Abstract
Background, aim, and scope
Water quality impairment by nutrient enrichment from agricultural activities has been a concern worldwide. Phytoremediation technology using aquatic plants in constructed wetlands and stormwater detention ponds is increasingly applied to remediate eutrophic waters. The objectives of this study were to evaluate the effectiveness and potential of water lettuce (Pistia stratiotes L.) in removing nutrients including nitrogen (N) and phosphorus (P) from stormwater in the constructed water detention systems before it is discharged into the St. Lucie Estuary, an important surface water system in Florida, using phytoremediation technologies.
Materials and methods
In this study, water lettuce (P. stratiotes) was planted in the treatment plots of two stormwater detention ponds (East and West Ponds) in 2005–2007 and water samples from both treatment and control plots were weekly collected and analyzed for water quality properties including pH, electrical conductivity, turbidity, suspended solids, and nutrients (N and P). Optimum plant density was maintained and plant samples were collected monthly and analyzed for nutrient contents.
Results
Water quality in both ponds was improved, as evidenced by decreases in water turbidity, suspended solids, and nutrient concentrations. Water turbidity was decreased by more than 60%. Inorganic N (NH4 + and NO3 −) concentrations in treatment plots were more than 50% lower than those in control plots (without plant). Reductions in both PO4 3− and total P were approximately 14–31%, as compared to the control plots. Water lettuce contained average N and P concentrations of 17 and 3.0 g kg−1, respectively, and removed 190–329 kg N ha−1 and 25–34 kg P ha−1 annually.
Discussion
Many aquatic plants have been used to remove nutrients from eutrophic waters but water lettuce proved superior to most other plants in nutrient removal efficiency, owing to its rapid growth and high biomass yield potential. However, the growth and nutrient removal potential are affected by many factors such as temperature, water salinity, and physiological limitations of the plant. Low temperature, high concentration of salts, and low concentration of nutrients may reduce the performance of this plant in removing nutrients.
Conclusions
The results from this study indicate that water lettuce has a great potential in removing N and P from eutrophic stormwaters and improving other water quality properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim, and scope
Chemical fertilizers have been playing a very important role in agricultural production in the modern society. Because of crops’ quick response to chemical fertilizers, to many farmers, fertilizer application seems to be the only guarantee of high crop yield. But the ever increasing use of fertilizer results in significant buildup of nutrients, such as N and P, in the soils (Smith et al. 2007). When the soils are saturated, these nutrients are subjected to losses by leaching and surface runoff. Water quality is impaired and water availability is reduced because of accelerated eutrophication (Carpenter et al. 1998).
Water quality throughout south Florida has been a major concern for many years. Nutrient enrichment has been considered to impact ecological functions of the Everglades National Park, Lake Okeechobee, and Indian River Lagoon (Capece et al. 2007; Ritter et al. 2007). Various water quality problems affect the Indian River Lagoon (IRL), most of which are associated with the development of an intricate network of the canals that drain the surrounding urban and agricultural lands. Canals C-23, C-24, and C-44 in the St. Lucie Basin, which are connected to the IRL, are estimated to collectively deliver at least 8.6 × l05 kg of N, 9.1 × 105 kg of P, and 3.6 × l08 kg of suspended solids (SS) to the estuary annually (Graves and Strom 1992). Overall IRL total N load is projected (year 2010) to increase by 32% (Woodward-Clyde Consultants 1994).
The St. Lucie Estuary is facing challenges of eutrophication due to increased inputs of nutrients, especially N and P from nonpoint sources. Results from a recent monitoring study by He et al. (2006) indicate that more than 50% of the surface runoff water samples contained a total N of 1 to 5 mg l−1 and total P above 1.0 mg l−1. Mean concentrations of total N and total P in the runoff were 4.1 and 1.6 mg l−1, respectively, which are much greater than the USEPA critical levels for surface waters (1.5 mg l−1 for total N and 0.1 mg l−1 for total P) (U.S. Environmental Protection Agency 1976).
Best management practices have been used to reduce N and P export from urban areas and agricultural fields, approximately 10–15% reduction may be realized based on our previous BMPs project (He et al. 2005). This reduction is still far below the goals (30–70% reduction in N and P) established in the Surface Water Improvement and Management Plan (SWIM plan) (SFWMD and SJRWMD 1994) for the St. Lucie Estuary watershed. The stormwater needs to be further cleaned before it is dischargeable to the St. Lucie Estuary.
