrations of Ni in the liver during study 3 in the MM treatment relative to the RW and 45 % PWE treatments. Bioaccumulation of Ni was observed in the gills of the 45 % PWE and MM treatments relative to the RW treatment in study 3; however, these concentrations were lower than the concentrations measured in gill tissues in any of the treatments during study 2.
3.4.3 Selenium
Concentrations of Se were elevated in the biofilm of the Se-only treatment compared to all other treatments (Fig. 3a). Concentrations of Se were elevated in the C. dilutus of Se-only, 45 % PWE, and MM treatments relative to the reference treatments (Fig. 3a and b). The ovaries and livers tended to accumulate Se. Concentrations of Se were significantly elevated in the ovaries of 45 % PWE and the Se-only treatments in study 2 (although also significantly different from one another), and in the ovaries of 45 % PWE treatment in study 3. Concentrations of Se were also significantly elevated in the livers of 45 % PWE and Se-only treatments relative to the RW treatment in study 2, and in the livers of 45 % PWE from study 3. No bioaccumulation of Se was observed in the MM treatment FHM tissues in study 3.
3.5 Reproduction
3.5.1 Study 1 — Copper vs. PWE
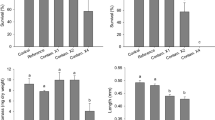
Although there were some differences in egg production among treatments, there were no statistically significant differences in cumulative mean egg production or cumulative spawning events between any of the treatments in study 1 (Fig. 4a and b).
3.5.2 Study 2 — Nickel or Selenium vs. PWE
In the second study, there was a statistically significant decrease in cumulative mean egg production in the 45 % PWE treatment relative to the RW, Ni-only, and Se-only treatments, small but non-significant change in the egg production of the Ni-only exposed FHMs relative to the RW FHMs, and no significant difference in the egg production of Se-only exposed FHMs relative to the RW FHMs (Fig. 5a). Cumulative total spawning events showed a similar trend to cumulative mean egg production. FHMs exposed to 45 % PWE showed a decrease in cumulative total spawning events relative to the RW, Ni-only, and Se-only FHMs (Fig. 5b). There were no significant differences between Ni-only and Se-only FHMs compared to the RW FHMs; however, there was a significant difference between both single metal treatment with Ni-only FHMs at a rate of nearly 50 % fewer spawning events than Se-only FHMs.
3.5.3 Study 3 — Copper, Nickel, and Selenium Mixture vs. PWE
There was a significant decrease in cumulative mean egg production for the 45 % PWE exposed FHMs in study 3 relative to the RW and MM FHMs; however, no change in the MM exposed FHMs relative to the RW FHMs (Fig. 6a). There was also a decrease in cumulative total spawning events in the 45 % PWE relative to the RW and MM FHMs (Fig. 6b). There was no significant decrease in spawning events between the RW and MM FHMs in this study.
3.6 Biological Endpoints
Mean adult survival (%) ranged from 71.5 % to 100 % per treatment and was not statistically different between any of the treatments within any of the three studies (Kruskal–Wallis, p = 0.783, p = 0.919, and p = 0.586). There were also no statistically significant differences in LSI, GSI, or condition factor (K) in males or females among treatments within any of the three studies (ANOVA; p > 0.05) (Online Resource 1, Online Resource 2). Rates of larval deformities ranged from 1.7 % to 27.8 % and primarily consisted of edema; however, no statistically significant differences in larval deformities among treatments were observed in any of the three studies (ANOVA; p > 0.05).
There was a statistically significant difference in the egg sizes of FHMs exposed to 45 % PWE in the first study relative to the RW eggs (−7.7 %) (Tukey’s HSD post hoc test, p = 0.027), with no significant difference in egg size between the 45 % PWE and eggs from the Cu-only treatment FHMs (Tukey’s HSD post hoc test, p = 0.173), nor the Cu-only FHMs relative to the RW eggs (Tukey’s HSD post hoc test, p = 0.536). There was a trend of smaller egg sizes in the 45 % PWE, Ni-only, and Se-only treatments, and the 45 % PWE and MM from studies 2 and 3; however, these were each not statistically significant (ANOVA, p = 0.432 and p = 0.079, respectively).
3.7 Densities of C. dilutus
There were no significant differences in densities of C. dilutus larvae among treatments at the end of the 21-day exposure period during the first study (ANOVA; p = 0.807) (Fig. 7). There was a trend of lower densities of C. dilutus larvae in the 45 % PWE treatment relative to the other treatments in the second study (0.5 larvae/cm2 fewer in 45 % PWE relative to the other treatments), however this difference was not statistically significant (p = 0.264). In study 3, the 45 % PWE treatment had significantly fewer C. dilutus larvae on day 21 than the RW treatment (Tukey’s HSD post hoc test, p = 0.003).
