Abstract
Sediment and porewater samples from an enclosed bay receiving stormwater discharge (Skutviken) near the centre of Luleå, northern Sweden were analysed for major and trace elements and 16 polycyclic aromatic hydrocarbons (PAHs). Among the studied metals Cd, Cu, Pb and Zn were enriched at Skutviken. Also, the PAH content was enriched, in particular for phenantrene, anthracene, fluoranthene and pyrene which are regarded as common constituents in stormwater. The use of trace metal ratios provided indications about pollutant sources for the sediment. Cs-137 dating was used to determine historical changes in metal and PAH fixation in the sediment. The bay Skutviken is enclosed through the construction of a road bank since 1962. The enclosure led to reduced water circulation in the bay that promotes the occurrence of anoxic conditions with sulphate reduction within the bay. As a consequence of these conditions, metals are trapped in the sediments as sulphides. This study suggests that enclosed bays with restricted water circulation may be efficient traps for urban pollutants, reducing the present-day input of pollutants to the sea. In areas with postglacial land uplift, where such bays are common, bay sediments are a potential future source of pollutants when uplift results in erosion and oxidation of the sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Urban hydrosphere and pedosphere are parts of an urban natural system (Endlicher 2004), which is intensely affected by human activities. In 2008, for the first time in history, more than half the human population in the world lives in urban areas, possibly increasing to 80% in 2030 (UNFPA 2007). In Sweden, today 84% of the population already lives in urban areas (Statistics Sweden 2006), and thus this environment and its own “natural driving forces and patchwork patterns” (Endlicher and Simon 2005) for the society are very important.
Rivers are important for both natural systems and human societies (Simmons 1991). Hauer and Lamberti (2006) use the term riverscape to describe the “expansive view of a stream or river and its catchment, including natural and cultural attributes and interactions”, which may change with time.
In a riverscape, surface waters and groundwater as well as sediments and soils will be affected by stormwater discharge, which is an important contamination source for trace metals and polycyclic aromatic hydrocarbons (PAH) (Brown and Peake 2006). Accumulation of metals and organic pollutants in recipients are a risk for living organisms (Wildi et al. 2004; Munch Christensen et al. 2006). Kayhanian et al. (2008) report grab and composite samples from urban highway runoff in Los Angeles to be toxic on freshwater and marine species, where in general the first samples taken during a storm event were found more toxic than those collected later. Previous studies of stormwater and gully pot sediments in Luleå in northern Sweden (Westerlund 2007; Karlsson and Viklander 2008a) indicated particle-related transport of metal and organic pollutants with seasonal variations. McKenzie et al. (2008) point out that trace metals from anthropogenic sources were enriched together with stormwater transported particles, where enrichment increased with decreasing particle size.
The objective of this study was to investigate how an enclosed bay of the Lule River in northern Sweden affects the transport of urban metal and organic pollutants to the nearby Lule River estuary. The objective is based on two hypotheses: (1) that metals are trapped as sulphides in the bay sediment and (2) that sediment grain size may be important for the sequestering of organic pollutants. To define the urban impact in Skutviken, its sediment and porewater geochemistry was compared with a reference sampling site unaffected by stormwater discharge.
2 Materials and Methods
2.1 Sampling Site
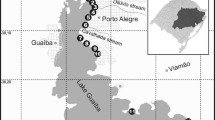
The bay Skutviken (Fig. 1) is located north of the city centre of Luleå (73,000 inhabitants) in northern Sweden. The most characteristic hydrodynamic patterns of Luleå are the Lule River and former shallow bays of the brackish Bothnian Bay, which are partially enclosed due to the postglacial rebound (8–9 mm a−1 (Lindén et al. 2006)) or artificial banks. The Lule River enters the Bothnian Bay passing the centre of Luleå. The 25,000-km2 large catchment area of the 460-km-long river has an annual average discharge of around 500 m3 s−1 (Raab and Vedin 1995). However, the water bodies situated close to Luleå are also affected by smaller local catchments, which contain urbanised and industrial areas as well as rural and forested areas (Erixon 1996; Hübinette 1998; Olofsson 2002).
