Abstract
Soil is one of the compartments most affected by the accumulation of pollutants from anthropic sources. The present study allowed the identification of the sensitivity of the Allium cepa test system to evaluate solubilized soils from two points in the area contaminated by heavy metals, as well as a point of reference. They are all located in the municipality of Triunfo, state of Rio Grande do Sul, Brazil. The parameters used for evaluation were germination index, mitotic index, chromosomal aberrations (CA), and index of mutagenicity index (IMUT) presented by A. cepa. Significant responses of CA were observed in the two samples of contaminated soil, but IMUT was significant only for soil 3. The toxicity and cytotoxicity indexes did not show significant responses. The results indicate that the A. cepa plant test system was sensitive to investigate the genotoxicity of the soil samples and can be used as an alert in studies on soil contamination. It was partially concordant with the mutagenic responses already detected for the Salmonella/microsome assay in previous studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil is a highly dynamic ecological interface, which is in a constant exchange with the atmosphere, hydrosphere, and biota; its nature is complex and depends on its physical and chemical composition, geological aspects, and interactions with the biotic medium (White and Claxton 2004). Because of the wealth of relations that exist between the different compartments, soil is highly vulnerable to human activities, since it can concentrate and retain the contaminants received permanently (Silva-junior and Vargas 2008a, b, c).
The great number of agricultural and industrial practices has contributed to modifying all environmental compartments. Soil, often considered the deposit and sinkhole of wastes generated, has received tons of contaminants directly and indirectly (Monarca et al. 2002), altering its composition and becoming potentially toxic. Thus, because it inappropriately becomes a pollutant reservoir characterizing a strong potential for contamination, it may be one of the main sites of final fixation of heavy metals.
The metals found in the environment are from different natural and mainly industrial processes. The latter are different from toxic compounds in that they are difficult to degrade, so that they may accumulate in the environmental components where they manifest their toxicity (Baird 2002).
Most heavy metals are water soluble and can be easily absorbed by plants or by animal tissue. After being absorbed by the organism, they tend to combine with biomolecules, such as proteins and nucleic acids, impairing their functions (Yu 2001), and they may alter the genetic material of the organisms.
When the mutagenic compounds are distributed in the environmental compartments, they tend to accumulate in soil and then generate genotoxic effects on the exposed organisms. These DNA lesions can occur through the action of chemical agents of different classes, including some heavy metals.
Therefore, biological tests to evaluate toxicity, genotoxicity, and mutagenicity are essential for the investigation of organism reactions to complex mixtures of environmental contaminants (Vargas et al. 1995; Smaka-Kincl et al. 1996). Over the years, specific methodologies using different test organisms have been selected and applied to enable the detection and to evaluate impairment to the DNA molecule caused by exposure to toxic agents in samples from different environmental compartments (Vargas 2003, Vargas et al. 1998, 1993; Silva-junior and Vargas 2008a, b, c; Claxton et al. 2004) including soil (White and Claxton 2004; Pohren et al. 2012).
In this context, plant test systems are an appropriate alternative in which some plants that are sensitive to groups of pollutants have been widely used to monitor the presence of mutagenic substances (Chandra et al. 2005), and it is an adequate genetic model for environmental studies (Leme and Marin-Morales 2009). These bioassays, besides calling attention to the possible effects of chemicals, also aim at ensuring the quality of the ecosystem as a whole. Assays using plants are very important when evaluating soils, precisely because these organisms develop directly in the soil. Hence, plant test systems can be used to qualify the studies to assess soil quality and for investigations of contaminated soil sites, since an intensive follow-up is necessary in these studies.
Plant roots are used in biological tests, because they are exposed to chemical variations in water and soil (Fiskesjö 1988). These tests supply significant parameters to evaluate the toxicity of complex mixtures without prior knowledge of their chemical composition. They can be used as the first indicator of damage and environmental pollution of water, air, and soil, which are elements essential to life (Cabrera and Rodriguez 1999). Moreover, this can be considered a low-cost, simple, and easy-to-perform test (Grant 1994; Nielsen and Rank 1994).
