Abstract
Ariidae catfish species are numerically abundant along the coast of Brazil. Their benthic habits and broad spectrum diets make them good potential candidates for sub-lethal biomonitoring studies. In this study, we assess the levels of various metal contaminants (Pb, Cd, Hg, Cu and Zn) in the muscle tissues of two Ariidae species, Cathorops spixii and Genidens genidens, from three sites in São Paulo State, Southeast Brazil: two polluted sites in the Santos-São Vicente estuary and a relatively unpolluted site in the Cananéia estuary. The Zn levels observed in the polluted areas in the Santos-São Vicente estuary were similar to those obtained for Ariidae from the reference site in Cananéia. The concentrations of Hg and Cu in the muscle tissue of both fish species were higher in individuals from the Santos-São Vicente than those from the Cananéia estuary. Both Ariidae species were observed to accumulate the Cu, Zn and Hg in their tissues; however C. spixii showed a more stable response suggesting its potential utility as a bioindicator species. The Zn and Cu concentrations probably reflect normal levels without a significant influence of anthropogenic contamination. The levels of Cd and Pb in muscle tissue of C. spixii and G. genidens were relatively low, but the PCA indicated the presence of the levels of these metals in the reference area (thereby supporting the need for monitoring in Cananéia estuary). The detection of Hg in fish from Santos-São Vicente and from the unpolluted site (Cananéia) is of particular concern as scientists frequently use this area as a reference site for biomonitoring studies on the Southwest coast of Brazil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The first use of the term ‘biological indicator’ in the context of aquatic systems referred to the detection and monitoring of changes in organisms that reflected (deleterious) changes in the quality of their environment (Wilhm and Dorris 1968). The definition of bioindication has subsequently broadened to encompass three separate contexts (McGeoch 1998): (1) environmental indicators—this is the most traditional use of the term and refers to taxa that respond predictably to environmental disturbance or to a change in environmental state. This group of indicators includes ‘sentinel species’, sensitive organisms introduced into the environment as an ‘early warning’ system, and ‘accumulators’ which naturally occur in the environment and readily take up and accumulate contaminants in quantities that can be easily measured. (2) Ecological indicators—taxa that are used to demonstrate or monitor the impacts of ecological change such as habitat fragmentation or climate change. (3) Biodiversity indicators—sometimes referred to as species-based surrogates, organisms used in conservation planning that are used as an indicator of ecosystem functioning (e.g. keystone species) or as indicators of overall biological diversity (reviewed in Watson et al. 2011).
One of the main uses of environmental indicator species has been to monitor the levels of exposure to contaminants such as metals, especially within the aquatic environment. A wide range of species have been used as environmental indicators in the marine environment. For example, invertebrates (mainly molluscs and crustaceans) have been extensively used as sentinel species (Bayen et al. 2004; Bellotto and Miekeley 2007; Turkmen and Ciminli 2007; Tang et al. 2009), while a wide variety of fish species have been utilized in environmental monitoring studies (Burger et al. 2005; Birungi et al. 2007; Dural et al. 2007; Linde-Arias et al. 2008; Azevedo, Fernandez et al. 2009; Barhoumi et al. 2009; Yılmaz et al. 2010).
Fish are particularly appropriate as environmental indicator species (Phillips 1977; Adams et al. 1989) because they are relatively easy to sample, frequently bioaccumulate toxins and often show predictable associations between contaminant levels in tissues and various morphological or life-history traits (Al-Yousuf et al. 2000). In this context, benthic species such as Ariidae catfish may be especially useful since they are eurihaline, feed on organisms present in the sediment where the bioavailability of contaminants is typically higher, have relatively low mobility, and are abundant and geographically widespread. In South America, the Ariidae are found all along the Atlantic coast, from Belize to southern Brazil (Figueiredo and Menezes 1978).
There have been very few studies on the accumulation and effects of inorganic pollutants in the Ariidae, or specifically on the two species used in the current study: Cathorops spixii and Genidens genidens. Here, we assess the level of metal (Pb, Cd, Hg, Cu and Zn) contamination in the muscle tissues of these two species using individuals sampled from three estuarine sites (two from a polluted estuary and one from a relatively unpolluted reference site) in southeast Brazil.
The presence of metals in the muscle tissue of fish is well-known (Joyeux et al. 2004; Meche et al. 2010; Bilandzic et al. 2011), and is of considerable importance for food safety and public health. In a biological context, metals are frequently categorized as essential or non-essential. Essential metals such as zinc (Zn) and copper (Cu) are very important for the metabolism of organisms (Heath 1990; Halliwell and Gutteridge 1999; Lehninger et al. 2005), but can be toxic in high concentrations due to their influence on physiological processes such as the redox balance of the cell (Jomova and Valko 2011). In contrast, non-essential such as lead (Pb), cadmium (Cd) and mercury (Hg) have no clear biological function and exposure to them can impair vital processes such as enzymatic activity, reproductive capacity and development (Burger et al. 2005; Yilmaz et al. 2010).