The surface area of Skutviken is ∼12 ha, and the mean and maximum depths of the bay are 1.6 and 3.4 m, respectively. It is separated from the Lule River by a ca 360-m-long road bank constructed in 1962 (Fig. 1). At the southern end of the road bank, a channel (8 m in width, 3 to 4 m in depth and 35 m in length) through the bank permits a limited water exchange with the Lule River. These physical conditions give the bay similarities with shallow naturally enclosed bays in the region. The bay is surrounded by the road bank and one more highly frequented road with traffic intensities of 23,500 and 13,600 vehicles per day, respectively (Luleå Kommun 2007). The sewer drainage area contains 0.53-km2 industrial area and 0.73-km2 housing area (Fig. 1).
Since parts of the road bank runoff and six stormwater channels enter the bay, it almost functions as a large stormwater pond where a high amount of stormwater sediment is trapped, resulting in a reduced sediment supply to the Lule River. All outlets are located below the water surface, except during periods of very low water level. A reference sampling site was chosen beside the main stream of the Lule River in front of the spit Gültzauudden (Fig. 1).
The annual precipitation in the Luleå area is about 500 mm of which 40% to 50% falls as snow between November and April/May (Hernebring 1996), and is thus discharged during snowmelt. From November until May the Lule River and the bays close to the city centre are ice covered.
2.2 Sampling
The sampling station in Skutviken was chosen in the deeper part of the bay with fine grained sediment. Sediment cores (25–30 cm long) were collected from Skutviken and Gültzauudden in March 2007 and 2008 using a Kajak gravity corer with a core tube diameter of 64 mm. Sampling was performed from the winter ice, and the sediment core surfaces were judged to be undisturbed (no resuspended sediment in core tubes and apparently undisturbed surface sediment structures). The cores were sectioned in subsamples (0.5 cm thick for the uppermost 3 and 1 cm thick until the core ends). For porewater analyses, the sediment samples were put into plastic bags immediately after core collection and sectioning in the field. All air was pressed out of the bag before it was placed in an Ar-filled container to keep the sediments in an oxygen free environment until the porewater was extracted within the following 6 h. The porewater was separated by vacuum filtration (0.22 μm Millipore® membrane filters) arranged in an Ar-flushed glove box. The porewater samples were collected in 60-ml acid-washed polyethylene bottles and refrigerated until further analysis. Bottom water was sampled from the core tube immediately after retrieval, 3 cm above the sediment surface. The water was drawn with a small plastic tube fixed on a syringe and filtered through a 0.22 μm Millipore® membrane filter.
2.3 Analytical and Chemical Analyses
The total carbon (TC) and total nitrogen (TN) of the sediment was analysed by Umeå Marine Sciences Centre. Analyses of carbon and nitrogen in sediments were performed with a Carlo Erba model 1108 high temperature combustion elemental analyzer, using standard procedures and a combustion temperature of 1,030°C. For standardisation Acetanilide was utilised.
Metal and PAH analyses were accomplished by the accredited laboratory ALS Scandinavia AB in Luleå. The sediment and porewater was analysed for major elements and trace metals. Sediment samples for determination of As, Cd Co, Hg, Ni, Pb and S were dried at 50°C and digested in a microwave oven in closed Teflon bombs with a nitric acid: water ratio of 1:1. For other elements, 0.125 g dried matter was melted with 0.375 LiBO2 and dissolved in HNO3. Metal determinations were made by inductively coupled plasma atomic emission spectrometry (ICP-AES) and inductively coupled plasma mass spectrometry. To the porewater samples 1 ml nitric acid (suprapur) was added per 100 ml. Analyses were made with ICP-AES and inductively coupled plasma sector field mass spectrometry. The following 16 PAHs were analysed in the sediment: naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, dibenz(a,h)anthracene, benzo(ghi)perylene and indeno(1, 2, 3-cd)pyrene. The PAH sediment samples were leached with acetone/hexan/cyclohexan (1:2:2), and measurements were performed with gas chromatography mass spectrometry.
Particle size analyses were performed with a Cilas 1064 laser diffraction particle size analyser in wet mode for four samples from a profile at Skutviken and a profile at Gültzauudden.
