Abstract
In the present study Cathorops spixii, was evaluated as a bioindicator fish for trace metal pollution. Concentrations of cobalt (Co), iron (Fe), selenium (Se) and zinc (Zn) were determined by Instrumental Neutron Activation Analysis in liver. Mercury (Hg) and methyl-mercury (MeHg) were analyzed by Cold Vapor Atomic Absorption Spectrometry in muscles and livers. High concentrations of Co, Fe, Se and Zn were observed in C. spixii from Santos Bay in comparison to fish collected in a non-polluted site in the same Brazilian coast. These trace metal concentrations were out of the permissible levels for human consumption. Although, Hg and MeHg levels were low, the C. spixii could still be used as an effective bioindicator to observe trace metal behaviors in the environment in function of the bioaccumulation process observed mainly by other analyzed trace metals. Thus, the use of this species is strongly recommended to monitor the effects and behavior of trace metal pollution in aquatic ecosystems in Brazil due to its bioaccumulation function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The coastal environment receives a large amount of trace metal pollution from different natural and anthropogenic sources, such as industrial and domestic sewage, storm runoff, leaching from landfills, shipping and harbor activities, and atmospheric deposits (Salomons and Förstner 1984; Lacerda 1998). Some trace metals represent a serious problem due to their toxicity and their ability to accumulate in the biota (Islam and Tanaka 2004). Therefore, trace metal concentration determination in organisms should be part of any assessment and monitoring program in the marine coastal environment (Marcovecchio 2004).
The Baixada Santista estuarine system receives an intensive and continuous industrial and domestic effluent. Some authors (Cetesb 1979, 2001; Fúlfaro et al. 1983; Boldrini and Navas-Pereira 1987; Montone 1987; Braga et al. 2003; Aguiar and Braga 2007) verified high concentrations of different chemical compounds introduced into the Santos Bay. In 1983, the total flow of Santos and São Vicente waste reached 2,000 L/s most being deposited through a submarine emissary (Homem 1983) into the Santos Bay. This emissary is about 5,800 m long and deposits mainly the domestic waste into the Santos Bay which also receives other kinds of pollutants.
Little is known about the effects of the sewage disposal on demersal fish populations (Otaway et al. 1995). However, some studies show changes in abundance and in diversity (Puffer et al. 1982; Grigg 1994) with some different consequent alterations overall in the reproductive system, histopathological changes and organic enrichment and others not only exactly from sewage disposal, but from other sources that reach the estuarine system and follow to the Santos Bay.
Organisms used as pollution monitors have numerous advantages over the chemical analyses of abiotic compartments. Organisms only accumulate the biological available forms of the pollutant Furthermore, they are always present in the marine ecosystem, consequently enabling continuous monitoring of pollutants (Bryan 1979; Phillips and Segar 1986).
Organisms are able to integrate the fluctuations of concentration pollutants through time and to accompany the magnification afforded by bioaccumulation. This is advantageous due to the precision and expense of analyses of trace metal pollutants near the limits of analytical detection (Phillips 1977; Jones and Walker 1979).
As a consequence of trace metal excess, the organism can present alterations in growth rate, reproductive phases, cellular mutations, modifications in the enzymatic processes, behavioral alterations and even death (Heath 1987). These effects can influence individual health, population and community survival (Furness and Raibow 1990).
Most studies published on marine pollution bioindicator organisms are concentrated on invertebrates, mainly crustaceans and mollusks. However, the use of fish as bioindicators of marine pollution monitoring is widely recognized (Reddy et al. 2001).
Fish tissues have high capacity to bio-accumulate trace metals and organic compounds being sensitive to aquatic pollution (Fisk et al. 2001). Concentrations of trace metals can vary in different degrees depending on the considered tissues in the same individual; as result of the different biochemical characteristics of the tissues. Therefore, fishes represent good bioindicators for environmental studies. They tend to accumulate pollutants in their tissues, transferring the pollutants to local trophic levels (Boon et al. 2002).