C. dilutus densities (no./cm2) measured on day 21 of the exposure period from each of the three studies. Treatments were reference water (RW), 45 % process water effluent (PWE), Cu, Ni, Se, or metal mixture (MM). Optimal densities are considered 3/cm2 (or 1 g/pair of FHM per day). The asterisk indicates a significant difference from RW (p = 0.004)
4 Discussion
4.1 Conditions of the Reference Water
It should be noted that Cu concentrations ranged from 7.03 to 11.20 μg/l in the RW used in the three present studies. Concentrations of Cu in water above 2–4 μg/l, depending on the water hardness, are considered elevated as per the Canadian Water Quality Guidelines for the Protection of Aquatic Life (CCME Canadian Council of Ministers of the Environment 2008). However, a mean Cu concentration of approximately 9.0 μg/l was recorded in the RW obtained from the Vermillion River during a field-based mesocosm study (Rozon-Ramilo et al. 2011a), which suggests that the Cu concentration in the RW used in present studies was similar to background Cu concentrations in the field and environmentally realistic. Concentrations of Cu in multi-trophic systems performed in the laboratory at the University of Saskatchewan have typically been in the range of 2.10 μg/l (Rozon-Ramilo 2011b) to 9.31 μg/l (Rickwood et al. 2006), as well. Furthermore, the free ion concentrations of Cu under the existing RW conditions is expected to be low (<0.01 %) as derived by geochemical speciation model Visual MINTEQ, version 3.0 (KTH, Department of Land and Water Resources Engineering, Stockholm, Sweden), suggesting Cu is not readily bioavailable in the water.
The present studies also had relatively high levels of DOC in the RW, which ranged from 4.0 to 7.2 mg/l. DOC concentrations in our studies were a result of the conditions of the multi-trophic mesocosms, which contained live C. dilutus larvae, dead C. dilutus adults, biofilm, and uneaten Tetramin™. It has previously been suggested that C. dilutus, biofilm, and Tetramin™ could be potential sources of organic matter and carbon (Rozon-Ramilo et al. 2011b), and we suspect this to be the case in the present studies as well. The average DOC concentration in lakes near Sudbury, ON has been reported to be approximately 3.7 (±3.7) mg/l (Valois et al. 2011), which is quite similar to the DOC concentrations of our laboratory RW.
4.2 Variability in Metal Concentrations and Water Chemistry
Overall, concentrations of Cu, Ni, and Se in our single metal, mixed metal and 45 % PWE treatments were variable within and among studies. Concentrations of Cu, Ni, and Se in the 45 % PWE ranged from 12.83 to 80.10, 77.33 to 120.20, and 4.17 to 7.27 μg/l, respectively. Typically, metal concentrations in PWE varies over time (see Dubé et al. 2006; Rickwood et al. 2006; Rozon-Ramilo et al. 2011a, b for values from 2001 to 2011). PWE composition from all metal mines depends on the types of ores being mined, rates of metal extraction, processing and water recycling, effluent treatment, as well as water quality (Clarke 1974). A temporary shutdown at the mining site in the present study during the Ni and Se single metal exposures resulted in unusually low concentrations of several metals in 45 % PWE, particularly Cu and Se. Other water chemistry and quality parameters showed marginal variation among 45 % PWE treatments from different studies but were not affected by the mining shutdown. This variability in metal concentrations and water chemistry could contribute to some differences in effects among treatments and is discussed below.
4.3 Reproductive Impacts
Previously, one of the most consistent changes observed in FHMs exposed to 45 % PWE has been a decrease in cumulative egg production and spawning events. As reported in Rozon-Ramilo (2011), four out of the five previous 45 % PWE exposure studies observed these types of decreases. Other MME studies have found increases in egg production from uranium mine effluent (Driessnack et al. 2011) and surface water effluent (Rozon-Ramilo et al. 2011a). Previous studies have also found decreases in egg sizes from 45 % PWE exposures (Rozon-Ramilo et al. 2011a, b). As a result, there is strong evidence that FHM egg production is a sensitive endpoint to certain MMEs, and these MMEs may have either a stimulatory or inhibitory effect.
Decreases in cumulative mean egg production were observed in FHMs exposed to 45 % PWE in two of our studies (80 % decrease in study 2 and 60 % decrease in study 3, relative to the respective RW exposures). FHMs exposed to single metal or mixed metal treatments did not suffer from any significant reproductive effects, except a moderate difference in egg production between the Ni and RW treatments in study 2 (35 % decrease in Ni treatment). However, cumulative mean egg production in the RW was also 2-fold higher than that observed in the other two studies (study 1 and study 3), and as a result, it is possible that the difference in egg production between Ni and RW in study 2 is an artifact of above normal RW breeding. In past studies, decreases in egg production and egg sizes resulting from exposure to Cu or Ni (Pickering 1974; Geckler et al. 1976; Horning and Neiheisel 1979), and reduced swelling in fish eggs from Cu exposure have been observed (Jezierska et al. 2009). Se has also contributed to increased larval deformities in fish (Lemly 1997; Muscatello et al. 2006; Driessnack et al. 2011). Overall, metal toxicity is highly related to water chemistry and bioaccumulation; therefore, toxic effects, such as changes to reproductive output, are likely to be very study-specific.