The meristematic cells of plant roots are appropriate indicators to detect the clastogenic effects of environmental pollutants, especially to monitor water and soil contaminants (Leme and Marin-Morales 2008; Math et al. 1995). One of the plants that can be used as part of a battery of environmental monitoring tests is the Allium cepa species, considered an efficient organism to indicate environmental mutagens (Fiskejö 1985; Fernandes et al. 2007).
These are detected by several parameters that measure genotoxicity, including point mutations and chromosomal aberrations in plant cells (Grant 1994). In the analysis, mainly metaphase and anaphase are used. These are the stages during which the chromosomes are more visible and moving in migration. These characteristics are easier to observe in A. cepa (Leme and Marin-Morales 2009; Villela et al. 2003). Therefore, plant test systems such as A. cepa may be very useful in the initial stages and to monitor treatments in soil contamination studies.
This study aimed at investigating the sensitivity of the A. cepa test as an alert system to evaluate soils contaminated by heavy metals from the industrial process of treating woods with chromated copper arsenate as a wood preservative, associating these results with the responses obtained in the Salmonella/microsome assay.
2 Materials and Methods
2.1 Area of Study
The area studied lies in Rio Grande do Sul region, close to the confluence of the Taquari and Jacuí rivers, in the municipality of Triunfo, on the flood plain of the left bank of Taquari River. The site is characterized by soils consisting of gravel interspersed with sand, clayey sands, and clays, and soil contamination is specifically a result of the industrial activity of a wood preservatives company which operated there between 1960 and 2004 (FEPAM/CNPq and V. M. F. coord. 2010).
The chemicals used by the company to treat wood during that period were the following: a solution of pentachlorophenol in oil and/or creosote oil; the alternate use of creosote and chromated copper arsenate (CCA) hydrosalt, from 1982 onwards; and use of hydrosalt exclusively from 1998 onwards. This compound characterizes one of the main types of contamination in the area because of its heavy metals composition.
2.2 Sample Collection
The samples were collected in May 2007 at the contaminated sites, according to the recommendations of Health and Environmental Guidelines for Selected Timber Treatment Chemicals (1997). The samples were collected simply, at a depth of 0 to 20 cm, and the excess of plant residues was removed. The samples were collected using inox spatulas, dried at ambient temperature for 2 days, selected in a sieve (2 mm), and stored at 4 °C. Sampling was performed at the following points:
-
Point 1 (29°52′34.99″ S; 51°42′30.26″ W): Area of reference in the present study, located in the municipality of Triunfo (Triunfo reference), about 1.2 km from the contaminated sites;
-
Point 2 (29°52′16.18″ S; 51°43′2.66″ W): A site that was used to store treated wood, where the CCA hydrosalt residues ran directly onto the underlying soil;
-
Point 3 (29°52′17.13″ S; 51°43′8.8″ W): Site within the industrial area proper.
2.3 Preparation of the Solubilized Extract
The solute was prepared according to NBR 10006 (2004), adapted according to laboratory experience. Fifteen grams of the soil samples were weighed, and distilled water was added. These mixtures were placed in a bath with agitation at a low speed for 30 min at ambient temperature. After settling for 1 h, they were centrifuged for 30 min at 2,500 rpm, filtered in a membrane with 0.45 μm of porosity, and the filtrates were the solubilized extracts of the soil samples. These extracts were used for analysis of genotoxic activity.
2.4 Procedures of the Test Using A. cepa
The genotoxicity assays on meristematic cells of A. cepa were performed following Grant’s protocol (1982), with modifications according to previous work. Seeds of the A. cepa species (piriform baia variety marca “Top Seed”®, lot 18570C) were used as test organisms, 100 seeds being placed on each Petri plate lined with filter paper and germinated in the solubilized extracts obtained from the soils collected. The negative control (NC) was performed with distilled water, and the positive control (PC) 4 × 10−4 M of methyl methanesulfonate; CAS no. 66-27-3 obtained from Merck) because the latter is an agent with proven mutagenic action.