In the present study, C. spixii and G. genidens were assumed to have been exposed to the assayed metals as a result of various industrial activities in the Santos-São Vicente estuary. Therefore, the main objectives of this study were: (1) to investigate if the Ariidae catfish are effective bioindicators of exposure to Pb, Cd, Hg, Cu and Zn, and (2) to provide information on the concentrations of some essential and non-essential metals in muscle tissue of two Ariidae catfish species, C. spixii and G. genidens, from an estuary with low levels of anthropogenic influence (Cananéia) and an estuary (Santos-São Vicente) subject to pollution from industrial effluents and domestic sewage.
2 Material and Methods
2.1 Site Descriptions
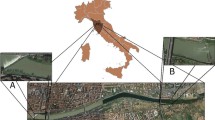
Two estuaries in southeast Brazil (São Paulo State) were chosen for the collection of samples: the polluted Santos-São Vicente estuary (24 °00′ S; 46 °21′ W) and the relatively pristine Cananéia estuary (25 ° S; 48 ° W).
The Santos-São Vicente estuary is an important economic area and contains the largest commercial harbour in South America. Moreover, it is home to the most important petrochemical and metallurgical complex in the region, comprising more than 1,100 industries. The rapid increase in urbanization and industrialization over the last 50 years has caused the degradation of coastal mangrove habitat of the estuary through contamination with effluent from industrial and domestic sources, combined with large quantities of solid waste. Thus, over recent decades the area has been subject to a gradual process of chemical contamination as a direct result of an increase in industrial activities and the release of sewage. As a consequence of these many anthropogenic activities, this region is exposed to potentially toxic metals such as Pb, Cd, Hg, Ni, Mn, Cr, Cu and Zn (Lamparelli et al. 2001), with potentially negative impacts on the resident estuarine organisms.
Two sample sites were chosen within the Santos-São Vicente estuary (Fig. 1) on the basis of different sources of pollution: site 1 (industrial sewage—23 °53.458′ S; 46 °22.604′ W)—industrial area close to the Cosipa, located in the inner part of the estuary and affected by intense industrial activity; site 2 (domestic sewage—23 °56.13′ S; 46 °24.42′ W)—area of raised (stilted) housing known as Araçarãna and previously the location of an off dump;
In contrast to the Santos-São Vicente estuary, the Cananéia estuary is subject to minimal anthropogenic influences and is frequently used as an environmental reference site for marine biomonitoring studies in São Paulo State (Azevedo, Serafim et al. 2009). However, even in this relatively undisturbed ecosystem, some recent studies have indicated the presence of toxic metals, including Hg, in fish and sediment (Amorim et al. 2008; Azevedo, Serafim et al. 2009). Over the last 150 years the Cananéia estuary has been subject to important environmental changes, mainly due to the construction of an artificial channel (the Valo Grande), connecting the Ribeira de Iguape River to the estuary (Mahiques et al. 2009). With the completion of this structure, organisms that inhabit the estuary were potentially exposed to Hg from an abandoned gold mine further upstream. There is also a fertilizer industry located in Iguape in the Northern area of the estuary. One sampling site (site 3—24 °58.946′ S; 46 °54.092′ W) was chosen in the Cananéia estuary.
Cananéia estuary is widely recognized as an aquatic environmental with low human influence as reflected by the low levels of some metals in the sediment (Amorin et al. 2008; Mahiques et al. 2009) and water chemistry (nutrients, pH, oxygen dissolved and organic matter) indicative of natural conditions (Azevedo, Serafim et al. 2009). In contrast, the Santos-São Vicente estuary is a polluted area that is subject to miscellaneous toxic agents (Bícego et al. 2006; Hortellani et al. 2008; Azevedo et. al., 2011). The data collected from these sites for the present study will contribute to a major database for the monitoring of Brazilian coastal areas under human influence.
2.2 Sample Collection
A total of 46 specimens of C. spixii and 48 of G. genidens were collected in two consecutive sample periods during July and August 2009 using gill nets of 20-mm mesh. Individuals were collected from two sites within the Santos-São Vicente estuary (n = 67) and one site from the Cananéia estuary (n = 27).
After collection, the fish were identified, maintained in water from the sampling site of origin and transported to the laboratory. Individuals were then anesthetized with benzocaine (2 % in water) and killed through spinal section. Morphometric data were recorded for total length (L) and total weight (W). Samples of the epaxial muscle from the dorsal surface were removed from each specimen, washed with distilled water, packed in polyethylene bags, labeled, and stored at −20°C for subsequent analysis.
