Abstract
Despite numerous urban pollution sources, research on aquatic species bioaccumulation in the Han River is scarce. In this longitudinal study, we assessed baseline heavy metal (HM) concentrations in Cyprinidae, a major freshwater fish family in the Han River. Specifically, were evaluated copper (Cu), total mercury (THg), cadmium (Cd), lead (Pb), and chromium (Cr) levels in the muscle of common carp, crucian carp, and barbel steed. Common carp had the highest HM accumulation, with baseline concentrations of Cu, THg, Cd, Pb, and Cr at 0.877, 0.060, 0.003, 0.032, and 0.178 mg/kg, respectively. Larger fish exhibited greater bioaccumulation, with THg levels significantly correlated with fish length (correlation coefficients: 0.57 (p < 0.05)–0.74 (p < 0.001)). Notably, Cr accumulated more extensively in fish muscle than Pb, and the metal selectivity index (MSI) of THg in barbel steed was 2–3 times higher than in other fish species. The baseline concentrations determined in this study can serve as identifiers of the initial point of abnormal HM bioaccumulation in fish and provide foundational data for future long- or short-term fish monitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To prepare for The Minamata Convention on Mercury, a global treaty to protect the environment and human health from the harmful effects of mercury (Hg) that entered into force in 2017, the Ministry of Environment of South Korea conducted two surveys on Hg levels in the blood of adult humans in Korea from 2009 to 2014. The Hg level found in the survey conducted in 2009–2011 was 3.08 µg/L, while that in the survey conducted in 2012–2014 was 3.11 µg/L. The accompanying report stated that these values were 4–5 times higher than those recorded in the U.S.A. or Canada because the consumption of seafood in Korea was relatively high [29]. Hg from fish is extremely harmful because methylmercury (MeHg) causes neurotoxicity [26]. Furthermore, the interactions of HMs with microplastics, which are widely used and produced in modern society, lead to greater toxicity than the sum of the toxicities of individual HMs [4]. Furthermore, fish deaths attributed to HM accumulation account for over 13% of the fish deaths in Korea [3], indicating the potential occurrence of fish mortality in the downstream areas of the Han River as a result of exposure to various pollutants. The aforementioned facts underscore the necessity for substantial interest and research in the context of heavy metal bioaccumulation in fish in South Korea.
Accurate HM bioaccumulation assessment and monitoring are important to identify the starting points of various bioaccumulation events and establish reference or baseline concentrations for pollution cleanup [27, 41]. The repeated inspection and documentation of metal bioconcentrations in fish can provide a reliable reference for research and long-term monitoring. These efforts are meaningful because the results of such surveys can accurately evaluate the risks of HMs to humans and ecosystems [5, 11]. In this regard, Han et al. [12] highlighted that fish monitoring is a global research trend.
However, studies on freshwater fish in the Han River in South Korea are limited, and the available reports are outdated. Indeed, the most recent study on this subject was published before 2010 [14]. Moreover, most studies were not conducted on fish inhabiting the Han River and, thus, do not reflect up-to-date information on its bioindicators. In addition, previous research was limited to the simple examination of metal concentrations [19, 38]. More importantly, the lack of environmental guidelines and historical data on HM accumulation in fish renders administrative decisions on their bioaccumulation, pollution, and purification quite challenging.
Typically, two species of factors affect the bioaccumulation of HMs in fish: extrinsic factors, including bioavailability, water temperature, and water alkalinity; and intrinsic factors, including species, age, size, feeding pattern, and physiological status [6]. These variables must be controlled to obtain reliable baseline concentration. The current research involves a longitudinal study conducted over 10 years to obtain reliable results without the effects of influencing factors such as seasons, habitats, and fish species [5, 11]. We collected 180 samples periodically from 2011 to 2020 because a large sample size is desirable to improve the reliability of the findings [25]. We investigated the influence of fish size on the concentration of heavy metals and evaluated bioaccumulation, using the Metal Selectivity Index (MSI) and Metal Pollution Index (MPI). MPI values were utilized to describe the degree of metal contamination within the muscle tissues, and MSI values were employed to determine which specific metals exhibited a higher affinity [7, 17, 35]. Furthermore, we assessed whether differences in bioaccumulation would occur depending on the habitat. This study is the first to present the baseline concentrations of HMs in fish in the Han River. The baseline concentrations determined in this research can help administratively identify specific points of bioaccumulation that differ from typical bioaccumulation patterns. Additionally, these baseline concentrations will serve as foundational data for long- and short-term fish monitoring.