Physical and chemical treatments to remediate eutrophication in waters are not cost effective, less flexible in terms of design modifications, and are targeted primarily to remove BOD and, to a lesser extent, to reduce N and P levels. Phytoremediation has been increasingly used to clean up contaminated soil and water systems because of its lower costs and fewer negative effects than physical or chemical engineering approaches (Gumbricht 1993; Kowalik et al. 1998; Mahujchariyawong and Ikeda 2001). The principles of phytoremediation systems for cleaning up stormwater include: (a) identification and implementation of efficient aquatic plant systems; (b) uptake of dissolved nutrients including N, P, and metals by the growing plant; and (c) harvest and beneficial use of the plant biomass produced from the remediation system.
Large constructed wetlands or stormwater treatment areas have been operating since the early 1990s to filter nutrients in eutrophic stormwater from Everglades Agricultural Area before they are drained into a water conservation area in the Everglades National Park. Similar wetland systems are also under construction in the Indian River area to reduce nutrients (N and P) before the stormwater from the agricultural areas is discharged into the IRL. Key to the performance of wetlands in reducing nutrient and metal loads is the establishment and sustainability of desired vegetation communities.
In many cases, especially in tropical or subtropical areas, invasive plants such as the water hyacinth (Eichhornia crassipes) and water lettuce (P. stratiotes L.) are used in these phytoremediation water systems (Karpiscak et al. 1994; El-Gendy et al. 2005). This is because, compared to native plants, these invasive plants show a much higher nutrient removal efficiency with their high nutrient uptake capacity, fast growth rate, and big biomass production (Reddy and Sutton 1984). In the active growth season, for instance, water hyacinth plants can double in number and biomass in 6 to 15 days (Lindsey and Hirt 1999). Thus, one of the large-leaved floating invasive plants, water lettuce was chosen in this study. And the primary objectives of this study were to evaluate the effectiveness of water lettuce (P. stratiotes L.) in removing nutrients, including N and P, from stormwater in the constructed water detention systems before it is discharged into the St. Lucie Estuary using phytoremediation technologies and to quantify the potential of this plant in improving stormwater quality in detention pond systems.
2 Materials and methods
2.1 Experimental design
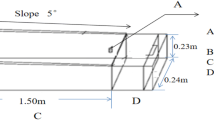
Two detention ponds (East and West Ponds) in the St. Lucie Estuary watershed, each with a control and a treatment plot, were selected. Water lettuce (P. stratiotes) was grown in the treatment plots, while no plant was maintained in the control plots.
Water samples were collected weekly from the control and the treatment plots and analyzed for water quality parameters, including total N and P, NO3–N, NH4–N, ortho-P, pH, electrical conductivity (EC), suspended solids, and turbidity.
Water lettuce was sampled monthly from the treatment plots. After being rinsed thoroughly with D.I. water and blotted dry, root and shoot were separated and their fresh weights were recorded. Plant parts were oven dried at 70°C for 3 days and ground to <1 mm using a stainless ball mill prior to analysis for total N and P.
Besides monthly sampling, plants were also periodically harvested to maintain an optimum plant density. For each harvest, the total fresh weight of the lettuce plant was recorded, plant moisture was determined, and total quantity of dry plant biomass yield was calculated for each plot. Harvested plant materials were applied to the field as organic amendments. Total amounts of N and P removed from the water by the harvested plant were quantified by multiplying the amounts of plant biomass by the concentrations of N and P in the plant.
2.2 Chemical analysis
Prior to filtration, pH and EC of the water samples were determined using a pH/ion/conductivity meter (pH/Conductivity Meter, Model 220, Denver Instrument, Denver, CO, USA) following EPA method 150.1 and 120.1, respectively. Turbidity of water samples was measured using a turbidity meter (DRT-100B, HF Scientific Inc., Fort Myers, FL, USA). Total P in the unfiltered water sample was determined by the molybdenum-blue method after digestion with acidified ammonium persulfate (EPA method 365.1). Sub-samples of the water were filtered through Whatman 42 filter paper. Portions of the sub-samples were filtered further through a 0.45-μm membrane for measuring total dissolved P and PO4–P. Concentrations of NO3–N and PO4–P were measured within 24 h after sample collection using an ion chromatograph (DX 500; Dionex Corporation, Sunnyvale, CA, USA) following EPA method 300. The concentrations of NH4–N and total Kjeldahl N (TKN) in the water sample were measured using a discrete autoanalyzer (EasyChem, Systea Scientific Inc., Italy) following EPA method 353.2. Total N in the water samples was calculated as the sum of TKN and NO3–N. Total dissolved P in water was determined using inductively coupled plasma atomic emission spectrometry (ICP-AES, Ultima, JY Horiba Inc. Edison, NJ, USA) following EPA method 200.7.