2.3 Determination of Metals
Concentrations of Pb, Cd, Zn and Cd were performed in accordance with Visnijic-Jeftic et al. (2010) with some modifications. Approximately 1.0 g of muscle tissue from each specimen was placed in pre-weighed 100-mL PFA Teflon vessels. Five milliliter of concentrated HNO3 and 3 mL H2O2 were then added, and the muscle tissue was pre-digested for 4 h at room temperature. Seven blank samples and two certified standard reference samples containing fish tissue (dogfish liver—DOLT-2—National Research Canada Council, NRCC; and oyster tissue—National Institute of Standard and Technology, NIST) were also analyzed. Two milliliter of milli-Q water was added to the individual samples which were then further digested by heating in a microwave (CEM Corporation, Mars 5 model) using the following parameters: 600 W, 100 %, 9 min of temperature ramp, 145 PSI, 145°C temperature and 5-min hold.
After digestion, the solutions were diluted by adding milli-Q water until 25 mL. Cu and Zn concentration was measured using flame mode of a Fast-Sequential Atomic Absorption Spectroscope Varian, model Spectr-AAS-220-FS. For Cd and Pb determinations, the samples were diluted again (4 mL sample until 10 mL milli-Q water). Indium (5 ppb) was added to each sample and Cd and Pb concentrations were then measured using a high resolution inductively coupled plasma mass spectrometer HR-ICP-MS (Element, Finnigan). Results were expressed on a wet weight basis as microgrammes per kilogramme. The limits of detection and quantification for each metal were as follows: 0.156 and 0.243 μg kg−1 for Cd; 1.43 and 2.00 μg kg−1 for Pb; 12 and 20 μg kg−1 for Cu; and 45 and 63 μg kg−1 for Zn. Data on quality control using the reference materials are shown in Table 1.
For Hg measurement, muscle tissues were digested in glass flasks in a solution of concentrated HNO3, H2SO4 and HClO4 (1:2:1). Measurements of metal concentration were taken using the flow injection cold vapor atomic absorption spectrometry (FI-CV-AAS) technique. The analytical procedure followed the methods described by Lima et al. (2005). The detection and quantification limits were 2 and 15 μg kg−1, respectively. The validation of total Hg determination was assessed against a certified the standard reference material—Dogfish muscle DORM-2 (National Research Canada Council, NRCC). Hg concentrations were also reported as microgrammes per kilogramme wet weight. Quality control was assured by performing Hg analysis of the certified reference material Dorm-2 with a certified Hg concentration of 4.64 ± 0.26 mg kg−1. Mean data obtained (n = 6) was 4.61 ± 0.07 mg kg−1 with an average recovery of 0.99 ± 0.02 at a 0.05 significance level within the confidence interval of the certified level. The analytical results showed good precision and accuracy.
2.4 Statistical Analysis
Morphometric data were characterized as mean, minimum and maximum values. Due to high individual variability, metal concentrations in the muscle tissue are presented as median, minimum and maximum values. A cluster analysis was also used to classify areas in accordance with the different human influence in the estuaries and sites. The software (Multi-Variate Statistical Package) applied an average linkage method. In this method, different groups are generated in accordance with the distance or similarity between the clusters. The grouping between endpoints measured as biological variables and metals levels for each sampling site were verified with principal component analysis. A correlation matrix was used for standardization because the variables were measured on different scales or were of different orders of magnitude.
3 Results
Biometric data of fish collected in both estuaries are summarized in Table 2. Fish from Cananéia estuary showed higher total length and total weight (C. spixii: L, 302–411 mm; W, 236–690 g or G. genidens: L, 297–315 mm; W, 217–578 g) when compared to fish from industrial (C. spixii: L, 128–317 mm; W, 19–328 g or G. genidens: L, 170–382 mm; W, 38–234 g) and domestic (C. spixii: L, 171–224 mm; W, 51–101 g or G. genidens: L, 159–315 mm; W, 33–305 g) areas in Santos-São Vicente estuary (Table 2). All analyzed fish were adults with active gonadal maturation (including individuals in spawning and post-spawning conditions).
The median concentrations of Pb, Cd, Hg, Cu and Zn for C. spixii and G. genidens Ariidae from two estuaries under distinct anthropogenic influence are summarized in Table 3. In general, with exception of Cu levels, G. genidens from the Santos-São Vicente estuary sites subject to industrial and domestic sewage (sites 1 and 2) showed a higher concentration of metals than C. spixii from these same regions. The metals concentrations obtained for all samples were in accordance with results observed for other estuarine benthic fish species (Has-Schön et al. 2006; Mansilla-Rivera and Rodríguez-Sierra 2011), but were below international (FAO 1983; MAFF 1995; HC 2007) and national (ANVISA 1998) guidelines (Table 4).