In the present investigation, the concentrations of some trace metals were measured in different tissues of Cathorops spixii from the Santos Bay. Cobalt (Co), iron (Fe), mercury (Hg), selenium (Se) and zinc (Zn) concentrations were determined in liver samples; mercury (Hg) and methyl-mercury (MeHg) were also determined in muscle of C. spixii. For comparison, the same trace metals were determined in C. spixii from a non-polluted site. An attempt was made to understand variations of the trace metal concentrations among organisms, as well as the relationship between metal concentrations and fish sizes. These analyses will subsidize the hypothesis that C. spixii is a good bioindicator species of trace metal pollution to a system strongly impacted by domestic and industrial sewage (Santos Bay) in comparison to a non-polluted site in the Brazilian coast.
Materials and methods
Studied sites
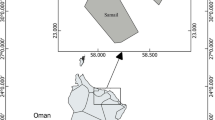
The Santos Bay is located in São Paulo state (24°00′S; 46°21′W), at the central part of this state coast. The climate is typically tropical (subtropical) with humid forest and a rainy summer season. This region represents an important economic area. Besides tourism, the secondary activity of the city, the largest commercial harbor of South America operates in the city. Brazil’s most important petrochemical and metallurgical complex composed of more than 1,100 industries, is located in the region. The increase of urbanization and industrialization along banks of estuary, especially over the last 50 years, has been responsible for the degradation of the mangrove vegetation due to the industrial and domestic effluents and the disposal of solid residues. The input of pollutants acts as an unbalancing agent of the ecosystem. The presence of some human activities contributes directly or indirectly to the input of trace metals in this area.
Cananéia estuarine–lagoon complex is located at south of São Paulo State coast (25°S; 48°W) and was used as non-polluted site due to the low human activity. The inner section of the estuarine–lagoon complex is subject to tidal cycles and to fresh water inputs. Externally, drift currents exists. The tidal movements and the fresh water discharges define the general circulation, the distribution processes and the water mixture in this estuarine–lagoon complex. This region is an important area for monitoring and contamination studies due to low anthropogenic influence.
Sampling
The sampling took place in January 2005, during the summer period corresponding to the rainy season. Fish were collected on board of the R/B Albacora ship, using a bottom Otter Trawl (1.6″ mesh wall and 1.2″ mesh cod end) 11 m length and, set at 8.8 m depth. Specimens of C. spixii were collected in Cananéia estuarine–lagoon complex (n = 05) and in Santos Bay (n = 23; Fig. 1) and the fish captured were transported to the laboratory in a thermal flask with ice. The identification followed Figueiredo and Menezes (1978) descriptions. In the laboratory, morphometric data of each fish was taken and ~1.0 g of the epaxial muscle from the dorsal fish surface and the liver sample were dissected, washed with distilled water, packed in polyethylene identified bags and kept at −15°C until analysis, in the Neutron Activation Analysis Laboratory.
Analytical procedures
Mercury and methyl-mercury were determined using Cold Vapor Atomic Absorption Spectrometry (CV AAS) using FIMS from Perkin Elmer. About 200–500 mg of fish muscle and liver were digested with a mixture of concentrated HNO3 and H2SO4 acids in Teflon vials. For MeHg determination, the methodology was based on the leaching of the sample with 6 M HCl. The separation of organic from inorganic mercury was performed by the use of an ionic exchange resin. Finally, the measurement of mercury content followed by CV AAS determination. The analytical procedure used (wet digestion) followed Horvat (1996) with some modifications. All reagents were of analytical grade with low levels of mercury. High purity water, of 18 MΩ cm−1 conductivity was obtained using Milli-Q system. The Hg stock solution (1,255 mg L−1) was acquired by dissolving HgO (Johnson Matthey Chemicals Limited) in HNO3.
Methodology validation for total Hg and MeHg determination was carried out by means of reference material analyses of Dogfish liver (DOLT-1, NRCC), Dogfish liver (DOLT-3, NRCC) and Dogfish muscle (DORM-1, NRCC). The detection limit for this method was established in agreement with studies found in the literature for Hg determination (Skoog et al. 2002). The detection limit was calculated using the formula (LD = X + 3s) and the value 0.5 ng mL−1 was found. The quantification limit (LQ = X + 5s) was 0.7 ng mL−1. The detection limit of this procedure, considering sample mass and dilution of solutions was 0.03 μg g−1.