Once the A. cepa roots had growth to a length of approximately 2 cm, the germinated seeds were counted. They were fixed in Carnoy 3:1 v/v for at least 24 h, before the slides were made. The roots were hydrolyzed in HCl 1 N at 60 °C for 8 min and then transferred to a Schiff reactive for 1 h, in a dark place. The root tips were cut to extract their meristematic regions. The material was covered by a laminule, finalized with synthetic resin, for analysis in an optical microscope after at least 24 h with a × 40 magnification. Approximately 5,000 cells from each sample were observed, 500 per slide for a total of ten slides.
2.5 Analysis of Genotoxic and Mutagenic Activity
To evaluate the genotoxic potential, cell alterations were analyzed, such as the following: binucleated and/or multinucleated cells, c-metaphases, chromosomal losses, irregular anaphases (disorganized, multipolar anaphases with bridges), and cells with chromosomal adhesions. Cells with micronuclei and chromosomal breaks were analyzed for the mutagenic potential. Toxicity and cytotoxicity analyses were also performed.
The chromosomal aberrations (CA) and index of mutagenicity (IMUT) were calculated according to the ratio of number of damaged cells by the total number of cells analyzed. The toxicity of the samples was evaluated by macroscopic parameters of the roots and using the germination index (GI) calculated according to the relation between the number of seeds that germinated and the total number of seeds. The toxicity parameter adopted was 70 % in relation to the result obtained for the NC. The cytotoxic activity of the samples was evaluated from the mitotic index (MI), through the relation between the number of cells in cell division and the total number of cells analyzed. Statistical analysis was performed using the Kruskal–Wallis test followed by Tukey’s test which enabled comparing the treatment to the negative control and also the treatments to each other, with a level of significance of 0.05.
2.6 Quantification of Metals
The results of analyses of metals performed in leached extracts of these soil samples obtained by mechanical agitation with acid solution in the previous study performed by the work group were used (Meyer et al. 2013).
3 Results and Discussion
3.1 Analyses Using the A. cepa Test System
The study allowed the identification of the sensitivity of the A. cepa test as an alert system to evaluate soils contaminated by heavy metals at a site impacted by wood preservatives for the effects of genotoxicity and mutagenesis, indicating the importance of using plant test systems such as A. cepa in evaluations of contaminated soil areas.
The analysis consisted of the study of the abnormalities observed in the cell chromosomes at different phases of the cell cycle—interphase and mitotic division (Fig. 1). These damages (Fig. 2a–d) are characterized by the fact that they can still undergo cellular repair.
The mutagenic effect was evaluated by frequency analysis of cells carrying micronuclei and chromosomal breaks (Fig. 2e, f), and these alterations are characterized by not having cellular repair mechanisms.
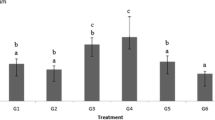
According to the results in Table 1, the samples of solubilized extracts of soils 1 (reference soil for the area of study), 2, and 3 did not show any toxic (GI) and cytotoxic (MI) values compared to those observed in NC (0.84).
The germination and mitotic indexes did not indicate the respective measurable toxic or cytotoxic effect of the test system in relation to the solubilized extracts being analyzed. Although, in this study, it did not respond significantly, some studies report that the mitotic index may reflect the quality of the compartment analyzed and was proved to be an important parameter to evaluate the effects of chemical agents on the cellular cycle (Philp 2001).
To evaluate the genotoxic potential, cell alterations that could still be repaired were analyzed, and for the mutagenic potential, the cells that carried micronuclei and chromosomal breaks (Fig. 2). These alterations could occur spontaneously or as a result of exposure to chemical or physical agents by means of different clastogenic mechanisms and actions involving aneugenic mechanisms.
The abnormalities found were multipolar anaphases, metaphases with adherence, binucleated cells, anaphases with bridges, chromosomal losses (genotoxic potential) besides chromosomal breaks, and cells with micronuclei (mutagenic potential). The data found (Table 1) revealed the absence of a significant response for chromosomal aberrations and low indexes of damage observed in soil 1, which is considered a point of reference for the area studied in this work. On the other hand, the indexes of alterations of the cellular cycle of samples of soils 2 and 3 presented statistically significant mean values indicating that existing pollutants in the area may be causing significant cell damage to the organisms exposed to soil. It should also be mentioned that the values of soils 2 and 3 were compared to those observed in the reference soil (soil 1). This analysis detected a significantly different result compared to soil 3, which suggests a higher chromosomal aberrations index in soil at point 3.