Plant N content was determined using a CN analyzer (vario Max CN, Elemental Analysensystem GmbH, Hanau, Germany). Sub-samples (each 0.400 g) of plant material were digested with 5 ml of concentrated HNO3 in a digestion tube using a block digestion system (AIM 500-C, A.I. Scientific Inc., Australia), and P concentration in the digester was determined using the ICP-AES.
3 Results and discussion
3.1 General water quality improvement
Total suspended solids, turbidity, EC, and pH in the waters of both plots changed seasonally; increasing during the rainy season of May to November and decreasing during the dry season of November to May with values in the rainy season several times, or up to three units of pH higher than for those in the dry season (Figs. 1, 2, 3, and 4). The increase in these parameters during the rainy season was likely to be due to the large input of stormwaters, which bring soil particles and solutes, including nutrients. The growth of water lettuce improved water quality. Total suspended solids in the water column were decreased by an average of approximately 10% in the treatment plots as compared with those in the control plots (see Fig. 1 and Table 1) due to sedimentation in a more favorable environment provided by the plants (Brix 1997). On average, water turbidity was reduced by 65.5% and 63.3% in treatment plots as compared with the controls in East and West Ponds, respectively, in the period from August 2005 to August 2007 (see Fig. 2 and Table 1). Water lettuce growth decreased water EC in both ponds (see Fig. 3), due to salt removal from the waters by plant uptake or root adsorption. In a similar trend, water lettuce growth decreased water pH (see Fig. 4), which was not expected for it is well known that water pH rises with plant photosynthesis. One explanation is that nearly complete coverage of the water surface by the floating lettuce effectively blocked out sunlight for the growth of other plants (such as submerged plants and algae) which carry out photosynthesis in the water and contribute to the pH rise. On the contrary, some algae might grow in the control plot due to higher N and P concentrations and thus caused a pH increase. It is also well known that oxygen oversaturation happens concurrently with pH rise, but dissolved oxygen (DO) monitoring results did not show an oxygen oversaturation scenario in the water during the day with DO concentration <1.5 and 0.7 mg/l in East and West Ponds, respectively. Thus, pH decrease here was probably due to reduced or eliminated growth of algae or other submerged vegetation by the floating plants.
3.2 N and P concentration reduction
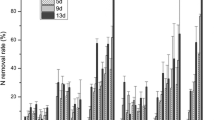
Changes of inorganic N (NH4–N plus NO3–N), total N, PO4 3−, and total P concentrations in water for the period from August 2005 to August 2007 are shown in Figs. 5, 6, 7, and 8. Like total suspended solids and water turbidity, nutrient concentrations in the waters showed seasonal changes during the year, which were affected by external input from stormwaters during the rainy season.
Although there are many reports showing that aquatic plants, such as Salvinia molesta and Elodea densa, preferred NH4–N to NO3–N (Reddy et al. 1987; Shimada et al. 1988) and theoretically NH4 + uptake is energetically more efficient than that of NO3 −, there were no differences in concentration reductions between NH4–N and NO3–N in both ponds with reduction rates of approximately 50–60% (see Table 1). Besides plant uptake, denitrification may also contribute to the decreased NO3–N concentration in the treatment plots as a more anaerobic condition (dissolved oxygen <1.5 and 0.7 mg/l in East and West Ponds, respectively) was created by the growing plants at the water’s surface and other anaerobic micro-sites (Gumbricht 1993; Reddy 1983).
Inorganic P (PO4 3−) removal (14% and 23% in East and West Ponds, respectively) was not as efficient as inorganic N (NH4–N+NO3–N) in both remediation systems (see Table 1), which was also the case in Sheffield’s research with a reduction rate of 40–55% in ortho-P compared to a reduction rate of 94% in inorganic N in a water hyacinth system (Sheffield 1967). Total P had a higher reduction than inorganic P (see Table 1), which indicates that the role aquatic plants play in such a remediation system is far more than uptake. Instead, nutrient uptake is only of quantitative importance in low-loaded systems (surface flow systems). More importantly, the aquatic plants play a crucial role by creating a favorable environment for a variety of complex chemical, biological, and physical processes that contribute to the removal and degradation of nutrients, which was thought by Brix (1997) to be the most important functions of aquatic plants. A higher removal rate in total P than in dissolved total P can come from the additional sedimentation effect on particulate P.
3.3 Plant N and P removal potential
Total N and P concentrations in the plant were approximately 17 and 3 g kg−1, respectively, with minimal differences between root and shoot (Figs. 9 and 10). Nitrogen and P content typically average 15–40 g N and 4–10 g P kg−1 for such large-leaved floating plants as water lettuce and water hyacinth (E. crassipes) (Aoi and Hayashi 1996).