The Co, Fe, Zn and Se chemical determination by Instrumental Neutron Activation Analysis (INAA) used ~150 mg of fish tissues and reference materials accurately weighed and sealed in pre-cleaned double polyethylene bags, for irradiation. Synthetic standard was prepared by pipetting convenient aliquot of each metal standard solution (SPEX CERTIPREP) onto small sheets of Whatman no. 41 filter. Fish tissue samples, reference materials and Co, Fe, Zn and Se synthetic standards were irradiated for 8 h, under a thermal neutron flux of 1012 cm−2 s−1 in the IEA-R1 nuclear research reactor at IPEN. Details for the INAA experimental procedure have been described by Fávaro et al. (2000).
Methodology validation for metals (Co, Fe, Se and Zn) determination by INAA was carried out by means of the reference material analyses Oyster tissue (NIST SRM 1566b) and Dog fish muscle (DOLT-1, NRCC).
All metal concentrations in fish tissues were reported in mg kg−1 dry weight and wet weight for comparison with other authors. Water contents were determined in the sample tissues by weight determination before and after the freeze drying process. Fifteen replicates of each tissue were evaluated with standard deviation smaller than 3%.
Statistical analysis
All statistical tests were performed using Bioestat version 4.0. Summarized data as a mean, standard deviation and ranges.
Results
Fish sampling in Santos Bay showed high values of total length and weight ranging of 188–290 mm and 59.5–148.8 g, respectively. For C. spixii from Cananéia, the ranges to total length and weight were of 175–296 mm and 55.3–152.6 g, respectively. The obtained data indicated only adult individuals according to Rios (2001) value proposed for the first maturation (LC50 = 95.9 mm) of C. spixii. Therefore, above of that value the individuals can be considered as adults.
Trace metal determinations
Methodology validation for metals (Co, Fe, Se and Zn) determination by INAA was carried out by means of the reference material analyses Oyster tissue (NIST SRM 1566b) and Dogfish muscle (DOLT-1, NRCC) showing relative standard deviation from 1.0 to 28% and relative error from 1.0 to 13% (Table 1). The methodology validation for total and methyl-mercury by CV AAS was carried out by means of reference materials analyses showing relative standard deviation from 4.3 to 8.5% and relative error from 0.8 to 9.3%, indicating good precision and accuracy (Table 2). The results indicated good agreement between the certified and the analytical values.
The trace metals Co, Fe, Se, Zn and total mercury (Hg) were determined in liver samples, being that, the latter was also determined in muscle. Methyl-mercury (MeHg) was analyzed in muscle and in liver samples. The average metal concentrations in liver of C. spixii from Cananéia and Santos Bay are given in Table 3. The concentrations of Hg in liver were lower than those of other metals (Table 4). On the other hand, Fe concentrations showed the highest level in examined tissues for Santos Bay and for Cananéia fish. The Brazilian environmental legislation (Anvisa 1998) only considers limit values for toxic inorganic metals such as Pb, Cd, Cu and Hg. Thus, due to the absence of limit values for the analyzed metals (Co, Zn, Fe), the results were compared with C. spixii collected in a non-polluted site (Cananéia estuarine–lagoon complex), with other fish species and considering the international maximum values proposed by EPA (1999).
The results obtained for Co, Fe, Se, Zn and Hg contents in liver of C. spixii are also presented in Fig. 2. For comparison, the maximum values proposed by the Environment Protection Authority (1999), established for human consumption were presented together with each trace metal concentration. Cobalt levels were not compared with the national or international limits due to the absence of values for this metal. In general, it was observed that the concentrations of trace metals in C. spixii specimens exceeded the limit for human use.
Table 5 shows the mean values of some trace metals in liver and muscle for different fish species. The data analyses showed that Co, Zn, and Fe concentrations were greater than those found in other fish from some different areas, in the world.
Comparing fish size and trace metal concentrations (Fig. 2) it is possible to verify that the relation between length and all trace metal concentrations were not significant.