In order to observe the relationship between sampling sites, cluster analysis was applied. Since fish from the reference site were significantly different to those from the contaminated sites in Santos-São Vicente estuary in terms of length and weight, these biometric data were not considered in this analysis. The dendogram revealed that the areas could be grouped into two main clusters. For C. spixii, the groups were related with the different anthropogenic influences (Fig. 2a): cluster 1 was the reference area (site 3) and cluster 2 contained both the domestic and industrial areas (sites 1 and 2) in the Santos-São Vicente estuary. The dendogram for G. genidens also indicated two clusters, but in this case one cluster was associated with the domestic area (site 2) and the other grouping contained the industrial and reference areas (sites 1 and 3).
A correlation matrix of all studied variables was analyzed for both species (Table 5). These data were used as a guide to clarify the possible correlations among the variables. However, cross correlations between species were not considered. For C. spixii, four positive and statistically significant correlations were found between the biometric data and the contents of Zn and Hg (L vs Zn: r s = 0.56, p < 0.01; W vs Zn: r s = 0.52, p < 0.01; L vs Hg/L vs Zn: r s = 0.56, p < 0.01; W vs Hg: r s = 0.33, p < 0.05). Another significant, though negative, correlation was observed between Cu and Zn (r s = −0.36, p < 0.05). For G. genidens, a unique negative significant correlation was found between the level of Hg and Cu (r s = −0.39, p < 0.05). As would be anticipated, significant and positive correlations were observed between the length and weight of both species (C. spixii—L vs W: r s = 0.97, p < 0.01; G. genidens—L vs W: r s = 0.93, p < 0.01).
The influence, dependence and associations of the variables (e.g. length, weight and metal concentrations) in each sampling site for both Ariidae species were verified using Principal Component Analysis (PCA). The analysis takes into account the metals contents (Zn, Cu, Cd, Pb and Hg) and the biological parameters (length and weight) for each catfish species sampled in the three studied sites (Fig. 3a). PC1 explained 86 % of the variance and 11 % was explained by PC2. Greater variability was observed in axis 2, where there were both significant positive and negative correlations among the variables. Two groups were clearly separated in PC2, representing the reference site (length, weight, Pb and Zn for C. spixii) and the industrial and domestic pollution sites (Cu for C. spixii and; length, weight, Zn, Cu, Hg, Cd and Pb for G. genidens). When the PCA was performed without the biometric data (Fig. 3b) there was an inversion of position between the two groups reference (now in the negative axis) and domestic and industrial sites (now in the positive axis) in PC2. In this latter analysis, 87 % of the variance was explained by PC1 and 8 % by PC2.
Principal components analysis (PCA) of metals (Cd, Pb, Zn, Cu and Hg) in C. spixii (Cs) and G. genidens (Gg) from two polluted sites (site 1: industrial; and site 2: domestic) in the Santos-São Vicente estuary and in .the reference site (site 3: Cananéia estuary). a PCA only to metals/areas; b PCA to metals and biological parameters of the fish (length and weight)/areas
4 Discussion
The significant positive correlations found in this study have been observed by other authors analyzing the relationship between biometric data and metal contamination in various fish species. Specifically, negative correlations between length/weight and Cu levels were reported by Canli and Atli (2003) in their study of metal concentrations in Scomberesox saurus (Atlantic saury). Moreover, significant positive correlations between THg and biometric data are commonly described in the literature (e.g. Anan et al. 2005; Rabitto et al. 2011; Zhu et al. 2012). However, it is important to note that different species can show different profiles of metal incorporation and it should therefore be cautiously applied in the context of biomonitoring.
In general, studies with natural population are problematic due to the inability to statistically control for the variation in the biological characteristics of the organisms. One potential solution is to only use organisms in the same length/weight classes. However, by doing this, sample size may be dramatically reduced thereby decreasing the ability of statistical tests to detect significant differences between samples. Moreover, it is also very important to apply the most appropriate statistical tools to partition out the influence of biological traits in the analysis, especially when assessing the potential utility of a species as a bioindicator. The pragmatic choice in studies in which sampling is constrained by limited time and resources, and the one adopted in the current study, is to use the greatest sample size and to be rigorous in the application of statistical tests in order to detect any trait related biases. On this basis, even though there was a slight difference in size characteristics of fish from the reference estuary and the two polluted sites, the analysis indicates that this did not compromise the main goal of the study. Thus, the data clearly show the separation of C. spixii and G. genidens in different areas, although it should be noted that C. spixii varied less in the assessed traits than G. genidens.
It is important to distinguish between the essential metals (Zn and Cu) that naturally occur in animal tissues and the non-essential metals (Pb, Cd and Hg) that reflect exogenous influences and which may be related to environmental pollution (Yilmaz et al. 2010). Indeed, toxic effects of Zn in the environment are rare, and this metal may even have a protective effect against the toxicity of some metals such as Cd and Pb.