Annual removal of N and P by plants were 190 and 24.6 kg ha−1, respectively, in East Pond and 329 and 34.1 kg ha−1, respectively, in West Pond, with dry matter being approximately 9 Mg ha−1 (East Pond) and 15 Mg ha−1 (West Pond). Much research has been performed on another invasive, large-leaf floating aquatic plant, water hyacinths (E. crassipes). Very high uptake rates have been reported in this research, for instance, 1,980 kg N and 322 kg P ha−1 year−1 by Boyd (1970), 2,500 kg N and 700 kg P ha−1 year−1 by Rogers and Davis (1972), and up to 5,350 kg N ha−1 year−1 and 1,260 kg P ha−1 year−1 by Reddy and Tucker (1983). The reasons behind this big difference in nutrient uptake rate between our research and the above research can be: (1) it is known that water hyacinth has a higher nutrient uptake and biomass yield potential than water lettuce; (2) this research was done using nutrient medium whose nutrient contents were much higher than that in the stormwater retention ponds; and (3) these high reported values were based on short-term experiments and extrapolated to 1 year, which often overestimates the nutrient uptake rate of the plant. On the other hand, the low nutrient uptake values from this research also indicated that the water lettuce was far from reaching their maximum nutrient uptake potential in these stormwater retention ponds.
3.4 Physiological limits
Plant growth is influenced by many environmental factors such as solar radiation, rainfall, and temperature, so is nutrient removal efficiency, as reflected in both nutrient concentrations in the plant (see Figs. 9 and 10) and plant yield of water lettuce (data not shown), showed strong seasonal dependence. This seasonal variability in plant growth and nutrient removal capacity was also discussed by Reddy and Sutton (1984).
West Pond worked better than East Pond in removing total N and total P from the waters (see Table 1), which could be related to the differences in total organic carbon (NPOC, averages of 30 and 12 mg l−1 in East and West Ponds, respectively) and EC of waters (180–2,000 and 100–400 μS cm−1 in East and West Ponds, respectively; see Fig. 3) between these two ponds. It was reported that an EC of 2,683 μS cm−1 was toxic to water lettuce (Haller et al. 1974). High EC in East Pond negatively affected water lettuce’s growth, leading to low efficiency in nutrient removal from the water.
Water lettuce is an invasive species, which means that it grows very well in nutrient-rich waters, but may not work well to our purpose in low nutrient waters.
4 Conclusions
Phytoremediation can be an important approach for cleaning eutrophicated stormwaters from agriculture and urban areas via man-made wetlands such as STAs and water detention/retention systems. Water lettuce has a great potential for removing N and P, reducing water suspended solids and turbidity from stormwaters, and improving water quality.
5 Recommendations and perspectives
For efficient water purification, grown-up biomass of aquatic macrophytes must be removed from water bodies to keep an optimum plant density. If not harvested, the vast majority of the nutrients that have been incorporated into the plant tissue will be returned to the water by decomposition processes (Brix 1997). Harvested plant biomass can be used as soil amendment or processed into livestock feed.
As water lettuce is an invasive species, it is important that the plant be strictly confined in the remediation system so that we can make full use of its nutrient scavenging ability without bringing unnecessary damage to the ecosystem.
More studies on how a variety of aquatic plants perform in different waters (with different nutrient ranges, pH, EC, or OC) under different environments (temperature, solar radiation, etc.) are needed for applying the right plant to the right water to achieve a maximum purification of the water.