Materials and methods
Sampling and sampling sites
The Han River is a large-scale river that crosses Seoul. The capital city of South Korea, Seoul, is inhabited by approximately 9.8 million people, which constitutes around 20% of the total population of South Korea [36, 40]. The annual average temperature of Seoul is 12.8 °C, with the coldest month being January at − 1.9 °C, and the hottest month being August with a temperature of 26.1 °C [21]. The construction of Seoul’s water supply and sewage system, which includes water purification plants and sewage treatment plants, as well as the expansion of transmission and drainage pipes, was completed in 2010. With the aid of this system, the city’s population is served with water from the Han River; however, sewage is also discharged back into this river [36].
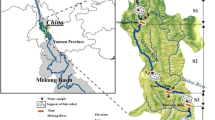
Cyprinidae is a major fish family in the Han River that includes common carp, crucian carp, and barbel steed. The Seoul Metropolitan Government and Seoul Metropolitan Government Research Institute of Public Health and Environment have monitored HM levels in this river for several years. The monitoring data from 2011 to 2021 was utilized as the total dataset for this study (Table 1). The data (n = 180) collected from 2011 to 2020 were used to determine HM baseline concentrations in common carp, crucian carp, and barbel steed inhabiting the Han River. We measured the weight, length, and thickness of each fish for 3 years (2019–2021) to estimate the impact of fish size on HM bioaccumulation in Cyprinidae living in the Han River. All fish were captured using triangular nets at three points (S1–S3) in March and September each year. To determine whether the habitat affects the accumulation of HMs, we collected 40 water samples from five points (W1–W5) in the upstream and downstream areas of the Han River from June to August 2021. Between sampling points S1 and S2, a submerged weir is installed, creating a structure that hinders the movement of fish. Furthermore, the transition from S1 to S3 indicates downstream flow. At points beyond the underwater barrier, the areas were designated as water source protection zones, making it temporally challenging to access for water sampling. Therefore, sampling was conducted downstream. The locations are indicated in Fig. 1.
Heavy metal analysis
We analyzed lead (Pb), cadmium (Cd), and total Hg (THg), which, according to the MFDS, should be restricted in fish. We also investigated copper (Cu), and chromium (Cr), which are essential nutrients. The HMs in both fish muscle and water media were analyzed. The Cyprinidae caught in the Han River were transported to the laboratory in a frozen state for HM analysis. The fish were thawed at room temperature and washed clean with distilled water (PURELAB Pima/Ultra RESERVOIR, ELGA, Germany). Fish muscle tissues were collected using stainless steel knives, taking care not to include bones, scales, blood, etc. The bones present between the muscles were carefully removed one by one using forceps. Prior to their use in the next dissection, the chopping boards and knives were washed. The tissues were homogenized using a stainless-steel mixer. Approximately 1 g of each tissue was digested with 4 mL of 63% nitric acid (Wako, Japan) in a microwave for Cu, Pb, Cr, and Cd analyses. The transparent residue was diluted to 50 mL with distilled water. The four HMs in the diluted solutions were determined using inductively coupled plasma-atomic emission spectrometry (ICP-OES 5110, Agilent, USA + Ciros CCD, Spectro, USA) and inductively coupled plasma-mass spectrometry (ICP-MS 7900, Agilent). THg analysis was conducted using 0.01 g of the homogenized tissue without further treatment via thermal decomposition and amalgamation/atomic absorption spectrophotometry (HydraII, Teledyne, USA). The fish were analyzed according to the methods of the Ministry of Food and Drug Safety (MFDS) in Korea [28]. To analyze HMs in water, we collected samples from the Han River using polyethylene bottles. The samples were transported to the laboratory in an ice-filled cooler, after which 45 mL of each sample was taken and mixed with 5 mL of nitric acid in a disposable polypropylene Falcon tube. Cu, Pb, Cr, and Cd in the diluted solutions were determined using ICP-MS (7900, Agilent). For the validation of heavy metal analysis, a certified standard solution (Cat. No. IS-11651-R2-1, Accustandard, USA) and certified reference material (ERM-CE278k, European Reference Material) were utilized. The water was analyzed according to the Water Quality Pollution Treatment Test Standards in Korea (National Institute of Environment Research [31]).