4.4 Waterborne Exposures and Water Chemistry
Hardness is likely an important factor in regulating metal bioavailability and toxicity in MMEs. Major hardness cations, calcium and magnesium ions compete with free metal ions in the water for binding to the uptake sites on the fish gill, and thereby reduce metal accumulation and toxicity (Pyle et al. 2002; Niyogi et al. 2008). The high hardness levels in our single metal, mixed metal, and 45 % PWE treatments suggest that bioavailability of metals that compete with calcium and magnesium (e.g., Cu2+, Ni2+) would be relatively low to C. dilutus or FHMs due to increased competition. Other water chemistry parameters which differed between treatments, such as pH, alkalinity, and DOC, are also known to affect metal bioavailability and toxicity by complexation of free metal ions, and thus could contribute to observed differences in reproductive effects in fish among treatments. However, our results suggest that the various water chemistry parameters were not major factors in contributing to differences in metal bioaccumulation and toxicity in FHM.
Increases in pH, alkalinity, and DOC are known to decrease the free ion concentrations and bioavailability of waterborne metals by complexation during acute exposures, which therefore reduce the toxicity of the metals (Campbell and Stokes 1985; Meador 1991; Allen and Hansen 1996; Pyle et al. 2002; Niyogi and Wood 2004). For example, metals may bind to DOC and become less bioavailable relative to the conditions of lower DOC, or metal complexes may dissociate under conditions of low alkalinity and pH and become available in their most bioavailable free ion form (e.g., Cu2+ and Ni2+). Free ion concentrations and bioavailability tend to be more closely linked with metal toxicity than total dissolved metal concentrations, particularly when considering waterborne exposures (Vijver et al. 2004). In 45 % PWE, pH has been observed to be as low as 6.69 ± 0.43 (Rozon-Ramilo et al. 2011a) and as high as 7.8 ± 0.1 (Hruska and Dubé 2005). This would suggest that MMEs under more acidic conditions are likely to result in increased toxicity. Interestingly, we did not observe a correlation between pH and reproductive toxicity. The 45 % PWE from our first study had a consistently lower pH than the 45 % PWE from either of our two other studies, but did not contribute to reduced egg production. It could also be expected that FHM egg production would be lower in 45 % PWE treatments with low DOC due to higher metal bioavailability. However, based on results from the present studies, it seems unlikely that there is any relationship between lower DOC and decreased egg production. For example, at DOC concentrations of approximately 5 mg/l in 45 % PWE, FHMs produced fewer eggs than the RW treatment in one exposure (study 3) yet produced a similar number of eggs relative to the RW treatment in another exposure (study 1).
There were some differences in pH, alkalinity, DOC, nitrates, and magnesium concentrations between the 45 % PWE, single metal, and mixed metal treatments in our studies. These factors can influence metal toxicity, primarily by influencing metal speciation. However, the metal speciation results suggest similar species of Cu, Ni, and Se among all treatments. Cu was generally not in the free ionic form (Cu2+). The concentration of Cu2+ in the exposure water was estimated to be ~5 % or less of total copper in each treatment. Selenium existed primarily as selenate (which has been confirmed in the 45 % PWE; unpublished data), which is much less toxic relative to the other common inorganic form of selenium, selenite (Brix et al. 2001). These findings also suggest that the dietary pathway was a more important uptake route for copper and selenium in fish relative to the gills.
The free Ni2+, conversely, was found to be the most common form of nickel in our studies (~50 %); therefore, its uptake through the gills may have been important. Toxicity in fish due to exposure to Ni is primarily observed through the gills and ultimately affects respiration. Typically, high concentrations of dissolved Ni are required in order to see acute effects. Bioaccumulation of Ni in the gills has been shown to cause damage to gills and cells involved in oxygen exchange at levels in acute tests ranging from 9.7 mg/l to 64 mg/l dissolved Ni (Nath and Kumar 1989; Pane et al. 2004a). These types of studies are generally done at high Ni concentrations, approximately 100-fold higher than levels found in the 45 % PWE or our metal treatments and are not environmentally realistic. Chronic Ni exposures at environmentally realistic levels of 243–394 μg/l over longer time frames (40–90 days) have also been shown to cause decreases in gas exchange and respiratory impacts in rainbow trout, although these effects were recorded at a much lower water hardness levels (~140 mg/l CaCO3) (Pane et al. 2004b) relative to that in PWE, nickel-only, or metal-mixture treatments in our studies.
4.5 Metal Bioaccumulation
Although free ion concentrations in the water may be an important source of toxicity, gill–metal binding and bioaccumulation are believed to better predict toxicity when compared to waterborne Cu2+ and Ni2+ concentrations, particularly in fish species such as rainbow trout (Oncorhynchus mykiss) and FHMs (Meyer et al. 1999; Pane et al. 2003, 2004a). Furthermore, tissue-specific metal accumulation provides a good indication of the routes of exposure and toxicity. Waterborne exposure is likely to lead to increased metal accumulation in the gills, whereas dietary uptake is likely to lead to increased metal accumulation mainly in the liver and insignificant or marginal increase in the gills.