In C. spixii from Cananéia, Co content ranged from 0.50 to 0.88 mg kg−1, with an mean of 0.6 ± 0.14 mg kg−1. For Santos Bay Co concentrations ranged from 0.45 to 1.33 mg kg−1, with an average of 0.85 ± 0.29 mg kg−1 (Fig. 2a).
Fe concentrations in the fish from Santos Bay showed an average of 3,082 ± 975 mg kg−1 ranging from 1,518 to 5,141 ± 124 mg kg−1. The average value obtained was three times larger than allowed by the Environment Protection Authority (1999; 946.29 mg kg−1). For Cananeia, Fe concentrations ranged from 3,419 to 5,501 mg kg−1, with an average of 4,838 ± 580 mg kg−1 (Fig. 2b). However, it is very important to consider the micronutrient function of this metal. Additionally, it should also consider the liver biotransformation function.
The average value observed for Se concentration was 15.39 ± 2.51 mg kg−1 for Santos Bay and 12.73 ± 1.61 mg kg−1 for Cananéia, with a variation from 11.87 to 19.21 and 10.99 to 14.96 mg kg−1, respectively. The selenium concentrations showed values twice as much as allowed by the Environment Protection Authority (1999; 6.76 mg kg−1; Fig. 2c). It is important to consider the relation between Se and Hg because the literature indicates a regulation mechanism among these two trace metals, when the organisms are exposed to a high mercury concentration (Farias et al. 2005).
The zinc concentrations ranged between 107 and 566 mg kg−1 in C. spixii from Santos Bay and 0.95 to 1.21 mg kg−1 in the fish from Cananéia. The average values were of 261 ± 137 and 1.11 ± 0.14 mg kg−1, respectively. For this metal, the Environment Protection Authority (1999) recommends a maximum value of 405.55 mg kg−1. The data showed that Zn concentrations were lower than recommended by EPA (1999; Fig. 2d).
Total Hg concentrations in livers of C. spixii from Santos Bay and Cananéia showed an average of 0.33 ± 0.17 and 0.25 ± 0.001 mg kg−1, respectively. The range observed were 0.10 to 0.92 mg kg−1 to Santos Bay and 0.25 to 0.26 mg kg−1 to Cananéia. The smallest values were found in the muscle, with a variation from 0.01 to 0.11 mg kg−1 to Santos and 0.06 to 0.08 mg kg−1 to Cananéia. However, total Hg concentrations were not significant among this sites (Fig. 2e).
MeHg contents in C. spixii muscles ranging between 0.01 and 0.08 mg kg−1, show an average concentration of 0.05 ± 0.02 mg kg−1. MeHg was not detected in the Cananéia fish (Fig. 2f). There is no limit established for MeHg in fish muscles in the Brazilian environmental legislation. However, the Environment Protection Authority (1999) allows a maximum value of 0.14 mg kg−1. The data show MeHg values below recommended by EPA.
Discussion
The use of fish as biological indicators in pollution monitoring program is highly recommended but not effectively used. Its use for environmental monitoring can provide continuous and reliable information of the environmental quality and can verify the contamination level in the fish consumed by humans. In both cases, a high contamination level can be dangerous for human health (Markert et al. 1999).
Few studies on trace metal concentrations exist for C. spixii tissues. Therefore, it is very difficult to realize a direct comparison of the obtained results in the present work with specimens from the other places. Boldini and Navas-Pereira (1987) and Farias et al. (2005) presented some results of a bioaccumulation process using C. spixii. These authors also did the studies in Santos Bay and Santos/São Vicente estuary. In this study, the authors chose to compare the results obtained in C. spixii from a polluted area (Santos Bay) with fish collected in a non-polluted estuary (Cananéia). Additionally, the data were to compare with other species (Table 5) that have the same habits as the C. spixii.