Statistical analysis
The data were statistically analyzed using R software (version 4.2 for Windows). Shapiro–Wilk tests were used to evaluate the data distribution, and the Kruskal–Wallis rank-sum test was used to determine significant differences in HM concentration according to (1) fish species and (2) habitat. Spearman’s rank correlation was also used to verify the correlation between fish size and HM level.
Baseline concentration
The baseline concentration is defined as the 10-year average concentration of HMs in the muscle of Cyprinidae inhabiting the Han River with a unit of milligrams per kilogram (mg/kg) of a sample (wet weight).
Bioaccumulation indices
MSIs were used to assess HM levels in the muscle of different fish species. This index reflects the relative sensitivity and capacity of fish tissues for accumulating specific HMs [30, 35] and is calculated as follows:
The total metal accumulation in the three species of fish was evaluated using the MPI, which is calculated as follows [44]:
where CCu, CTHg, Ccd, CPb, and CCr denote the concentrations (mg/kg) of the corresponding HMs in the fish muscle.
Results
Table 2 presents the concentrations of HMs in the muscle of freshwater fish recorded in earlier reports and the present study. Figure 2 illustrates the distribution of HMs in the different species of fish considered and at the sampling sites. Among the HMs investigated, Cu showed the highest accumulation in common carp, barbel steed, and crucian carp (0.877, 0.747, and 0.730 mg/kg, respectively). The HM levels decreased in the order of Cu > Cr > THg > Pb > Cd in common carp and crucian carp and Cu > THg > Cr > Pb > Cd in barbel steed. The THg concentrations were 0.060 and 0.040 mg/kg in common carp and crucian carp, respectively, and 0.141 mg/kg in barbel steed. Analysis of the HM distribution according to fish species and sampling site using the Shapiro–Wilk test indicated that the HM concentration did not have a normal distribution (p < 0.05). The Kruskal–Wallis rank-sum test confirmed that only the average THg level significantly differed according to the fish species (p < 0.05).
The concentrations of Cu, Cd, Pb, and Cr in the surface water of the Han River are illustrated in Fig. 3. The THg concentration was below the detection limit (0.0001 mg/L). The Pb (Fig. 3c) and Cr (Fig. 3d) levels in the surface water were 243 and 258 µg/L, respectively. The MSI of Cr in the fish was 6–10 times greater than that of Pb (Fig. 4), and the MSI of THg was 2–3 times higher in barbel steed than in the two other species. As indicated in Table 3, the highest MPI was 0.062 for common carp; the MPIs for crucian carp and barbel steed were 0.052 and 0.051, respectively. To investigate the variations in MPI based on fish size, we used data collected from 2019 to 2021; the results are shown in Table 4. The fish were classified into two groups based on the median length (32 cm), and the MPIs for large and small fish were 0.050 and 0.042, respectively. Especially in the case of Common carp, it was notable that there was a significant difference in MPI between size categories, with differences exceeding twofold.
Next, we assessed the extent of HM bioaccumulation in the fish according to their size and habitat. The ranges of correlation coefficients between THg level and fish length and THg level and fish weight were 0.57–0.74 and 0.6–0.69, respectively (Table 5). For crucian carp, the correlation coefficients between Cu level and fish length and Cu level and fish weight were 0.53 (p < 0.05) and 0.62 (p < 0.01), respectively. Moreover, the correlation coefficient between Pb level and barbel steed weight was 0.47 (p < 0.005). The results of the Kruskal–Wallis rank-sum tests indicated the absence of significant differences in HM concentration among the habitats (p > 0.005). However, all HM concentrations in the surface water were higher in downstream samples (W5) than in upstream samples (W1) (Fig. 3).
Discussion
This study aimed to determine the baseline concentrations of HMs in Cyprinidae inhabiting the Han River and interpret HM bioaccumulation from various aspects. To this end, a longitudinal study was conducted because such a study offers large amounts of data without the influences of fish species, habitat, and season.