Chromosomal aberrations are cited as major consequences of the genotoxic actions of chemicals (Natarajan 2002), to which many organisms, including man, are exposed. They are characterized by alterations in the structure and chromosomal number, both by clastogenic or aneugenic effects. Clastogenic action is characterized by the induction of the chromosomal break during cell division, while aneugenic action consists of the inactivation of a cell structure, such as the mitotic fuse, leading to chromosomal losses (Fenech 2000).
The results of the mutagenicity index (Table 1) showed nonsignificant values compared to the negative control, corresponding to the absence of significant mutagenic potential for soil 1. It should be highlighted that soil 1 presented nonsignificant genotoxic responses in all parameters evaluated. These findings confirm the need to get to know the typical response of the environment without the presence of contaminants, to have a reference baseline that will allow the definition of significant values.
The responses for soil 2 did not show significant effects. On the other hand, soil 3 presented a statistically significant mutagenicity response. When these values were compared to soil sample 1, soil 3 again presented a significant mutagenicity result, and this is possibly related to the presence of pollutants causing mutagenicity in the test organism at the site sampled, which serves to indicate the existing environmental risks.
These results found for mutagenicity are considered based on the observation of the significant presence of chromosomal breaks and micronuclei in the cells analyzed. There is evidence that chromosomal breaks that occur in specific regions of oncogenes and/or suppressor genes of somatic cell tumors may be involved in cancer induction. There are also estimates of association between this damage and different types of problems that impair human health (Villela et al. 2003). On the other hand, micronuclei are masses with cytoplasmic chromatin which appear as small nuclei formed from chromosome fragments in the anaphase phase of cell division or due to loss of whole chromosomes; they are induced by agents that are able to break the DNA or interfere in fuse formation (Zagatto and Bertoletti 2008; Fenech et al. 1999). The micronuclei may appear spontaneously, but generally, they are induced by mutagenic agents, characterizing genetic damage.
3.2 Contribution of the Metals
The samples of solubilized soils tested contain a mixture of different classes of chemical compounds. However, this study prioritized a look at effects caused by heavy metals because of the contamination in the area investigated due to the use of the CCA hydrosalt characterizing this contaminant profile.
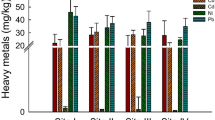
Aluminum, iron, and zinc were the metals identified in the three soils evaluated at higher concentrations in soil 1 (reference) compared to soils 2 and 3. They may be considered natural constituents of the area (Table 2). A high concentration of manganese was seen in soil 2 (11.320 mg/kg), showing a marked enrichment compared to soil 1 (0.150 mg/kg). Also, concentrations of copper were found only in soils 2 and 3, and specifically for soil 2, chromium and nicke.
There is a considerable variation in the concentration of some metals between the reference soil and the other soils collected. The main difference is the presence of the metal aluminum in soil 1 at higher concentrations than in soils at points 2 and 3, the same occurring for iron and zinc. This information suggests that these metals are found as constituents of the soil in the region and may already be naturally present at high concentrations. Although these metals are present in soil 1, the response of absence of mutagenic activity suggests that the chemical forms with which these metals are associated do not allow them to become available to the environment. Thus, it might be useful to investigate the influence of other properties inherent to the soil, since these characteristics may interfere in the possible interactions between the different soil components and may modify the genotoxicity and mutagenicity responses.
From the results of metals obtained for the reference soil, it can be inferred that the concentrations presented provide a baseline for investigations of the genotoxic potential, based on samples of soil contaminated with heavy metals in this area. Consideration of genotoxic activity is based only on the concentrations of metals above the values found for soil 1. Thus, metals Mn, Cr, Cu, and Ni may have influenced the genotoxic activity detected in soil 2; on the other hand, mutagenic activity in soil 3 may be associated with metals Mn and Cu (Table 2). Another significant difference is the concentration of manganese in soil 2, almost 100 times higher, which may be related to the genotoxic response found.