Zinc is an integral component of a large number and variety of proteins that are involved in a multiplicity of vital metabolic processes (Vallee and Auld 1993). Several functions have been attributed to metallothionein (MT) in response to exposure to high levels of Cu, Zn, Cd, Pb and Hg (Viarengo 1989), especially those concerned with the detoxification and antioxidative responses of the cell (Valle 1995; Jacob et al. 1999; Livingstone 1993). Moreover, MT plays a critical role in zinc metabolism, both in homeostasis and distribution, as well as in neural and metabolic networks (Bell and Vallee 2009). Such essential metal “clusters” such as MT are very important for cell functioning. For example, during homeostasis, MT makes Cu+2 and Zn+2 available for the biosynthesis of many metalloenzymes and metalloproteins (Valle 1995; Gao et al. 2008). MT also decreases the input of metals to the cell, thereby decreasing the toxic effects of some metals. The Zn levels observed in the polluted areas in the Santos-São Vicente estuary were similar to those obtained for Ariidae from the reference site in Cananéia, suggesting that these concentrations probably reflect normal levels without a significant influence of anthropogenic contamination.
The concentrations of Hg and Cu in the muscle tissue of both fish species were higher in individuals from the Santos-São Vicente than those from the Cananéia estuary. These data can suggest an anthropogenic input of these metals into the estuary, with potentially negative impacts on the health of the fish populations—Pb, Cd and Hg are highly toxic, even in trace amounts. Lead (Pb) is especially toxic for fish and is associated with deficits or decreases in survival, growth rates, development and metabolism (Yilmaz et al. 2010). It should be noted, however, that all the concentrations of metals observed in the present study are below the maximum advisable international and national limits for muscle tissue in fish (FAO 1983; ANVISA 1998).
Amorim et al. (2008) analyzed sediments from several stations in Cananéia estuary and observed low values of THg, Zn, Co, Cr—interpreted as being indicative of natural conditions. In relation to the station and season where catfish in the present study were sampled, the authors obtained the following values: THg 0.032 μg g−1, Zn 25 μg g−1, Co 3.7 μg g−1 and Cr 24 μg g−1. By contrast, a recent study in the Santos-São Vicente estuary obtained much higher concentrations of metals in sediment than those from the Cananéia estuary (Hortellani et al. 2008). In sample sites corresponding to the domestic (site 2) and industrial (site 1) sites metal concentrations were as follows: Cr 97.5 μg g−1, Zn 284.4 μg g-1, Ni 22.2 μg g−1, Co 10.3 μg g−1, Cd 0.92 μg g−1, Pb 23.5 μg g−1, Hg 0.23 μg g−1, Al 5.21 μg g−1 and Fe 2.68 μg g−1 (domestic site equivalent). Cr 97.5 μg g−1, Zn 312 μg g−1, Ni 44.2 μg g−1, Co 15.3 μg g−1, Cd 0.98 μg g−1, Pb 89.9 μg g−1, Hg 0.75 μg g−1, Al 6.19 μg g−1 and Fe 7.99 μg g−1 (industrial site equivalent). Hortellani et al. 2008 considered these data to be indicative of an input of Al, Fe, Ni, Co and Cr into the industrial area, high Zn, Hg and Pb were connected with the industrial and port regions, and Cd and Zn were the unique metals indicative of domestic disposal.
THg concentrations in sediments and water chemistry (NO −3 , NO −2 , NH4 and PO −34 , dissolved oxygen, pH, temperature and salinity) have been previously determined in the Santos-São Vicente estuary and in Cananéia. Specifically, Azevedo et al. (2009, 2011) demonstrated the deteriorating condition of water and sediment and the deleterious influence of the human activities in the Santos-São Vicente estuary as compared with the Cananéia estuary.
The presence of Pb, Hg and Cd was also detected in the relatively unpolluted Cananéia estuary, albeit at lower concentrations. The influence of these metals in the reference site was also confirmed by the PCA analysis when the influence of the biometric differences were not considered (Fig. 3b). Their presence is somewhat surprising in an area that is frequently used as a reference for biomonitoring studies, but can be explained in part by the proximity of the estuary to an abandoned gold mine and the location of a fertilizer factory in Iguape city in the northern portion of the estuary. Moreover, these metals can also come from a wide variety of more distant and diffuse sources, such as transport in river sediments, air and in the bodies of migrating aquatic species (Wiener and Spry 1996; Fitzgerald et al. 1998). However, given the presence of these toxic metals in the study area the choice of the Cananéia estuary as a reference site for environmental assessment and monitoring should be made with reservations according to the type of study and the contaminants of interest.