Baseline concentration
In areas that have undergone industrialization and urbanization without specific contamination events, it is possible to estimate the anthropogenic background concentration of contaminant levels in the habitat of fish [27, 41]. We suggest that the mean HM concentrations presented in Table 2 be used as the baseline concentrations of these metals for Cyprinidae living in the Han River. Among the HMs investigated, Cu presented the highest concentration in the fish species considered in this study. This finding is consistent with an earlier study that indicated a pronounced tendency for nutritive elements, such as Cu, to accumulate in omnivorous species [15]. The average concentration of Cd was 0.001–0.003 mg/kg, which is similar to or lower than that obtained in the studies presented in Table 2 [13, 19, 22, 39]. The concentration of Pb was 0.013 mg/kg for common carp and 0.027 mg/kg for crucian carp; these values are 3–10 times lower than the corresponding values of 0.113 and 0.063 mg/kg, respectively, found by Kim and Han [19]. Therefore, we can conclude that the level of Pb accumulation in Cyprinidae inhabiting the Han River, except barbel steed, is lower than that in commercial fish. Table 2 indicates that the accumulation level of Cr was lower than that noted in other studies, except those conducted by Qiao-Qiao [33] and Rajeshkumar and Li [34].
MSI and MPI
Among the three fish species, common carp demonstrated the highest accumulation of the five HMs, with an MPI of 0.062. Further, a significant difference in THg levels was noted among the fish species (p < 0.005). The highest THg level, 0.141 mg/kg, was observed in barbel steed, and the THg MSI of this species was 2–3 times higher than that of the two other species (Fig. 4). In a study conducted by Kim and Han [19], THg levels were also found to be 2–3 times higher in barbel steed than in other species. Nair et al. [30] and Rather et al. [35] revealed that the MSI in target tissues differed significantly among fish species. A higher MSI reflects greater interactions and affinity between HMs and the target tissues. The THg MSI results revealed that Hg accumulation in the muscle differed according to fish species, moreover, barbel steed demonstrated greater sensitivity to this metal than the two other species.
Although the average concentrations of Pb and Cr in the water samples were found to be similar at 0.243 and 0.258 mg/L, respectively, we confirmed that the Cr MSI was 6–7 times greater than the Pb MSI. Because the MSI reflects the sensitivity of a species to an HM and their capability for selective accumulation, we can conclude that Cr was more selectively accumulated than Pb in Cyprinidae inhabiting the Han River.
THg
Hg originates from fossil fuel combustion or mines and can be released into the atmosphere in the form of inorganic mercury (Hg0). Hg can flow into water and combine with organic matter, thereby allowing it to convert into a toxic form of organic Hg called MeHg [26]. The MFDS in Korea allows a maximum level of 0.5 mg Hg/kg as MeHg, while the EU allows a maximum level of 1 mg Hg/kg as THg [16, 28]. Thus, the THg levels detected in this study were below the maximum allowable limit for human consumption. However, the THg concentration in barbel steed was higher than that reported in similar studies, as indicated in Table 2, except those conducted by Li et al. [23], Siraj et al. [39], and the U.S. EPA [43]. The drifting nature of barbel steed suggests that it is likely to be significantly influenced by the bottom sediment in the river. According to research conducted by the Seoul government, the average Hg concentration in sediments passing through the downtown area of Seoul is higher than that in the upstream region of the Han River or other domestic watersheds [37]. Based on these findings, we can infer that freshwater fish in the Han River are exposed to sources of Hg pollution.
Previous studies confirmed higher Hg accumulation in muscle tissue than in other tissues, and fish serve as both a major dietary source of proteins and a significant route of MeHg intake for humans [8, 10]. In particular, the lipophilic nature of MeHg can promote the bioaccumulation of the metal in top predators [2]. Thus, expanding research efforts to include not only omnivorous but also herbivorous and carnivorous fish is necessary owing to the risk of biomagnification and the possibility of accumulation.
Fish size
We examined the influence of fish size on HM bioaccumulation and summarized the results in Table 4. The correlation between fish size and HM concentration revealed that THg was significantly accumulated in all three fish species (Table 5). Pb and Cu were also positively correlated with fish size. Moreover, larger fish had higher MPIs than smaller fish. These findings, although limited to data between 2019 and 2021, suggest a contradiction compared with a previous study conducted by Rather et al. [35], which showed that older fish had higher concentrations of HMs than younger fish.