Pane et al. (2004b) reported concentrations of approximately 3 μg/g in the gills of rainbow trout following 42 days of waterborne exposure to 384 μg/l at a moderate hardness (~140 mg/l CaCO3) in a study designed to examine the effects of Ni on gill functions. The levels of Ni bioaccumulation in the gills in our studies were low at approximately 1 μg/g suggesting that respiratory effects were probably not an issue. Pane et al. (2004b) also reported that Ni bioaccumulation occurred primarily in the gills, plasma and kidneys, with no increases in muscles or livers. Our results showed a similar trend, with little bioaccumulation of Ni occurring in the carcass or livers of FHM despite increases in the biofilm and C. dilutus, which suggests that dietary uptake of Ni was not significant. Therefore, our results indicate that it is unlikely that Ni bioaccumulation is contributing to toxic effects in fish observed in our studies.
Similarly to Ni, our studies frequently resulted in bioaccumulation of Cu, and Se in biofilm, and bioaccumulation of Cu in C. dilutus from single metal, mixed metals, and 45 % PWE exposures. These metals have also been found to increase consistently in the biofilm and C. dilutus of 45 % PWE treatments in past studies (Rickwood et al. 2006; Rozon-Ramilo et al. 2011a). Despite these consistent increases, Cu and Se did not necessarily result in trophic transfer into the fish tissues.
In fish, liver is the primary organ involved in bioaccumulation and homeostasis of Cu; therefore, we would expect any significant increases in Cu to occur in this type of tissue (Bury et al. 2003). However, Cu is a highly regulated essential metal and concentrations in fish have been suggested to vary between 7 and 50 μg/g dry weight (d.w.) in the liver under conditions that would not cause adverse effects to fish (or approximately 1.75–12.5 μg/g wet weight [w.w.], assuming water content in the liver of fish is approximately 0.75 g water/g wet liver) (Couture and Rajotte 2003). Concentrations of Cu in FHM livers from our studies did not exceed 20 μg/g w.w. and were similar among treatments. Concentrations of Cu of 20 μg/g w.w suggest that Cu could be reaching concentrations great enough to cause effects; however, our present studies did not find any significant reproductive or morphometric effects in FHMs during exposure to Cu. Other studies have reported greater concentrations of Cu in livers of yellow perch (Perca flavescens), ranging from ~27 to 62 μg/g w.w. (Brodeur et al. 1997; Sherwood et al. 2000), which suggests that the concentrations of Cu in the livers of FHMs is on the lower range of Cu contamination that could cause energetic effects or stress responses in fish.
Bioaccumulation of Se, like Cu, is known to occur in the livers of fish. To prevent reproductive impairment, 12 μg/g d.w. concentrations of Se have been suggested as a maximum threshold in liver tissues. The maximum concentration of Se bioaccumulation that was observed in our studies was 2.67 μg/g d.w. (again, assuming water content in the liver is approximately 75 %). Bioaccumulation of Se in the ovaries and eggs of fish is also quite common; however, to observe impacts, accumulation levels have been suggested to be at least 17 μg/g d.w. in cutthroat trout and greater than 20 μg/g d.w. in FHMs (Chapman et al. 2010). We did not measure Se concentrations in eggs; however, levels higher than 2.5 μg/g d.w. were not observed in FHM ovaries from any metal, mixed metal, or MME exposure in our study. As a result, it is not surprising that we did not observe increases in larval deformities in any of our treatments.
Perhaps the most significant finding in these studies is that Cu, Ni, and Se resulted in similar bioaccumulation in C. dilutus and FHM tissues when alone, in combination, or when in a more complex MME mixture. These bioaccumulation patterns occurred even with some variation in Cu, Ni, and Se concentrations and differences in water chemistry across treatments. To our knowledge, this is the only study to have examined and compared the bioaccumulation patterns in fish between single metals, metal mixtures, and a complex MME under simultaneous waterborne and dietary exposures. These types of exposures are extremely complex and predicting responses to multiple mixtures is difficult, although necessary for environmental monitoring and risk assessment (Norwood et al. 2003). The metals we examined (Cu, Ni, and Se) do not appear to influence the bioaccumulation of each other in more complex mixtures (either metal mixture or MME), particularly in high hardness water conditions. However, MMEs contain a variety of other metals which may still contribute to additive or more than additive effects on fish.
Other metals, such as Cd and Zn have been shown to contribute to reduction in FHM egg production when in combination with Cu in water at moderate hardness (~200 mg/l as CaCO3) (Eaton 1973). Effects on FHM eggs in soft waters for zinc alone have been reported at concentrations of approximately 145 μg/l in soft water (Benoit and Holcombe 1978). However, Cd and Zn were not elevated in 45 % PWE, and therefore were not examined in the present studies. In moderately hard water (~200 mg/l as CaCO3), with Zn concentrations of ~20 μg/l and Cd at <1 μg/l, Cu concentrations of approximately 34 μg/l and above have been shown to reduce FHM egg production (Mount 1968). These Cu, Zn, and Cd concentrations were nearly identical to the mixed metals component of the current study, further suggesting that hardness is a major factor responsible for reducing toxic responses in MMEs. Whether these effects occur due to other metals and bioaccumulation should be explored in future studies. Despite the similar patterns of Cu, Ni, and Se bioaccumulation, reproductive impacts were not comparable between metal treatments and the 45 % PWE.