Some of the metals present in the area which are of greatest concern and an environmental risk because they are easy to solubilize and mobilize are, by order of priority, lead (Pb), chromium (Cr), copper (Cu), nickel (Ni), zinc (Zn), and aluminum (Al) (Volesky 2001). From the activity that used to take place in the area studied, it is known that one of the main contaminants is the product used as a wood preservative, formed by the association of several salts at proportions that vary according to the type of product. In this case, it is CCA (CrO3 · CuO · As2O5), a salt with characteristics known to be carcinogenic as recognized by the International Agency for Research on Cancer (IARC 2009).
Arsenate, the organic species present in CCA, is one of the forms most toxic to organisms (Jedynak et al. 2009). It has been a matter of concern, worldwide, due to its likely role in the development of cancer of the bladder, lung, skin, and prostate, among others, in human beings (Peralta-Videa et al. 2009). The possible mobilization of As in soil and subsequent leaching to ground or surface water should be considered. To evaluate the potential risks of mobilizing this metal in the environment, it is necessary to perform a detailed investigation to estimate the total concentration of As in the soil in the area, its chemical fractionation, and potential solubility (Bhattacharya et al. 2007) since its toxicity depends highly on the chemical form and species under which it is found in the environment. As(III) are more mobile than As(V), and the presence of iron and manganese oxide also has an influence. It can diminish the mobility and availability of As in soil, factors that may be related to the genotoxicity results found in soil 2, with high Mn content.
Arsenic metal was not detected in this study, but in an overall study of this field, the concentration of this element in its total form was defined by X-ray fluorescence. The values observed (FEPAM/CNPq and V. M. F. coord. 2010) were increased from soil 1 (11.1 mg/kg) compared to soils 2 (18.0 mg/kg) and 3 (60.0 mg/kg). Although these values do not correspond to those observed in the solubilized extract and only to the portion of stable metal in the soil, which is therefore not bioavailable, they present a major gradient. However, because of the composition of CCA hydrosalt, a considerable contribution of the main component of this mixture of salts can be recognized, arsenic pentoxide (CAS no. 66-1303-28-2), since the latter is considered carcinogenic. According to Jedynak et al. (2009), 6 % of the total arsenic found in the soil in his study is in the bioavailable form, indicating a considerable contribution of this metal in its potentially toxic form.
It is clearly documented that the genotoxic effects of heavy metals such as As, Cd, and Cr are related to inhibitory effects in the DNA repair enzymes (Majer 2002). Specifically, regarding exposure to As, there is a relationship between a variety of serious effects on health, such as cancer, neurological disorders, and reproductive problems (White and Claxton 2004). Chromosomal breaks have been observed experimentally based on the presence of tri- and pentavalent forms of arsenic compounds (Philp 2001).
According to IARC, copper is not carcinogenic (Peralta-Videa et al. 2009) but, at higher concentrations, may trigger toxic responses with potential bioaccumulation (Zagatto and Bertoletti 2008). Chromium(III) and Cr(VI) are the most stable states of chromium oxidation in the environment and, also biologically, the most important (Tagliari et al. 2004). Cr(VI) is the most toxic species, since it has a high oxidative potential, solubility, and mobility through the membranes of the living organisms. Many studies have shown that chromium(VI) compounds induce aberrations and mutations. The Cr(III) form is easily linked to the DNA molecules (Philp 2001). This reduction process is accompanied by the formation of oxygen radical species which are considered responsible for inducing DNA damage (Yu 2001).
The heavy metals (iron, nickel, and chromium) are among the contaminants commonly found in environmental mixtures. They act as genotoxic agents and may influence the mitotic index, induce chromosomal aberrations, and the formation of micronuclei in tests performed with A. cepa (Rank and Nielsen 1998; Chandra et al. 2005).
Polluting substances such as heavy metals can act on the organisms when they form oxygen free radicals, beginning degenerative processes and causing genotoxic effects. This test is especially useful to look at the damage induced by reactive oxygen species in chromosomes (Fatima and Ahmad 2006), since the A. cepa cells present an oxidase enzyme system that can metabolize some chemical compounds (Fiskejö 1985).