The concentrations of HMs in tissues depend on their absorption and elimination rates [15]. Younger fish, owing to their higher metabolic activities, tend to accumulate higher levels of HMs compared with older, larger fish [24]. Farkas et al. [8] found a negative correlation between the concentrations of Cu and Pb and fish size, indicating that smaller fish generally exhibited higher HM concentrations than larger fish. However, this finding does not apply universally. MeHg, which is lipid soluble, demonstrates higher accumulation in larger fish owing to bioconcentration [1, 42]. Yi and Zhang [45] observed a positive correlation between fish size and HM concentration in the majority of the fish they investigated. Jia et al. [15] also discovered a significant correlation between Pb concentration in muscles and fish size.
Muscles, being less metabolically active than other organs such as the liver, are expected to have a slower HM accumulation rate [1, 9]. However, once accumulated, HMs are not easily excreted because of the low metabolic activity of muscle tissue [18]. The results obtained in this study may be attributed to the continuous uptake of HMs from the surrounding water environment over time, leading to their accumulation in fish muscles.
The relationship between fish size and HM concentration varies depending on the study area, fish species, HM type, feeding habit, and target tissue. Therefore, future studies should consider fish size as an additional factor in calculating the baseline concentrations of HMs to obtain more representative data. This consideration will help obtain a better understanding of the comprehensive relationship between fish size and HM concentration. Overall, the results of this study suggest that the inclusion of fish size as an additional influencing factor will enhance the representativeness and comprehensiveness of the baseline HM concentrations in fish inhabiting the Han River.
Habitat
To assess the influence of habitat on HM accumulation, we examined HM concentrations in the surface water collected from five different sites (W1–W5) along the Han River. The HM concentrations obtained in downstream regions were 1.5–2 times higher than those obtained in upstream regions. However, the HM levels in the fish did not significantly differ among the different sites (p > 0.05). Thus, we can infer that habitat has little influence on HM accumulation in Cyprinidae in the Han River.
Conclusions
This paper reports the baseline concentrations of five HMs in Cyprinidae inhabiting the Han River using average levels observed over the past 10 years. The average concentrations of Cu, THg, Cd, Pb, and Cr in common carp were 0.877, 0.060, 0.003, 0.032, and 0.178 mg/kg, respectively. In crucian carp, the average concentrations of the above HMs were 0.730, 0.040, 0.003, 0.027, and 0.179 mg/kg, respectively. Finally, the average concentrations of Cu, THg, Cd, Pb, and Cr in barbel steed were 0.747, 0.141, 0.001, 0.023, and 0.136 mg/kg, respectively.
In terms of MSIs, Cu most selectively accumulated in the fish, with MSIs ranging from 71.2 to 74.7; by contrast, Cd, with MSIs ranging from 0.3 to 0.1, presented the lowest accumulation. Similar concentrations of Pb and Cr were found in the water samples; however, the Cr MSI of the fish was 6–8 times higher accumulation than their Pb MSI. THg was 2–3 times more concentrated in barbel steed than in the other fish species, and its concentration was higher than that reported in previous studies. The range of correlation coefficients between THg concentration and fish size was 0.57 (p < 0.05)–0.74 (p < 0.001). Such comprehensive assessments are crucial for obtaining accurate evaluations of HM levels in freshwater fish. The baseline concentrations determined in this study can serve as valuable indicators of the initial point of abnormal HM bioaccumulation in fish, providing foundational data for future long- or short-term fish-monitoring endeavors.
Availability of data and materials
All data generated or analyzed during this study are included in this publication.