The trace metals as cobalt, iron and zinc are essential since they play an important role in biological systems (Ansari et al. 2004; Turkmen and Ciminli 2007) because they have a micronutrient function. On the other hand, metals such as mercury are nonessential and have toxic effects (Turkmen and Ciminli 2007). The deficiency of zinc can provoke serious consequences, as decrease of growth and sexual immaturity (Ansari et al. 2004). Some authors consider that the excess of some metals can cause harmful effects in fish as alterations in oxygen consumption, damages in the gills (Furness and Raibow 1990, Zagatto and Bertoletti 2006). There are indications that Se plays an important role in the organism protecting against toxic effects of Hg (Azevedo 2003; Farias et al. 2005) in some environmental conditions.
In the present study, the bioaccumulation processes was evaluated in a benthic feeder species that has an intrinsic association with the bottom sediment and it showed the capacity to be used as a bioindicator. The Co, Fe, Se and Zn concentrations found in C. spixii liver, from Santos Bay showed high values. Although total Hg in liver and muscle and MeHg in muscle showed lower values when compared to other species from different environments (Kakulu et al.1987; Hellou et al. 1992, 1996; Voigt 1999; Farkas et al. 2003; Karadede and Unlϋ 2000; Canli and Atli 2003; Agusa et al. 2005; Dural et al. 2007; Maršálek et al. 2007), significant differences were not observed among fish from Cananéia and Santos Bay, suggesting input absence of total Hg and MeHg in both systems. Additionally, Co, Fe, Se and Zn concentrations in liver from Santos Bay C. spixii were useful as a trace metal diagnosis in the specimens which could be used as a quantitative index for the analyses.
In three species of the Ariidae family (Bagre bagre, C. spixii and Netuma barba), Boldini and Navas-Pereira (1987) obtained Zn and total Hg contents in liver of fish from Santos Bay similar to the values obtained in the present study. The same authors observed total Hg concentrations in muscle tissue lower than that showed in this investigation.
The results in trace metal concentrations obtained in this study showed the capacity of C. spixii to bioaccumulate trace metals from the environment, since most of the found metal contents in fish from Santos Bay were greater than metal contents in fish from Cananéia and the national (Anvisa 1998) and international (EPA 1999) maximum values established for human consumption. Therefore, the data showed that C. spixii is a good bioindicator for trace metal accumulation.
The biometric relations of length versus all trace metal concentrations were not statistically significant (Fig. 2). This result can be influenced by: (1) the selection process in the sampling; (2) the level of efficiency of the fishing equipment in capturing fish of smaller length; (3) the biological characteristics of the species as related to the feeding habits; (4) the degree of association of C. spixii to the sediment and the capacity of metabolization and/or degradation of the pollutants by this species as described by Reuther (1994) and Sinderman (1996).
Metal concentrations can vary in different tissues in function of the biochemical affinities and metabolism. Trace metals accumulation are intense in metabolic organs as liver and kidney. In these organs, the metal concentrations can be 400% higher than the contents found in the muscle tissue (Parsons 1999; Kojadinovic et al. 2007; Tϋrkmen and Ciminli 2007). The results obtained in this study agree with first ones, since mercury levels showed significant differences in the tissues analyzed, accumulating more in the liver than in the muscle. Additionally, the largest Fe contents observed in C. spixii from Cananéia in comparison with Santos Bay can be related to a more effective metabolism on the fish from non-polluted site, considering the role of the Fe in the biological system with micronutrient element.
The use of fish as biological indicators of pollution is efficient when basic biological information and ecological aspects of the environment studied are available (Olsson and Jensen 1975). However, some aspects as abundance, the facility of yearly collection, non-migratory nature, easy identification and capacity to accumulate the contaminant are necessary to select the bioindicator organisms appropriately (Phillips 1977; Phillips and Segar 1986). Thus, it is possible to determine the correlations between the xenobiotic compounds in the environment and concentrations in the bioindicator organism (Markert 1996).
Conclusion
The results obtained in this study and the main requirements previously mentioned, the authors suggest that C. spixii is appropriate as a bioindicator species of trace metal pollution in Santos Bay, being recommended for biomonitoring programs, to evaluate the evolution of the contamination by trace metals.