Finally, the data presented strongly indicate that the two species of catfish, and specifically C. spixii, can serve as bioindicator species. The grouping revealed by the cluster analysis between the reference and domestic areas suggests that metal accumulation in C. spixii is less influenced by biological characteristics than G.genidens because, with exception for Cu contents, the level of metals recorded for both catfish species were very similar.
Even though levels of the metals analyzed are not yet critical from a human health perspective, the initiation of further monitoring programs in the study area is desirable. This is especially the case in the Cananéia estuary, which is frequently used as reference site for environmental assessment studies, but which is clearly subject to toxic metal pollution. The high concentrations of mercury in the muscle tissues of both catfish species from all the regions studied warrant regular monitoring of the environment and the fish inhabiting it. Because of its high toxicity, this element is of significance to the environment and to the health of fish and consumers. The other metals studied (Cu and Zn) occur at levels that are regulated by fish metabolism and do not pose threats.
References
Adams, S. M., Shepard, K. L., Greeley, M. S., Jimenez, B. D., Jr., Ryon, M. G., Shugart, L. R., & McCarrthy, J. F. (1989). The use of bioindicators for assessing the effects of pollutant stress on fish. Marine Environmental Research, 28, 459–464.
Al-Yousuf, M. H., El-Shahawi, M. S., & Al-Ghais, S. M. (2000). Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. The Science of the Total Environment, 256, 87–94.
Amorim, E. P., Favaro, D. T. I., Berbel, G. B. B., & Braga, E. S. (2008). Assessment of metal and trace element concentrations in the Cananéia estuary, Brazil, by neutron activation and atomic absorption techniques. Journal of Radioanalytical And Nuclear Chemistry, 278(2), 485–489.
Anan, Y., Kunito, T., Tanabe, S., Mitrofanov, I., & Aubrey, D. G. (2005). Trace element accumulation in fishes collected from coastal waters of the Caspian Sea. Marine Pollution Bulletin, 51, 882–888.
ANVISA. Portaria no. 685, de 27 de agosto de 1998. Brazilian Legislation. http://www.anvisa.gov.br/legis/portarias/685_98.htm. Accessed 27 Apr 2011.
Azevedo, J. S., Braga, E. S., Favaro, D. T., Perretti, A. R., Rezende, C. E., & Souza, C. M. M. (2011). Total mercury in sediments and in Brazilian Ariidae catfish from two estuaries under different anthropogenic influence. Marine Pollution Bulletin, 62, 2724–2731.
Azevedo, J. S., Fernandez, W. S., Farias, L. A., Favaro, D. T. I., & Braga, E. S. (2009). Use of the tropical Brazilian fish Cathorops spixii as a bioindicator of trace metals pollution in Santos Bay. Ecotoxicology, 18, 577–586.
Azevedo, J. S., Serafim, A., Company, R., Braga, E., Favaro, D. I., & Bebianno, M. J. (2009). Biomarkers of exposure to metal contamination and lipid peroxidation in the benthic fish Cathorops spixii from two estuaries in South América, Brazil. Ecotoxicology, 18, 1001–1010.
Barhoumi, S., Messaoudi, I., Deli, T., Said, K., & Kerkeni, A. (2009). Cadmium bioaccumulation in three benthic fish species, Salaria basilisca, Zosterisessor ophiocephalus and Solea vulgaris collected from the Gulf of Gabes in Tunísia. Journal of Environmental Sciences, 21, 980–984.
Bayen, S., Thomas, G. O., Lee, H. K., & Obbard, J. P. (2004). Organochlorine pesticides and heavy metals in green mussel, Perna viridis in Singapore. Water, Air, and Soil Pollution, 155, 103–116.
Bell, S. G., & Vallee, B. L. (2009). The metallothionein/thionein system: an oxidoreductive metabolic zinc link. ChemBioChem, 10, 55–62.
Bellotto, V. R., & Miekeley, N. (2007). Trace metals in mussel shells and corresponding soft tissue samples: a validation experiment for the use of Perna perna shells in pollution monitoring. Analytical and Bioanalytical Chemistry, 389, 769–776.
Bícego, M. C., Taniguchi, S., Yogui, G. T., Montone, R. C., Silva, D. A. M., Lourenço, R. A., Martins, C. C., Sasaki, S. T., Pellizari, V. H., & Weber, R. R. (2006). Assessment of contamination by polychlorinated biphenyls and aliphatic and aromatic hydrocarbons in sediments of the Santos and São Vicente Estuary System, São Paulo, Brazil. Marine Pollution Bulletin, 52, 1784–1832.
Bilandzic, N., Dokic, M., & Sedak, M. (2011). Metal content determination in four fish species from the Adriatic Sea. Food Chemistry, 124, 1005–1010.
Birungi, Z., Masola, B., Zaranyika, M. F., Naigaga, I., & Marshall, B. (2007). Active biomonitoring of trace heavy metals using fish (Oreochromis niloticus) as bioindicator species. The case of Nakivubo wetland along Lake Victoria. Physics and Chemistry of the Earth, 32, 1350–1358.