References
Ali H, Khan E (2018) Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environ Chem Lett 16:903–917. https://doi.org/10.1007/s10311-018-0734-7
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J Chem 2019:6730305. https://doi.org/10.1155/2019/6730305
Byeon M, Kim S, Lee J, Kwon D, Jin Y, Lee Y, Hwang M, Lee J, Rhew D (2016) Study on the freshwater fish kill at municipal stream. Sci Res. https://doi.org/10.23000/TRKO201700008097
Cao Y, Zhao M, Ma X, Song Y, Zuo S, Li H, Deng W (2021) A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci Total Environ 788:147620. https://doi.org/10.1016/j.scitotenv.2021.147620
Caruana EJ, Roman M, Hernández-Sánchez J, Solli P (2015) Longitudinal studies J Thorac Dis 7:E537–E540. https://doi.org/10.3978/J.ISSN.2072-1439.2015.10.63
Dhanakumar S, Solaraj G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region. India Ecotoxicol Environ Saf 113:145–151. https://doi.org/10.1016/j.ecoenv.2014.11.032
Don Xavier ND, Nandan SB, Jayachandran PR, Neethu KV, Mohan D, Marigoudar SR (2021) Eliciting heavy metal contamination on selected native organisms from Cochin estuary using contemporary biomarker approach. J Earth Syst Sci. https://doi.org/10.1007/s12040-021-01676-1
Farkas, A., Salánki, J., Specziár, A., 2003. Age- and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. populating a low-contaminated site. Water Res. 37, 959–964. https://doi.org/10.1016/S0043-1354(02)00447-5.
Fernandes C, Fontainhas-Fernandes A, Peixoto F, Salgado MA (2007) Bioaccumulation of heavy metals in Liza saliens from the Esmoriz-Paramos coastal lagoon. Portugal Ecotoxicol Environ Saf 66:426–431. https://doi.org/10.1016/j.ecoenv.2006.02.007
Ferreira da Silva S, de Oliveira Lima M (2020) Mercury in fish marketed in the Amazon triple frontier and health risk assessment. Chemosphere 248:125989. https://doi.org/10.1016/J.CHEMOSPHERE.2020.125989
Göpferich S, Jakobsen AL, Mees IM, Eds. Behind the Mind: Methods, Models and Results in Translation Process Research. Samfundslitteratur, Denmark. 2009. pp. 11–12.
Han R, Zhou B, Huang Y, Lu X, Li S, Li N (2020) Bibliometric overview of research trends on heavy metal health risks and impacts in 1989–2018. J Clean Prod 276:123249. https://doi.org/10.1016/j.jclepro.2020.123249
Hong M, Yoon Y, Choi H, Yi H, Park S, Kim M, Jung K. Monitoring and risk assessment for heavy metals in freshwater fish (in Korean). 2015. https://opengov.seoul.go.kr/research/view/?nid=10098082 (accessed May 13, 2022).
Hong SU, Jong RH. Distribution of heavy metals in fresh-water fishes in Han River. Korean J. Limnol. 15, 7–14. 1982. https://kiss.kstudy.com/thesis/thesis-view.asp?key=94949 (accessed Nov. 5, 2022).
Jia Y, Wang L, Qu Z, Wang C, Yang Z (2017) Effects on heavy metal accumulation in freshwater fishes: Species, tissues, and sizes. Environ Sci Pollut Res 24:9379–9386. https://doi.org/10.1007/s11356-017-8606-4
Jinadasa BKKK, Jayasinghe GDTM, Pohl P, Fowler SW (2021) Mitigating the impact of mercury contaminants in fish and other seafood—A review. Mar Pollut Bull 171:112710. https://doi.org/10.1016/j.marpolbul.2021.112710
Ju YR, Chen CW, Chen CF, Chuang XY, Dong CD (2017) Assessment of heavy metals in aquaculture fishes collected from southwest coast of Taiwan and human consumption risk. Int Biodeterior Biodegrad 124:314–325. https://doi.org/10.1016/j.ibiod.2017.04.003
Kargin F, Cogun HY (1999) Metal interactions during accumulation and elimination of zinc and cadmium in tissues of the freshwater fish Tilapia nilotica. Bull Environ Contam Toxicol 63:511–519. https://doi.org/10.1007/s001289901010
Kim YC, Han SH (1999) A study on heavy metal content of the fresh water fish, and the shellfish in Korea. J Fd Hyg Safety 14:305–318
Kim H, Kim J, Kim S, Lee J, Jang Y, Lee M, Park J, Lee K (2007) Monitoring of Heavy Metals in Fishes in Korea - As, Cd, Cu, Pb, Mn, Zn, Total Hg