4.6 Other Possible Causes of Effects
As we found no evidence indicating that Cu, Ni, or Se, either alone or in mixture, contributed to reproductive impairment in FHMs during exposure to 45 % PWE, it is possible that other metals or elements were responsible for effects. The concentration of B, Ba, Li, Rb, Ca, and Na tend to be greater in 45 % PWE than RW in the present studies, which was also observed in previous studies (Rickwood et al. 2006; Rozon-Ramilo et al. 2011a). However, B, Ba, Li, and Rb are generally not believed to cause toxicity at low concentrations. Ca and Na were also present almost at similar concentrations in all treatments, including the single metal and metal mixture exposures that did not cause reproductive effects in FHMs, and therefore likely were not responsible for negative effects in the 45 % PWE.
Interestingly, densities of C. dilutus were reduced by approximately 27 % (study 2) and 53 % (study 3) in 45 % PWE, relative to RW, in two of the three present studies. The reduction in FHM egg production also occurred simultaneously with the reduction in C. dilutus densities in those 45 % PWE exposures. Fish can be directly impacted through impaired physiology from the accumulation of toxic metals; however, they may also be affected indirectly through altered prey abundance or diversity — a consequence of metal contamination in the aquatic environments (Campbell et al., 2003; Rasmussen et al., 2008). Densities of C. dilutus have been reported to decrease during exposure to 45 % PWE in several previous studies (Hruska and Dubé 2004, 2005; Rozon-Ramilo et al. 2011a); therefore, the role in food quantity should be examined in future studies to evaluate its role in metal bioaccumulation and influencing FHM egg production.
5 Conclusion
Although we were not able to establish whether a specific metal is contributing to reproductive effects in FHMs, this research has reduced the likelihood that any of the three metals we examined plays a significant role. There is some evidence that suggests that, under certain circumstances, Cu, Ni, and Se can be responsible for reproductive effects in fish; however, under conditions similar to 45 % PWE, single and mixed metals exposures alone did not result in any significant reproductive changes. Still, the 45 % PWE caused decreased egg production in two of our three studies. In fact, concentrations of Cu and Se were low in the 45 % PWE during the mining shutdown in one of our studies; however, a decrease in FHM egg production was still observed. This provides further evidence that Cu and Se are not responsible for reproductive effects in 45 % PWE. Although water chemistry parameters and metal concentrations were marginally variable between some of the treatments, tissue bioaccumulation patterns were similar between our single metals, mixed metals, and 45 % PWE FHMs, and generally low overall. Thus, it appears that exposure and bioaccumulation of these metals probably do not play a significant role in inducing reproductive effects in fish. As water chemistry parameters are known to influence toxicity of metals, artificially manipulating these parameters would be beneficial in order to further examine the role of metal bioaccumulation and toxicity in MME effluents.
References
Allen, H. E., & Hansen, D. J. (1996). The importance of trace metal speciation to water quality criteria. Water Environmental Research, 68, 42–54. doi:10.2175/106143096X127307.
Ankley, G. T., Jensen, K. M., Kahl, M. D., Korte, J. J., & Makynen, E. A. (2001). Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas). Environmental Toxicology and Chemistry, 20, 1276–1290. doi:10.1002/etc.5620200616.
Benoit, D. A., & Holcombe, G. W. (1978). Toxic effects of zinc on fathead minnows Pimephales promelas in soft water. Journal of Fish Biology, 13, 701–708.
Bhavsar, S. P., Gandhi, N., Diamond, M. L., Lock, A. S., Spiers, G., & de la Torre, M. C. A. (2008). Effects of estimates from different geochemical models on metal fate predicted by coupled speciation-fate models. Environmental Toxicology and Chemistry, 27, 1020–1030. doi:10.1897/07-406.1.
Brix, K. V., Henderson, D. G., Adams, W. J., Reash, R. J., Carlton, R. G., & Mcintyre, D. O. (2001). Acute toxicity of sodium selenate to two daphnids and three amphipods. Environmental Toxicology, 16, 142–150. doi:10.1002/tox.1018.
Brodeur, J. C., Sherwood, G., Rasmussen, J. B., & Hontela, A. (1997). Impaired cortisol secretion in yellow perch (Perca flavescens) from lakes contaminated by heavy metals: in vivo and in vitro assessment. Canadian Journal of Fisheries and Aquatic Sciences, 54, 2752–2758.
Bury, N. R., Walker, P. A., & Glover, C. N. (2003). Nutritive metal uptake in teleost fish. Journal of Experimental Biology, 206, 11–23. doi:10.1242/jeb.00068.