Burger, J., Campbell, K. R., Campbell, T. S., Shukl, T., Dixon, C., & Gochfeld, M. (2005). Use of central Stonerollers (Cyprinidae: Campostoma anomalum) from Tennessee as a bioindicator of metal contamination. Environmental Monitoring And Assessment, 110, 171–184.
Canli, M., & Atli, G. (2003). The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environmental Pollution, 121, 129–136.
Dural, M., Göksu, M. Z. L., & Özak, A. A. (2007). Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chemistry, 102, 415–421.
FAO (Food and Agriculture Organization). (1983). Compilation of legal limits for hazardous substances in fish and fishery products. FAO fishery circular, 464, 5–100.
Figueiredo, J. L., & Menezes, N. A. (1978). Manual de peixes marinhos do sudeste do Brasil. II. Teleostei (1) (p. 10). São Paulo: Museu de Zoologia da Universidade de São Paulo.
Fitzgerald, W., Engstrom, D. R., Mason, R. P., & Nater, E. A. (1998). The case for atmospheric mercury contamination in remote áreas. Environmental Science and Technology, 32(1), 1–7.
Gao, D., Wang, G. T., Chen, X. T., & Nie, P. (2008). Metallothionein-2 gene from the mandarin fish Siniperca chuatsi: cDNA cloning, tissue expression, and immunohistochemical localization. Comparative Biochemistry and Physiology. doi:10.1016/j.cbpc.2008.05.014.
Halliwell, B., & Gutteridge, J. M. C. (1999). Free radicals in biology and medicine (3ath ed.). Oxford: Oxford Science Publications.
Has-Schön, E., Bogut, I., & Strelec, I. (2006). Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Archives of Environmental Contamination and Toxicology, 50, 545–551.
HC (Health Canada). (2007). Canadian standards for various chemical contaminants in foods, Ottawa, Ontario. http://laws.justice.gc.ca/PDF/Readability/CRC870.pdf. Accessed 9 May 2011.
Heath, A. G. (1990). Water Pollution and Fish Physiology (2nd ed., p. 245). Boca Raton: CRC Press.
Hortellani, M. A., Sarkis, J. E. S., Abessa, D. M. S., & Sousa, E. C. P. M. (2008). Avaliação da contaminação por elementos metálicos dos sedimentos do estuário de Santos-São Vicente. Química Nova, 31(1), 10–19.
Jacob, C., Maret, W., & Vallee, B. L. (1999). Selenium redox biochemistry of zinc-sulfur coordination sites in proteins and enzymes. Proceeding of the National Academy of Sciences of the USA, 96, 1910–1914.
Jomova, K., & Valko, M. (2011). Advances in metal-induced oxidative stress and human disease. Toxicology, 283(2–3), 65–87.
Joyeux, J. C., Campanha Filho, E. A., & Coutinho de Jesus, H. (2004). Trace metal contamination in estuarine fishes from Vitória Bay, ES, Brazil. Brazilian Archives of Biology and Technology, 47(5), 765–774.
Lamparelli, M. C., Costa, M. P., Prósperi, V. A., Bevilacqua, J. E., Araújo, R. P. A., Eysink, G. G. J., & Pompéia, S. (2001). Sistema estuarino de Santos e São Vicente. São Paulo: Relatório Técnico; CETESB. 183p.
Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2005). Principles of biochemistry (4ath ed.). New York: Worth.
Lima, A. P. S., Sarkis, J. E. S., Shihomatsu, H. M., & Muller, R. C. S. (2005). Mercury and selenium concentrations in fish samples from Cachoeira do Piraí Municipality, Para State, Brazil. Environmental Research, 97(3), 236–244.
Linde-Arias, A. R., Inácio, A. F., Alburquerque, C., Freire, M. M., & Moreira, J. C. (2008). Biomarkers in an invasive fish species, Oreochromis niloticus, to assess the effects of pollution in a highly degraded Brazilian River. The Science of the Total Environment, 399, 186–192.
Livingstone, D. R. (1993). Biotechnology and pollution monitoring: use of molecular biomarker in the aquatic environment. Journal of Chemistry Technology and Biotechnology, 57, 195–211.
MAFF (1995). Monitoring and surveillance of non-radioactive contaminants in the aquatic environment and activities regulating the disposal of wastes at sea, 1993. Aquatic Environment Monitoring Report No. 44. Directorate of Fisheries Research, Lowestoft.
Mahiques, M. M., Burone, L., Figueira, R. C. L., Lavenere-Wanderley, A. A. D., Capellari, B., Rogacheski, C. E., Barroso, C. P., dos Santos, L. A. S., Cordero, L. M., & Cussioli, M. C. (2009). Anthropogenic influences in a lagoonal environment: A multiproxy approach at the Valo Grande mouth, Cananéia-Iguape system (Se Brazil). Brazilian Journal of Oceanography, 57(4), 325–337.