Korea Meteorological Administration, 2020. In South Korea, regional climate characteristics (in Korean). https://www.weather.go.kr/w/obs-climate/climate/statistics/regional-char.do (accessed Sep. 29, 2023)
Kwok CK, Liang Y, Wang H, Dong YH, Leung SY, Wong MH (2014) Bioaccumulation of heavy metals in fish and Ardeid at Pearl River Estuary. China Ecotoxicol Environ Saf 106:62–67. https://doi.org/10.1016/j.ecoenv.2014.04.016
Li P, Zhang J, Xie H, Liu C, Liang S, Ren Y, Wang W (2015) Heavy metal bioaccumulation and health hazard assessment for three fish species from Nansi Lake. China Bull Environ Contam Toxicol 94:431–436. https://doi.org/10.1007/S00128-015-1475-Y
Liu JL, Xu XR, Ding ZH, Peng JX, Jin MH, Wang YS, Hong YG, Yue WZ (2015) Heavy metals in wild marine fish from South China Sea: Levels, tissue- and species-specific accumulation and potential risk to humans. Ecotoxicology 24:1583–1592. https://doi.org/10.1007/s10646-015-1451-7
Liu X-B, Lin C, Wu Y-Y, Huang H-N, Zhu L-T, Jiang R, Huang Q (2022) Dataset-based assessment of heavy metal contamination in freshwater fishes and their health risks. Environ Sci Pollut Res 29:49985–49997. https://doi.org/10.1007/s11356-022-19427-0
Llull RM, Garí M, Canals M, Rey-Maquieira T, Grimalt JO (2017) Mercury concentrations in lean fish from the Western Mediterranean Sea: Dietary exposure and risk assessment in the population of the Balearic Islands. Environ Res 158:16–23. https://doi.org/10.1016/j.envres.2017.05.033
Mazzoni M, Buffo A, Cappelli F, Pascariello S, Polesello S, Valsecchi S, Volta P, Bettinetti R (2019) Perfluoroalkyl acids in fish of Italian deep lakes: Environmental and human risk assessment. Sci Total Environ 653:351–358. https://doi.org/10.1016/J.SCITOTENV.2018.10.274
Ministry of Food and Drug Safety, 2016. Food Code (No. 2021–54) (in Korean). https://www.mfds.go.kr/eng/brd/m_15/view.do?seq=72437&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (accessed Sept. 24, 2022).
Ministry of Environment, 2015. Mercury Management Comprehensive Measures (2016–2020) (in Korean). https://www.keco.or.kr/kr/business/research/communityid/203/view.do?idx=16577 (accessed May 23, 2022).
Nair M, Jayalakshmy KV, Balachandran KK, Joseph T (2006) Bioaccumulation of toxic metals by fish in a semi-enclosed tropical ecosystem. Environ Forensics 7:197–206. https://doi.org/10.1080/15275920600840438
National Institute of Environment Research, 2021. Water Quality of Pollution Treatment Standards (in Korean). https://me.go.kr/home/web/law/read.do?pagerOffset=0&maxPageItems=10&maxIndexPages=10&searchKey=lawTitle&searchValue=%EC%88%98%EC%A7%88%EC%98%A4%EC%97%BC&menuId=71&orgCd=&condition.typeCode=admrul&typeCode=admrul&lawSeq=1110 (accessed Apr. 10, 2023).
Pragnya M, Ajay B, Kumar SD, Byragi Reddy T (2021). Bioaccumulation of heavy metals in different trophic levels of aquatic ecosystems with fish as a bioindicator in Visakhapatnam, India. Mar. Pollut. Bull 165:112162. https://doi.org/10.1016/j.marpolbul.2021.112162
Qiao-Qiao C, Guang-Wei Z, Langdon A (2007) Bioaccumulation of heavy metals in fishes from Taihu Lake. China J Environ Sci 19:1500–1504. https://doi.org/10.1016/S1001-0742(07)60244-7
Rajeshkumar S, Li X (2018) Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake. China Toxicol Reports 5:288–295. https://doi.org/10.1016/j.toxrep.2018.01.007
Rather MY, Tilwani YM, Dey A (2019) Assessment of heavy metal contamination in two edible fish species Carassius carassius and Triplophysa kashmirensis of Dal Lake, Srinagar, Kashmir. India Environ Monit Assess 191:242. https://doi.org/10.1007/s10661-019-7382-7
Seoul Institute, 2016. 50 Years of Urban Spatial Policies of Seoul: Past and Present (in Korean). https://seoulsolution.kr/ko/seoul-history (accessed Jun. 5, 2022).