References
Aguiar VMC, Braga ES (2007) Seasonal and tidal variability of phosphorus along a salinity gradient in the heavily polluted estuarine system of Santos/São Vicente—São Paulo, Brazil. Mar Pollut Bull 54:464–488. doi:10.1016/j.marpolbul.2006.11.001
Agusa T, Kunito T, Yasunaga G, Iwata H, Subramanian A, Ismail A, Tanabe S (2005) Concentrations of trace elements in marine fish and its risk assessment in Malaysia. Mar Pollut Bull 51:896–911. doi:10.1016/j.marpolbul.2005.06.007
Ansari TM, Marr IL, Tariq N (2004) Heavy metals in marine pollution perspective—a mini review. J Appl Sci 4(1):1–20
Anvisa (1998) Legislação Brasileira, Portaria 685. Available in http://www.anvisa.gov.br/legis/portarias/685_98.htm. Accessed 26 Jan 2008
Azevedo FA (2003) Toxicologia do Mercúrio. Rima, São Carlos
Boldrini CV, Navas-Pereira DN (1987) Metais pesados na baia de Santos e estuário de Santos e São Vicente. Ambiente 1(3):118–127
Boon JP, Lewis WE, Choy MR, Allchin CR, Law RJ, de Boer J (2002) Levels of polybrominated diphenyl ether (PBDE) flame retardants in animals representing different trophic levels of the North Sea food web. Environ Sci Technol 36:4025–4032. doi:10.1021/es0158298
Braga ES, Fonseca ALO, Bosquilha GE, Ducatti GMF, Aguiar VMC, Lima CAC, Arasaki E (2003) Eutrophication and bacterial pollution assessment risks on the Santos Bay sandy beaches (Brazil): influence of seasonal conditions. J Coast Res 35:516–524
Bryan GW (1979) Bioaccumulation of marine pollutants. Philos Trans R Soc Lond B 289:273–305
Canli M, Atli G (2003) The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ Pollut 121:129–136. doi:10.1016/S0269-7491(02)00194-X
Cetesb (1979) Qualidade das praias paulistas 1976–1977. Cetesb, São Paulo, 181 p
Cetesb (2001) Sistema estuarino de Santos e São Vicente: Relatório Técnico, Agosto de 2001. CETESB, São Paulo, 178 p
Dural M, Göksu MZL, Özak AA (2007) Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chem 102:415–421. doi:10.1016/j.foodchem.2006.03.001
Eboh L, Mepba HD, Ekpo MB (2006) Heavy metal contaminants and processing effects on the composition, storage stability and fatty acid profiles of live common commercially available fish species in Oron Local Government, Nigeria. Food Chem 97:490–497. doi:10.1016/j.foodchem.2005.05.041
Environmental Protection Agency (EPA) (1999) Integrated risk information system (IRIS) on elemental mercury. National Center for Environmental Assessment, Office of Research and Development, Washington, DC. Available in http://www.epa.gov/reg3hscd/risk/human/index.htm. Accessed 03 Feb 2008
Farias LA, Azevedo J, Fávaro DIT, Saraiva ESBG (2005) Evaluation of mercury, selenium and methylmercury in fish consumed by Santos Bay Communities, São Paulo, Brasil. VII Encontro Nacional de Aplicações Nucleares—VII ENAN, vol 1. Anais do VII Encontro Nacional de Aplicações Nucleares, Santos, pp 1–9
Farkas A, Salánki J, Specziár A (2003) Age- and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. populating a low-contaminated site. Water Res 37:959–964. doi:10.1016/S0043-1354(02)00447-5
Fávaro DIT, Afonso C, Vasconcellos MBA, Cozzolino SMF (2000) Determinação de Elementos MInerais e Traços por Ativação Neutrônica, em refeições servidas no restaurante da Faculdade de Saúde Pública/USP. Cienc Tecnologia Alimentos 20(2):176–182
Figueiredo JL, Menezes NA (1978) Manual de peixes marinhos do sudeste do Brasil. II Teleostei (1). Universidade de São Paulo, Museu de Zoologia, São Paulo, Brazil, 110 p
Fisk AT, Hobson KA, Norstrom RJ (2001) Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the Northwater Polynya marine food web. Environ Sci Technol 35:732–738. doi:10.1021/es001459w