Mansilla-Rivera, I., & Rodríguez-Sierra, C. J. (2011). Metal levels in fish captured in Puerto Rico and estimation of risk from fish consumption. Archives of Environmental Contamination and Toxicology, 60, 132–144.
McGeoch, M. A. (1998). The selection, testing and application of terrestrial insects as bioindicators. Biology Reviews, 73, 181–201.
Meche, A., Martins, M. C., Lofrano, B. E. S. N., Hardaway, C. J., Merchant, M., & Verdade, L. (2010). Determination of heavy metals by inductively coupled plasma-optical emission spectrometry in fish from the Piracicaba River in Southern Brazil. Microchemical Journal, 94, 171–174.
Phillips, D. J. H. (1977). The use of biological indicator organisms to monitor trace metal pollution in marine and estuarine enviroments—a review. Environmental Pollution, 13(4), 281–317.
Rabitto, I. S., Bastos, W. R., Almeida, R., Anjos, A., de Holanda, I. B. B., Galvão, R. C. F., Filipak Neto, F., Menezes, M. L., Santos, C. A. M., & Oliveira Ribeiro, C. A. (2011). Mercury and DDT exposure risk to fish-eating human populations in Amazon. Environment International, 37, 56–65.
Summers, J. K., Paul, J. F., & Robertson, A. (1995). Monitoring the ecological condition of estuaries in the United States. Toxicology and Environmental Chemistry, 49, 93–108.
Tang, C. H., Lin, C. S., & Wang, W. H. (2009). Metal accumulation in marine bivalves under various tributyltin burdens. Environnmetal and Toxicology Chemistry, 28(11), 2333–2340. http://apps.isiknowledge.com/full_record.do?product=WOS&search_mode=GeneralSearch&qid=2&SID=1BBOdP8Hb@LMJn3fpiF&page=1&doc=4—address000270846900012-3.
Turkmen, M., & Ciminli, C. (2007). Determination of metals in fish and mussel species by inductively coupled plasma-atomic emission spectrometry. Food Chemistry, 103, 670–675.
Valle, B. L. (1995). The functions of metallothionein. Neurochemistry International, 27(1), 23–33.
Vallee, B. L., & Auld, D. S. (1993). Zinc: biological functions and coordination motifs. Accounts of Chemical Research, 26, 543–551.
Viarengo, A. (1989). Molecular mechanisms of heavy metal cytotoxicity in marine organisms. Marine Environmental Research, 28(1–4), 298.
Visnijic-Jeftic, Z., Jaric, I., Jovanovic, L., Skoric, S., Smederevac-Lalic, M., Nikcevic, M., & Lenhardt, M. (2010). Heavy metal and trace element accumulation in muscle, liver and gills of the Pontic shad (Alosa immaculata Bennet 1835) from the Danube River (Serbia). Microchemical Journal, 95, 341–344.
Watson, J. E. M., Grantham, H. S., Wilson, K. A., & Possingham, H. P. (2011). Systematic conservation planning: past, present and future. In R. J. Ladle & R. J. Whittaker (Eds.), Conservation Biogeography. Oxford: Wiley-Blackwell.
Wiener, J. G., & Spry, D. J. (1996). Toxicological significance of mercury in fresh water fish. In W. N. Beyer, G. H. Heinz, & A. W. Redmon-Norwood (Eds.), Environmental contaminants in wildlife interpreting tissue concentrations. Lewis: Boca Raton Publishers.
Wilhm, J. L., & Dorris, T. C. (1968). Biological parameters for water quality criteria. BioSciences, 8, 477–481.
Yılmaz, A. B., Sangün, M. K., Yaglıoglu, D., & Turan, C. (2010). Metals (major, essential to non-essential) composition of the different tissues of three demersal fish species from Iskenderun Bay, Turkey. Food Chemistry, 123, 410–415.
Zhu, L., Yan, B., Wang, L., & Pan, X. (2012). Mercury concentration in the muscle of seven fish species from Chagan Lake, Northeast China. Environment Monitoring and Assessment, 184, 1299–1310.
Acknowledgments
The authors would like to thank the São Paulo Foundation for Research Support—FAPESP for the postdoc fellowship to J.S. Azevedo (Process 2008/58261-6)—and the Institute of Energy and Nuclear Research for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azevedo, J.S., Sarkis, J.E.S., Hortellani, M.A. et al. Are Catfish (Ariidae) Effective Bioindicators for Pb, Cd, Hg, Cu and Zn?. Water Air Soil Pollut 223, 3911–3922 (2012). https://doi.org/10.1007/s11270-012-1160-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-012-1160-2