Campbell, P. G. C., & Stokes, P. M. (1985). Acidification and toxicity of metals to aquatic biota. Canadian Journal of Fisheries and Aquatic Sciences, 42, 2034–2049. doi:10.1139/f85-251.
Campbell, P. G. C., Hontela, A., Rasmussen, J. B., Giguère, A., Gravel, A., Kraemer, L., et al. (2003). Differentiating between direct (physiological) and food-chain mediated (bioenergetic) effects on fish in metal-impacted lakes. Human and Ecological Risk Assessment, 9, 847–866. doi:10.1080/713610012.
CCME (Canadian Council of Ministers of the Environment). (2008). Canadian Water Quality Guidelines. http://www.ccme.ca/assets/pdf/cwqg_pn_1040.pdf. Accessed 3 December 2012.
Chapman, P. M., Adams, W. J., Brooks, M. L., Delos, C. G., Luoma, S.N., Maher, W. A., Ohlendorf, H. M., Presser, T. S., & Shaw, D.P. (2010). Ecological assessment of selenium in the aquatic environment. Pensacola, FL: Society of Environmental Toxicology and Chemistry (SETAC).
Clarke, R. M. (1974). The effects of effluents from metal mines on aquatic ecosystems in Canada (p. 488). Winnipeg: Environment Canada Fisheries and Marine Service Technical Report No.
Couture, P., & Rajotte, J. W. (2003). Morphometric and metabolic indicators of metal stress in wild yellow perch (Perca flavescens) from Sudbury, Ontario: a review. Journal of Environmental Monitoring, 5, 216–221. doi:10.1039/B210338A.
Driedger, K., Weber, L. P., Rickwood, C. J., Dubé, M. G., & Janz, D. M. (2010). Growth and energy storage in juvenile fathead minnows exposed to metal mine waste water in simulated winter and summer conditions. Ecotoxicology and Environmental Safety, 73, 727–734. doi:10.1016/j.ecoenv.2010.04.004.
Driessnack, M. K., Dubé, M. G., Rozon-Ramilo, L. D., Jones, P. D., Wiramanaden, C. I. E., & Pickering, I. J. (2011). The use of field-based mesocosm systems to assess the effects of uranium milling effluent on fathead minnow (Pimephales promelas) reproduction. Ecotoxicology, 20, 1–16. doi:10.1007/s10646-011-0666-5.
Dubé, M. G., MacLatchy, D. L., Hruska, K. A., & Glozier, N. E. (2006). Assessing the responses of creek chub (Semotilus atromaculatus) and pearl dace (Semotilus margarita) to metal mine effluents using in situ artificial streams in Sudbury, Ontario, Canada. Environmental Toxicology and Chemistry, 25, 18–28. doi:10.1897/04-116R.1.
Dubé, M. G., MacLatchy, D. L., Kieffer, J. D., Glozier, N. E., Culp, J. M., & Cash, K. J. (2005). Effects of metal mining effluent on Atlantic salmon (Salmo salar) and slimy sculpin (Cottus cognatus): using artificial streams to assess existing effects and predict future consequences. Science of the Total Environment, 343, 135–154. doi:10.1016/j.scitotenv.2004.09.037.
Eaton, J. G. (1973). Chronic toxicity of a copper, cadmium and zinc mixture to the fathead minnow (Pimephales promelas Rafinesque). Water Research, 7, 1723–1736.
Geckler, J. R., Horning, W. B., Neiheisel, T. M., Pickering, Q. H., Robinson, E. L., & Stephan, C. E. (1976). Validity of laboratory tests for predicting copper toxicity in streams. Duluth: Environmental Protection Agency.
Hewitt, L. M., Dubé, M. G., Culp, J. M., MacLatchy, D. L., & Munkittrick, K. R. (2003). A proposed framework for investigation of cause for environmental effects monitoring. Human and Ecological Risk Assessment, 9, 195–211. doi:10.1080/713609859.
Hewitt, L. M., Dubé, M. G., Ribey, S. C., Culp, J. M., Lowell, R., Hedley, K., et al. (2005). Investigation of cause in pulp and paper environmental effects monitoring. Water Quality Research Journal of Canada, 40, 261–274.
Holm, J., Palace, V., Siwik, P., Sterling, G., Evans, R., Baron, C., et al. (2005). Developmental effects of bioaccumulated selenium in eggs and larvae of two salmonid species. Environmental Toxicology and Chemistry, 24, 2373–2381. doi:10.1897/04-402R1.1.
Horning, W. B., & Neiheisel, T. W. (1979). Chronic effect of copper on the bluntnose minnow, Pimephales notatus (Rafinesque). Archives of Environmental Contamination and Toxicology, 8, 545–552. doi:10.1007/BF01055035.
Hruska, K. A., & Dubé, M. G. (2004). Using artificial streams to assess the effects of metal–mining effluent on the life cycle of the freshwater midge (Chironomus tentans) in situ. Environmental Toxicology and Chemistry, 23, 2709–2718. doi:10.1897/03-508.