References
Aoi T, Hayashi T (1996) Nutrient removal by water lettuce (Pistia stratiotes). Water Sci Tech 34(7/8):407–412
Boyd CE (1970) Vascular aquatic plants for mineral nutrient removal from polluted waters. Econ Bot 23:95–103
Brix H (1997) Do macrophytes play a role in constructed treatment wetlands. Water Sci Tech 35(5):11–17
Capece JC, Campbell KL, Bohlen PJ, Graetz DA, Portier KM (2007) Soil phosphorus, cattle stocking rates, and water quality in subtropical pastures in Florida, USA. Range Ecol Manage 60(1):19–30
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
El-Gendy AS, Biswas N, Bewtra JK (2005) A floating aquatic system employing water hyacinth for municipal landfill leachate treatment: effect of leachate characteristics on the plant growth. J Environ Eng Sci 4(4):227–240
Graves GA, Strom DA (eds) (1992) Bessey Creek and the greater St. Lucie Estuary. Florida Department of Environmental Protection, Southeast District, West Palm Beach, FL
Gumbricht T (1993) Nutrient removal processes in freshwater submersed macrophyte systems. Ecol Eng 2:1–30
Haller WT, Sutton DL, Barlowe WC (1974) Effects of salinity on growth of several aquatic macrophytes. Ecology 55(4):891–894
He ZL, Stoffella PJ, Yang XE, Yu S, Chen G, Banks DJ, Yang YG, Yang JY, Calvert DV (2005) Assessment and evaluation of nitrogen, phosphorus, and heavy metals in surface runoff from citrus groves and vegetable fields in the Indian River area. Project Final Report, Florida Department of Environmental Protection, Tallahassee, FL
He ZL, Zhang MK, Stoffella PJ, Yang XE, Banks DJ (2006) Phosphorus concentrations and loads in runoff water under crop production. Soil Sci Soc Am J 70:1807–1816
Karpiscak MM, Foster KE, Hopf SB, Bancroft JM, Warshall PJ (1994) Using water hyacinth to treat municipal wastewater in the desert southwest. Water Resour Bull 30:219–227
Kowalik P, Toczylowska I, Scalenghe R, Boero V, Ambrosoli R, Zanini E, Vola G, Edwards AC (1998) Phytoremediation by constructed wetlands: assessment of biopedological techniques for resource-efficient farming with livestock. Acta Horticulturae 457:187–194
Lindsey K, Hirt HM (1999) Use water hyacinth! (A practical handbook of uses for the water hyacinth from across the world). Anamed, Winnenden
Mahujchariyawong J, Ikeda S (2001) Modelling of environmental phytoremediation in eutrophic river—the case of water hyacinth harvest in Tha-Chin River, Thailand. Ecol Model 142(1/2):121–134
Reddy KR (1983) Fate of nitrogen and phosphorus in a waste-water retention reservoir containing aquatic macrophytes. J Environ Qual 12(1):137–141
Reddy KR, Sutton DL (1984) Waterhyacinths for water quality improvement and biomass production. J Environ Qual 13(1):1–9
Reddy KR, Tucker JC (1983) Productivity and nutrient uptake of water hyacinth, Eichhornia crassipes I. effect of nitrogen source. Econ Bot 37(2):237–247
Reddy KR, Tucker JC, Debusk WF (1987) The role of Egeria in removing nitrogen and phosphorus from nutrient enriched waters. J Aquat Plant Manage 25:14–19
Ritter A, Munoz Carpena R, Bosch DD, Schaffer B, Potter TL (2007) Agricultural land use and hydrology affect variability of shallow groundwater nitrate concentration in south Florida. Hydrol Process 21(18):2464–2473
Rogers HH, Davis DE (1972) Nutrient removal by waterhyacinth. Weed Sci 20(5):423–428
Sheffield CW (1967) Water hyacinth for nutrient removal. Hyacinth Control J 6:27–30
Shimada N, Yajima S, Watanabe Y (1988) Improvement of water quality using Salvinia molesta (1). Absorption of nitrogen and phosphorus by Salvinia molesta. Technical Bulletin, Faculty of Horticulture, Chiba University, pp 15–21
Smith DR, Owens PR, Leytem AB, Warnemuende EA (2007) Nutrient losses from manure and fertilizer applications as impacted by time to first runoff event. Environ Pollut 147:131–137
SFWMD (South Florida Water Management District), SJRWMD (St. Johns River Water Management District) (1994) Indian River Lagoon surface water improvement and management (SWIM) plan
U. S. Environmental Protection Agency (1976) Quality criteria for water. USEPA Rep 440:19–76–023
Woodward-Clyde Consultants (1994) Loading assessment of the Indian River Lagoon. Final report to Indian River Lagoon National Estuary Program. Melbourne, FL
Acknowledgment
The authors thank Mr. Diangao Zhang for his assistance in water sampling and processing, and thank Drs. G.C. Chen, J.Y. Yang, Y.G. Yang, and W.R. Chen, Mr. D. Banks and Mr. B. Pereira, and Miss J.H. Fan for their help in lab analysis. This project was in part supported by a grant (contract# 4600000498) from South Florida Water Management District.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Lee Young
Rights and permissions
About this article
Cite this article
Lu, Q., He, Z.L., Graetz, D.A. et al. Phytoremediation to remove nutrients and improve eutrophic stormwaters using water lettuce (Pistia stratiotes L.). Environ Sci Pollut Res 17, 84–96 (2010). https://doi.org/10.1007/s11356-008-0094-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-008-0094-0