Seoul Metropolitan Government, 2019. Han River Water Source and Sediment Management Method Research Academic Service Report (in Korean). https://lib.seoul.go.kr/search/detail/CATLAZ000001296054?tr_code=m_lib (accessed Jun. 3, 2022).
Shin MJ, Park YM, Lee JE, Seo EW (2010) Heavy metal contents in tissues of fishes in Andong and Imha Reservoirs. J Life Sci 20:1378–1384. https://doi.org/10.5352/jls.2010.20.9.1378
Siraj M, Khisroon M, Khan A, Zaidi F, Ullah A, Rahman G (2018) Biomonitoring of tissue accumulation and genotoxic effect of heavy metals in Cyprinus carpio from River Kabul Khyber Pakhtunkhwa Pakistan. Bull Environ Contam Toxicol 100:344–349. https://doi.org/10.1007/s00128-017-2265-5
Song H, Shin WJ, Ryu JS, Shin HS, Chung H, Lee KS (2017) Anthropogenic rare earth elements and their spatial distributions in the Han River, South Korea. Chemosphere 172:155–165. https://doi.org/10.1016/j.chemosphere.2016.12.135
Southworth GR, Peterson MJ, Adams SM, Blaylock BG (1994) Estimation of appropriate background concentrations for assessing mercury contamination in fish. Bull Environ Contam Toxicol 53:211–218. https://doi.org/10.1007/BF00192035
Streit B (1998) Bioaccumulation of contaminants in fish, in: Braunbeck, T., Hinton, D.E., Streit, B. (Eds), Fish Ecotoxicology. Birkhäuser, Basel, pp. 353–387. https://doi.org/10.1007/978-3-0348-8853-0_12.
U.S. EPA (1999) The National Survey of Mercury Concentrations in Fish: Data Base Summary 1990–1995. https://www.epa.gov/sites/default/files/2018-11/documents/national-survey-mercury-fish-database-summary.pdf (accessed Nov. 5, 2022).
Usero J, González-Regalado E, Gracia I (1997) Trace metals in the bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic Coast of Southern Spain. Environ Int 23:291–298. https://doi.org/10.1016/S0160-4120(97)00030-5
Yi YJ, Zhang SH (2012) Heavy metal (Cd, Cr, Cu, Hg, Pb, Zn) concentrations in seven fish species in relation to fish size and location along the Yangtze River. Environ Sci Pollut Res 19:3989–3996. https://doi.org/10.1007/s11356-012-0840-1
Zhong W, Zhang Y, Wu Z, Yang R, Chen X, Yang J, Zhu L (2018) Health risk assessment of heavy metals in freshwater fish in the central and eastern North China. Ecotoxicol. Environ. Saf 157:343–349. https://doi.org/10.1016/j.ecoenv.2018.03.048
Zhou HY, Wong MH (2000) Mercury accumulation in freshwater fish with emphasis on the dietary influence. Water Res 34:4234–4242. https://doi.org/10.1016/S0043-1354(00)00176-7
Zupo V, Graber G, Kamel S, Plichta V, Granitzer S, Gundacker C, Wittmann KJ (2019) Mercury accumulation in freshwater and marine fish from the wild and from aquaculture ponds. Environ. Pollut 255:112975. https://doi.org/10.1016/j.envpol.2019.112975
Acknowledgements
Fish collection was completed under the guidance of the Seoul Metropolitan Hangang Project Headquarters. We thank everyone who contributed to this investigation over the last few years; in particular, we would like to thank Nak-Gyoung Choi, Soo-Seock Cho, and Jin-Hyo Lee. We would also like to thank Editage for providing support with their English editing and Dr. Bo-Ra Kim at the University of Seoul for their help in improving the language of this manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
H-RJ: Data curation, Investigation, Writing – original draft, Writing – review & editing, Investigation; J-SL: Investigation; I-SH: Writing – review & editing; M-jA: Writing – review & editing; Y-sC: Supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jung, HR., Lee, JS., Ahn, M. et al. Heavy metal baselines in cyprinidae of the Han River: a decade-long study on bioaccumulation trends and species-specific sensitivities. Environ Sci Eur 35, 110 (2023). https://doi.org/10.1186/s12302-023-00816-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-023-00816-2