The mobility of heavy metals is highly influenced by the pH of the samples, distribution of particle size, carbon content present in the soil, and other physical and chemical variables (Majer 2002). Hence, it would also be important to consider these types of parameters in the studies performed, to allow a better correlation with the genotoxic potential of the samples studied (Watanabe and Hirayama 2001). It is important to seek an integrated interpretation of the data from the chemical analyses and results obtained for mutagenic responses to trace an effective profile of the genotoxicity of contaminant mixtures, compared to the probable and main groups of substances present in the area (Silva-Junior 2008). It should be taken into account that the samples investigated may also contain a mixture of nonmetallic substances.
3.3 Relationship Between A. cepa Test System and Salmonella/Microsome
Investigations performed in the area of study, using the Salmonella/microsome assay monitoring damage at the molecular level enabled associating the results of the A. cepa test with data previously obtained for the same soil samples (Vargas et al. 2012). These studies used strains that detect base pair substitution (TA100), frameshift (TA98 and TA97a), and sensitivity to the action of heavy metals (TA97a) in tests in the presence and absence of liver metabolization (S9mix). Cytotoxicity was observed in the Salmonella assay in the different samples analyzed in absence (soils 2 and 3) and presence of S9mix (soils 1, 2, and 3), while no responses to toxicity were observed in any of the samples in the A. cepa system.
As to the mutagenic response, the reference soil, with negative responses in A. cepa, presented mutagenicity induction in the strain sensitive to heavy metals in the presence of metabolization, although at levels considered low.
On the other hand, for the contaminated soils, there was greater sensitivity of the Salmonella/microsome assay in the mutagenic potential of soil 2, detecting major damage caused by different mechanisms (TA98 + S9, 401 rev/g of soil; TA100 − S9, 1,869 rev/g of soil). In this sample, the A. cepa system detected significant genotoxic responses compared to the negative control for different types of chromosomal aberrations. In soil 3, the highest mutagenic potencies were detected in the A. cepa test system, compared with the Salmonella/microsome test, with less potent responses only for strain TA97a + S9mix (168 rev/g of soil) (Vargas et al. 2012), indicating damages as a consequence of the genotoxic and mutagenic effect of contaminants in the contaminated soils.
The plant test system of A. cepa was sensitive to investigate the genotoxicity of the soil samples and presented partial concordance with another already widely used and validated mutagenic test, the Salmonella/microsome assay.
4 Conclusions
It should be underscored that the A. cepa test system was highly sensitive and capable of identifying genotoxicity in soil samples. This agrees with the review of studies performed by White and Claxton (2004) in which they report that it was surprising that this quick, simple test has not been more widely applied to studies on environmental contamination. The results indicated that the test system responds to contaminants existing in the area of study, suggesting that A. cepa may be used as a general indicator of exposure to complex mixtures and could be very useful as an alert for an initial screening in biomonitoring.
References
ABNT - Associação brasileira de normas técnicas. (2004). NBR 10006: Procedimento para obtenção de extrato solubilizado de resíduos sólidos. Rio de Janeiro/Brazil: ABNT.
Baird, C. (2002). Química Ambiental (2nd ed.). Porto Alegre: Bookman.
Bhattacharya, W. B. A. H., Stollenwerk, C. K. G., Mclaughlin, D. M. J., Bundschuh, E. J., & Panaullah, F. G. (2007). Arsenic in the environment: biology and chemistry. Science of the Total Environment, 379, 109–120.
Cabrera, G. L., & Rodriguez, D. M. G. (1999). Genotoxicity of soil from farmland irrigated with wastewater using three plant bioassays. Mutation Research, 426, 211–214.
Chandra, S., Chauhan, L. K. S., Murthy, R. C., Saxena, P. N., Pande, P. N., & Gupta, S. K. (2005). Comparative biomonitoring of leachates from hazardous solid waste of two industries using Allium test. Science of Total Environ, 346, 56–59.
Claxton, L. D., Matthews, P., & Warren, S. (2004). The genotoxicity of ambient outdoor air, a review: Salmonella mutagenicity. Mutation Research, 567, 347–399.
Fatima, R. A., & Ahmad, M. (2006). Genotoxicity of industrial wastewaters obtained from two different pollution sources in northern India: a comparison of three bioassays. Mutation Research, 609, 81–91.