Fúlfaro VJ, Requejo CS, Landim PMB, Fúlfaro R (1983) Distribuição de elementos metálicos nos sedimentos da baía de Santos, SP. Atas do Simpósio regional de Geologia, vol 4. Atas da Sociedade Brasileira de Geologia, São Paulo, pp 275–289
Furness RW, Raibow PS (1990) Heavy metals in the marine environment, 1st edn. CRC, Boca Raton
Grigg RW (1994) Effects of sewage discharge, fishing pressure and habitat complexity on coral ecossystem and reef fishes in Hawaii. Mar Ecol Prog Ser 103:25–34. doi:10.3354/meps103025
Heath AG (1990) Water pollution and fish physiology, 2nd edn. CRC, Boca Raton, Boston
Hellou J, Warren WG, Payne JF, Belkhode S, Lobel P (1992) Heavy metals and other dements in three tissues of cod, Gadus morhua, from the Northwest Atlantic. Mar Pollut Bull 24:452–458. doi:10.1016/0025-326X(92)90345-7
Hellou J, Zitkob V, Friel J, Alkanani T (1996) Distribution of elements in tissues of yellowtail flounder Pleuronectes ferruginea. Sci Total Environ 181:137–146. doi:10.1016/0048-9697(95)04963-0
Homem JCM (1983) Balneabilidade das praias de Santos e São Vicente. In: Seminário sobre uma síntese do conhecimento sobre a Baixada Santista. CETESB, p 61–64
Horvat M (1996) Mercury analysis and speciation in environmental samples. In: Baeyens W et al (eds) Global and regional mercury cycles: sources, fluxes and mass balances. NATO ASI Series, Partnership Sub-Series, 2. Environment, vol 21. Kluwer Academic Publishers, Netherlands, pp 1–31
Islam MS, Tanaka M (2004) Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar Pollut Bull 48:624–649. doi:10.1016/j.marpolbul.2003.12.004
Jones WG, Walker KF (1979) Accumulation of iron, manganese, zinc and cadmium by the Australian freshwater mussel Velesunio ambiguus (Phillipi) and its potential as a biological monitor. Aust J Mar Freshwater Res 30:741–751. doi:10.1071/MF9790741
Kakulu SE, Osibanjo O, Ajayi SO (1987) Trace metal content of fish and shellfishes of the niger delta area of Nigeria. Environ Int 13:247–251. doi:10.1016/0160-4120(87)90136-X
Karadede H, Unlϋ E (2000) Concentrations of some heavy metals in water, sediment and fish species from the Atatϋrk Dam Lake (Euphrates), Turkey. Chemosphere 41:1371–1376. doi:10.1016/S0045-6535(99)00563-9
Karadede H, Oymak SA, Unlϋ E (2004) Heavy metals in mullet, Liza abu, and catfish, Silurus triostegus, from the Atatürk Dam Lake (Euphrates), Turkey. Environ Int 30:183–188. doi:10.1016/S0160-4120(03)00169-7
Kojadinovic J, Potier M, Le Corre M, Cosson RP, Bustamante P (2007) Bioaccumulation of trace elements in pelagic fish from the Western Indian Ocean. Environ Pollut 146:548–566. doi:10.1016/j.envpol.2006.07.015
Lacerda LD (1998) Biogeochemistry of trace metals and diffuse pollution in mangrove ecosystems. Mangrove ecosystems occasional papers no. 2. International Society for Mangrove Ecosystems, Okinawa, p 65
Marcovecchio JE (2004) The use of Micropogonias furnieri and Mugil liza as bioindicators of heavy metals pollution in La Plata river estuary, Argentina. Sci Total Environ 323:219–226. doi:10.1016/j.scitotenv.2003.09.029
Markert B (1996) Instrumental element and multielement analysis of plant samples—methods and applications. Wiley, Chichester, p 263
Markert B, Wappelhorst O, Weckert V, Herpin U, Siewers U, Friese K, Breulmann G (1999) The use of bioindicators for monitoring the heavy-metal status of the environment. J Radioanal Nucl Chem 240(2):425–429. doi:10.1007/BF02349387
Maršálek P, Svobodová Z, Randák T (2007) The content of total mercury and methylmercury in common carp from selected Czech ponds. Aquacult Int 15:299–304. doi:10.1007/s10499-007-9076-3