Hruska, K. A., & Dubé, M. G. (2005). Comparison of a partial life–cycle bioassay in artificial streams to a standard beaker bioassay to assess effects of metal mine effluent on Chironomus tentans. Environmental Toxicology and Chemistry, 24, 2325–2335. doi:10.1897/04-154R.1.
Jezierska, B., Ługowska, K., & Witeska, M. (2009). The effects of heavy metals on embryonic development of fish (a review). Fish Physiology and Biochemistry, 35, 625–640. doi:10.1007/s10695-008-9284-4.
Kamunde, C., Grosell, M., Higgs, D., & Wood, C. M. (2002). Copper metabolism in actively growing rainbow trout (Oncorhynchus mykiss): interactions between dietary and waterborne copper uptake. Journal of Experimental Biology, 205, 279–290.
Lemly, A. D. (1997). A teratogenic deformity index for evaluating impacts of selenium on fish populations. Ecotoxicology and Environmental Safety, 37, 259–266. doi:10.1006/eesa.1997.1554.
Levesque, H. M., Dorval, J., Hontela, A., Van Der Kraak, G. J., & Campbell, P. G. C. (2003). Hormonal, morphological, and physiological responses of yellow perch (Perca flavescens) to chronic environmental metal exposures. Journal of Toxicology and Environmental Health. Part A, 66, 657–676. doi:10.1080/15287390309353773.
Meador, J. P. (1991). The interaction of pH, dissolved organic carbon, and total copper in the determination of ionic copper and toxicity. Aquatic Toxicology, 19, 13–31. doi:10.1016/0166-445X(91)90025-5.
Meyer, J. S., Bobbitt, J. P., Debrey, L. D., Boese, C. J., Bergman, H. L., Santore, R. C., et al. (1999). Binding of nickel and copper to fish gills predicts toxicity when water hardness varies, but free-ion activity does not. Environmental Science and Technology, 33, 913–916. doi:10.1021/es980715q.
Mount, D. I. (1968). Chronic toxicity of copper to fathead minnows (Pimephales promelas, rafinesque). Water Research, 2, 215–223.
Muscatello, J. R., Bennett, P. M., Himbeault, K. T., Andrew, M., & Janz, D. M. (2006). Larval deformities associated with selenium accumulation in northern pike (Esox lucius) exposed to metal mining effluent. Environmental Science and Technology, 40, 6506–6512. doi:10.1021/es060661h.
Nath, K., & Kumar, N. (1989). Nickel-induced histopathological alterations in the gill architecture of a tropical freshwater perch, Colisa fasciatus (Bloch & Schn.). Science of the Total Environment, 80, 293–296, doi: 10.1016/0048-9697(89)90083-1.
Niyogi, S., & Wood, C. M. (2004). Biotic ligand model, a flexible tool for developing site-specific water quality guidelines for metals. Environmental Science and Technology, 38, 6177–6192. doi:10.1021/es0496524.
Niyogi, S., Kent, R., & Wood, C. M. (2008). Effects of water chemistry variables on gill binding and acute toxicity of cadmium in rainbow trout (Oncorhynchus mykiss): a biotic ligand model (BLM) approach. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 148, 305–314. doi:10.1016/j.cbpc.2008.05.015.
Norwood, W., Borgmann, U., Dixon, D., & Wallace, A. (2003). Effects of metal mixtures on aquatic biota: A review of observations and methods. Human and Ecological Risk Assessment, 9, 795–811. doi:10.1080/713610010.
Pane, E. F., Haque, A., Goss, G. G., & Wood, C. M. (2004). The physiological consequences of exposure to chronic, sublethal waterborne nickel in rainbow trout (Oncorhynchus mykiss): exercise vs resting physiology. Journal of Experimental Biology, 207, 1249–1261. doi:10.1242/jeb.00871.
Pane, E. F., Haque, A., & Wood, C. M. (2004). Mechanistic analysis of acute, Ni-induced respiratory toxicity in the rainbow trout (Oncorhynchus mykiss): an exclusively branchial phenomenon. Aquatic Toxicology, 69, 11–24. doi:10.1016/j.aquatox.2004.04.009.
Pane, E. F., Richards, J. G., & Wood, C. M. (2003). Acute waterborne nickel toxicity in the rainbow trout (Oncorhynchus mykiss) occurs by a respiratory rather than ionoregulatory mechanism. Aquatic Toxicology, 63, 65–82. doi:10.1016/S0166-445X(02)00131-5.
Payne, J. F., French, B., Hamoutene, D., Yeats, P., Rahimtula, A., Scruton, D., et al. (2001). Are metal mining effluent regulations adequate: identification of a novel bleached fish syndrome in association with iron-ore mining effluents in Labrador, Newfoundland. Aquatic Toxicology, 52, 311–317. doi:10.1016/S0166-445X(00)00166-1.