Fenech, M. (2000). The in vitro micronucleus technique. Mutation Research, 455, 81–95.
Fenech, M., Crott, J., Turner, J., & Brown, S. (1999). Necrosis, apoptosis, cytostasis and DNA damage in human lymphocytes measured assay: description of the method and results for hydrogen peroxide. Mutagenesis, 14(6), 605–612.
FEPAM/CNPq, Vargas, V. M. F. (coord.). (2010). Estratégias ecotoxicológicas para caracterizar áreas contaminadas como medida de risco à saúde populacional. Porto Alegre: FEPAM. Relatório do Projeto FEPAM/CNPq 555187/2006-3.
Fernandes, T. C. C., Mazzeo, D. E. C., & Marin-Morales, M. A. (2007). Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pesticide Biochemistry and Physiology, 88, 252–259.
Fiskejö, G. (1985). The Allium test as a standard in environmental monitoring. Hereditas, 102, 99–112.
Fiskesjö, G. (1988). The Allium test—an alternative in environmental studies: the relative toxicity of metal ions. Mutation Research, 197, 243–260.
Grant, W. F. (1982). Chromosome aberration assays in Allium. A report of the US Environmental Agency Gene-Tox program. Mutation Research, 99, 273–291.
Grant, W. F. (1994). The present status of higher plant bioassays for detection of environmental mutagens. Mutation Research, 310, 175–185.
Health and environmental guidelines for selected timber treatment chemicals. (1997). Wellington. Available at: http://www.mfe.govt.nz/publications/hazardous.
International Agency for Research on Cancer/EPA’s Genetic Activity Profile Database - IARC (2009). Available at: http://monographs.iarc.fr.
Jedynak, L., Kowalska, J., Harasimowicz, J., & Golimowski, J. (2009). Speciation analysis of arsenic in terrestrial plants from arsenic contaminated area. Science of the Total Environment, 407, 945–952.
Leme, D. M., & Marin-Morales, M. A. (2008). Chromosome aberration and micronucleus frequencies in Allium cepa cells exposed to petroleum polluted water—a case study. Mutation Research, 650, 80–86.
Leme, D. M., & Marin-Morales, M. A. (2009). Allium cepa test in environmental monitoring: a review on its application. Mutation Research, 682, 71–81.
Majer, B. J. (2002). Effects of heavy metal contamination of soils on micronucleus induction in Tradescantia and on microbial enzyme activities: a comparative investigation. Mutation Research, 515, 111–124.
Math, Xu, Z., Xu, C., McConnell, H., Rabago, E. V., Arreola, G. A., et al. (1995). The improved Allium/Vicia root tip micronucleus assay for clastogenicity of environmental pollutants. Mutation Research, 334, 185–195.
Meyer, D. D., Silva, F. M. R. J., Souza, J. W. M., Pohren, R. S., Rocha, J. A. V. & Vargas, V. M. F (2013). Using the Salmonella/microsome assay to delineate the background of mutagenicity from inorganic compounds in soil samples. In press.
Monarca, S., Feretti, D., Zerbini, I., Alberti, A., Zani, C., Resola, S., et al. (2002). Soil contamination detected using bacterial and plant mutagenicity tests and chemical analyses. Environmental Research, 88, 64–69.
Natarajan, A. T. (2002). Chromosome aberration: past, present and future. Mutation Research, 504, 3–16.
Nielsen, M. H., & Rank, J. (1994). Screening of toxicity and genotoxicity in wastewater by the use of the Allium test. Hereditas, 121, 249–254.
Peralta-Videa, A. J. R., Lopez, A. M. L., Narayan, A. M., Saupe, A. G., & Gardea-Torresdey, J. (2009). The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. The International Journal of Biochemistry & Cell Biology, 41, 1665–1677.
Philp, R. B. (2001). Ecosystems and human health: Toxicology and environmental hazards. Boca Raton: CRC Lewis Publishers.
Pohren, R. S., Rocha, J. A. V., Leal, K. A., & Vargas, V. M. F. (2012). Soil mutagenicity as a strategy to evaluate environmental and health risks in a contaminated area. Environment International, 44, 40–52.