Montone RC (1987) Hidrocarbonetos clorados no litoral do estado de São Paulo. MSc Dissertation, Instituto Oceanográfico, Universidade de São Paulo, 102 p
Olsson M, Jensen S (1975) Pike as the test organism for mercury, DDT and PCB pollution: a study of the contamination in the Stockholm Archipelago. Inst Fresh Res 54:20
Otaway NM, Gray CA, Craig JR, McVea TA, Ling JE (1995) Assessing the impacts of deepwater sewage disposal: a case study fro South Wales, Austrália. Mar Pollut Bull 33(4–12):347–354
Palheta D, Taylor A (1995) Mercury in environmental and biological samples from a gold mining area in the Amazon region of Brazil. Sci Total Environ 168:63–69. doi:10.1016/0048-9697(95)04533-7
Parsons ECM (1999) Short communication: trace element concentrations in whole fish from North Lantau waters, Hong Kong. J Mar Sci 56:791–794
Phillips DJH (1977) The use of biological indicator organisms to monitor trace metals in marine and estuarine environments—a review. Environ Pollut 13:282–317
Phillips DJH, Segar DA (1986) Use of bio-monitors in monitoring conservative contaminants, programme design imperatives. Mar Pollut Bull 17:10–17. doi:10.1016/0025-326X(86)90797-6
Porvari P (1995) Mercury levels of fish in Tucuruí hydroelectric reservoir and in River Mojú in Amazonia, in the state of Pará, Brazil. Sci Total Environ 175:109–117. doi:10.1016/0048-9697(95)04907-X
Puffer HW, Azen SP, Duda MJ (1982) Sportfishing activity and catches in polluted coastal regions of metropolitan Los Angeles. N Am J Fish Manage 1:74–79. doi:10.1577/1548-8659(1982)2<74:PHHFCO>2.0.CO;2
Reddy ML, Reif JS, Bachand A, Ridgway SH (2001) Opportunities for using Navy marine mammals to explore associations between organochlorine contaminants and unfavorable effects on reproduction. Sci Total Environ 274:171–182. doi:10.1016/S0048-9697(01)00741-0
Reuther R (1994) Mercury accumulation in sediments and fish from rivers affectedby alluvial gold mining in the Madeira river basin, Brazil. Ambio 19:11–15
Rios EP (2001) Papel do estuário no ciclo de vida das espécies dominantes da ictiofauna do Complexo Estuarino-lagunar de Cananéia-Iguape. M.Sc. Dissertation, Universidade de São Paulo, São Paulo, p 128
Salomons W, Förstner U (1984) Metals in the hydrocycle. Springer, Heidelberg, p 337
Sinderman CJ (1996) Ocean pollution: effects on living resources and humans. CRC Press, Boca Raton, p 275
Skoog DA, Holler FJ, Nieman N (2002) Princípios de Análise Instrumental, 5th edn. Bookman, São Paulo
Turkmen M, Ciminli C (2007) Determination of metals in fish and mussel species by inductively coupled plasma-atomic emission spectrometry. Food Chem 103:670–675
Voigt HR (1999) Concentrations of heavy metals in fish from coastal waters around the Baltic Sea (extended abstract). ICES J Mar Sci 56:140–141. doi:10.1006/jmsc.1999.0623
Zagatto PA, Bertoletti E (2006) Ecotoxicologia Aquática: Princípios e Aplicações. Rima Editora, São Carlos, p 464
Acknowledgments
The authors thank the R/v Albacora crew from Oceanographic Institute of University of São Paulo, for their help in the fieldwork and appreciate the financial support of the São Paulo Foundation for Research Support—FAPESP (Process 2005/50769-2) and Brazilian Agencies for Science and Technology—CAPES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azevedo, J.S., Fernandez, W.S., Farias, L.A. et al. Use of Cathorops spixii as bioindicator of pollution of trace metals in the Santos Bay, Brazil. Ecotoxicology 18, 577–586 (2009). https://doi.org/10.1007/s10646-009-0315-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0315-4