Pickering, Q. H. (1974). Chronic toxicity of nickel to the fathead minnow. Journal of the Water Pollution Control Federation, 46, 760–765.
Pyle, G. G., Swanson, S. M., & Lehmkuhl, D. M. (2002). The influence of water hardness, pH, and suspended solids on nickel toxicity to larval fathead minnows (Pimephales promelas). Water, Air, and Soil Pollution, 133, 215–226. doi:10.1023/A:1012973728628.
Pyle, G. G., Rajotte, J. W., & Couture, P. (2005). Effects of industrial metals on wild fish populations along a contamination gradient. Ecotoxicology and Environmental Safety, 61, 287–312. doi:10.1016/j.ecoenv.2004.09.003.
Rasmussen, J., Gunn, J., Sherwood, G., Iles, A., Gagnon, A., Campbell, P., et al. (2008). Direct and indirect (foodweb mediated) effects of metal exposure on the growth of Yellow Perch (Perca flavescens): implications for ecological risk assessment. Human and Ecological Risk Assessment, 14, 317–350. doi:10.1080/10807030801935017.
Ribey, S. C., Munkittrick, K. R., McMaster, M. E., Courtenay, S. C., Langlois, C., Munger, S., et al. (2002). Development of a monitoring design for examining effects in wild fish associated with discharges from metal mines. Water Quality Research Journal of Canada, 37, 229–249.
Rickwood, C. J., Dubé, M. G., Weber, L. P., Driedger, K. L., & Janz, D. M. (2006). Assessing effects of metal mining effluent on fathead minnow (Pimephales promelas) reproduction in a trophic-transfer exposure system. Environmental Science and Technology, 40, 6489–6497. doi:10.1021/es060636b.
Rozon-Ramilo, L. D. (2011). Examination of the exposure pathways and effects of metal mining mixtures in fathead minnow (Pimephales promelas). MS thesis, Saskatoon: University of Saskatchewan.
Rozon-Ramilo, L. D., Dubé, M. G., Rickwood, C. J., & Niyogi, S. (2011). Examining the effects of metal mining mixtures on fathead minnow (Pimephales promelas) using field-based multi-trophic artificial streams. Ecotoxicology and Environmental Safety, 74, 1536–1547. doi:10.1016/j.ecoenv.2011.05.005.
Rozon-Ramilo, L. D., Dubé, M. G., Squires, A. J., & Niyogi, S. (2011). Examining waterborne and dietborne routes of exposure and their contribution to biological response patterns in fathead minnow (Pimephales promelas). Aquatic Toxicology, 105, 466–481. doi:10.1016/j.aquatox.2011.07.006.
Sherwood, G. D., Rasmussen, J. B., Rowan, D. J., Brodeur, J., & Hontela, A. (2000). Bioenergetic costs of heavy metal exposure in yellow perch (Perca flavescens): in situ estimates with a radiotracer (137Cs) technique. Canadian Journal of Fisheries and Aquatic Sciences, 57, 441–450.
Spencer, P., Bowman, M. F., & Dubé, M. G. (2008). A multitrophic approach to monitoring the effects of metal mining in otherwise pristine and ecologically sensitive rivers in Northern Canada. Integrated Environmental Assessment and Management, 4, 327–343. doi:10.1897/IEAM_2007-073.1.
Valois, A. E., Keller, W. B., & Ramcharan, C. W. (2011). Recovery in a multiple stressor environment: using the reference condition approach to examine zooplankton community change along opposing gradients. Journal of Plankton Research, 33, 1417–1429. doi:10.1093/plankt/fbr036.
Vijver, M. G., van Gestel, C. A. M., Lanno, R. P., van Straalen, N. M., & Peijnenburg, W. J. G. M. (2004). Internal metal sequestration and its ecotoxicological relevance: a review. Environmental Science and Technology, 38, 4705–4712. doi:10.1021/es040354g.
Weber, L. P., Dubé, M. G., Rickwood, C. J., Driedger, K., Portt, C., Brereton, C., et al. (2008). Effects of multiple effluents on resident fish from Junction Creek, Sudbury, Ontario. Ecotoxicology and Environmental Safety, 70, 433–445. doi:10.1016/j.ecoenv.2007.08.001.
Acknowledgments
We thank Allison Merla and Christine Brereton at Vale for their support on the three studies, Lisa Rozon-Ramilo, Michelle Heggstrom, Allison Squires, Melissa Driessnack, Sara Pryce, Ashley Mahaffey, and Robyn Pollock for their assistance and support in the laboratory, and Dirk Wallschlaeger at the University of Trent for performing selenium speciation analysis. Funding was provided by Vale, Natural Sciences and Engineering Research Council of Canada, and the University of Saskatchewan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ouellet, J.D., Dubé, M.G. & Niyogi, S. A Single Metal, Metal Mixture, and Whole-Effluent Approach to Investigate Causes of Metal Mine Effluent Effects on Fathead Minnows (Pimephales promelas). Water Air Soil Pollut 224, 1462 (2013). https://doi.org/10.1007/s11270-013-1462-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1462-z