Rank, J., & Nielsen, M. H. (1998). Genotoxicity testing of wastewater sludge using the Allium cepa anaphase-telophase chromosome aberration assay. Mutation Research, 418, 113–119.
Silva-Junior F.M.R. (2008). Atividade mutagênica em solos sob influência de rejeitos de carvão. Dissertação de Mestrado em Ecologia. Universidade Federal do Rio Grande do Sul, Porto Alegre.
Silva-junior, F. M. R., & Vargas, V. M. F. (2008). Avaliação de áreas sob a influência de uma termelétrica a carvão através de ensaio de genotoxicidade. Journal of the Brazilian Society of Ecotoxicology, 2, 1–3.
Silva-Júnior, F. M. R., & Vargas, V. M. F. (2008). Using the Salmonella assay to delineate the dispersion routes of mutagenic compounds from coal wastes in contaminated soil. Mutation Research, Environmental Mutagenesis, 673, 116–123.
Smaka-Kincl, V., Stegnar, P., Lovka, M., & Toman, M. J. (1996). The evaluation of waste, surface and ground water quality using the Allium test procedure. Mutation Research, 368, 171–179.
Tagliari, K. C., Vargas, V. M. F., Zimiani, K. C., & Cecchini, R. C. (2004). Oxidative stress damage in the liver of fish and rats receiving an intraperitoneal injection of hexavalent chromium as evaluated by chemiluminescence. Environmental Toxicology and Pharmacology, 17, 149–150.
Vargas, V. M. F. (2003). Mutagenic activity as a parameter to assess ambient air quality for protection of the environmental and human health. Reviews in Mutation Research, 544(2,3), 313–319.
Vargas, V. M. F., Motta, V. E. P., & Henriques, J. A. P. (1993). Mutagenic activity detected by the Ames test in river water under the influence of petrochemical industries. Mutation Research, 319, 31–45.
Vargas, V. M. F., Guidobono, R. R., & Henriques, J. A. P. (1995). Use of two short term test to evaluate genotoxicity of river water treated with different concentration extraction procedure. Mutation Research, 343, 31–52.
Vargas, V. M. F., Horn, R. C., Guidobono, R. R., Mittelstaedt, A. B., & Azevedo, I. M. G. (1998). Mutagenic activity of airborne particulate matter from urban areas of Porto Alegre, Brazil. Genetics and Molecular Biology, 21, 1–7.
Vargas V. M. F., Pohren, R. S., Silva-Junior F. M. R, Moreira, J. W., Vaz, C. R., Rocha J. et al. (2012). Mutagenic potential and profile of PAHs in soils contaminated by wood preservatives: effects on the environment and on human health. In W. C. Flag & H. E. Meneses (Eds.), Polycyclic Aromatic Hydrocarbons: Chemistry, Occurrence and Health Issues. Nova Science Publishers: USA.
Villela, I. V., Lau, A., & Silveira, J. (2003). In A. Alcance (Ed.), Bioensaios para o Monitoramento de Genotoxicidade Ambiental. Porto Alegre: Genética Toxicológica.
Volesky, B. (2001). Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy, 59, 203–216.
Watanabe, T., & Hirayama, T. (2001). Genotoxicity of soil. Journal of Health Science, 47(5), 433–448.
White, P. A., & Claxton, L. D. (2004). Mutagens in contaminated soil: a review. Mutation Research, 567, 227–345.
Yu, M.-H. (2001). Environmental toxicology: Impacts of environmental toxicants on living systems. Boca Raton: CRC Lewis Publishers.
Zagatto, P. A, Bertoletti, E. (org.) (2008). Ecotoxicologia Aquática – princípios e aplicações. São Carlos, São Paulo, BR: RiMa Editora.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza Pohren, R., da Costa, T.C. & Vargas, V.M.F. Investigation of Sensitivity of the Allium cepa Test as an Alert System to Evaluate the Genotoxic Potential of Soil Contaminated by Heavy Metals. Water Air Soil Pollut 224, 1460 (2013). https://doi.org/10.1007/s11270-013-1460-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1460-1