Water fraction and porosity were determined through weighing before and after drying the sediment at 50°C for at least 7 days. The dissolved oxygen in the water column was determined with a Hydrolab® MiniSonde 5 water quality probe.
Radionucleide activity of 137Cs (mean standard deviation ± 5%) was determined by gamma spectrometry at Risø National Laboratory for Sustainable Energy, Denmark.
3 Results and Discussion
3.1 Sediment Characteristics
3.1.1 Particle Size and Sedimentation Rate
The particle size analyses showed that the 2–3 and 5–6 cm layers at both sites had very similar particle size distribution (Fig. 2). The main components (60% cumulative volume) in these layers had a grain size from 10 to 30 μm. At Skutviken, the 10–11 cm sample contains the overall finest sediment with 70% accumulated volume in particle size 2–10 μm. The 15–16-cm-layer particle size distribution at Skutviken falls between the two uppermost and third layer with respect to particle size. At Gültzauudden the 10–11-cm layer contains the finest material at this site with 60% cumulative volume containing grain size 5–11 μm. The deepest sample (15–16 cm) shows the coarsest grain composition with 60% cumulative volume consisting of material with the grain size 20–100 μm.
The activity of the radionuclide 137Cs shows 2 peaks (Fig. 3). The upper peak 4 cm upwards is interpreted to represent the Chernobyl fallout from the reactor accident in April 1986 (Ilus and Saxén 2005), while the lower peak is interpreted to be caused by the fallout from nuclear weapons testing in the early 1960s (Appleby 2002). However, this peak should be concurrent with the construction of the road bank in 1962, and may be displaced slightly downward in the sediment due to reworking of sediments during construction works. Caesium-137 data indicate that changes in sediment characteristics (particle size, concentrations of TC, TN, metals and PAHs) from 11 cm and upwards became apparent in the early 1960s.
3.1.2 Redox Conditions
Dissolved oxygen was measured in the water column to provide information on the redox conditions at the sediment-water interface. At Skutviken the oxygen saturation in the water 10 cm above the sediment surface is close to 0% in wintertime, when the bay is ice covered. In contrast, the water column is well oxygenated (saturation 85–90%) during the ice free season. These changing redox conditions can affect release or accumulation of pollutants through formation/dissolution of Fe–Mn oxyhydroxides in the surface sediment.
Sediment cores contain information about past and present processes in the sediment. It is possible to follow element concentrations back in time, assuming the stratigraphy is undisturbed. At Gültzauudden, the high Mn content in the sediment top layers (Fig. 4) can be related to the oxic environment at this site where Mn occurs mostly as Mn oxyhydroxides (Davison 1993). The decomposition of organic material and increasingly anoxic environment with sediment depth results in reduction of Mn oxyhydroxides and increased porewater concentration of Mn(II). A breaking point for the Mn in the solid phase is reached at 4 cm depth where the MnO content stabilises at 0.2%. Together with the increasing porewater Mn concentration, this suggests that anoxic conditions predominate below 4 cm. The porewater profile indicates Mn(II) flux upward, resulting in the oxidation of Mn(II) to Mn(IV) in the oxic parts of the sediment (Davison 1993; Wehrli 1991) (Fig. 4). The sediment content and porewater concentration of Fe at Gültzauudden comply with the Mn observations. The Fe2O3 peak in the sediment profile is situated below the MnO peak. In oxic sediment Fe occurs as Fe(III) in iron oxyhydroxides, resulting in a solid Fe peak at 3 cm depth. Below 5 cm the solid Fe content declines continuously. The porewater Fe concentration indicates that reduction of solid Fe(III) to the soluble Fe(II) occurs when porewater becomes more anoxic (Davison 1993, Wehrli 1991).
At Skutviken the Mn and Fe sediment and porewater concentrations differ from those at Gültzauudden (Fig. 4). The MnO content in the sediment is much lower than at Gültzauudden in the upper part of the sediment. The geochemical conditions where Mn(IV) is reduced to Mn(II) appear to be reached already in the bottom water above the sediment surface. During winter, when the bay is ice covered, the oxygen concentration in the bottom water is <0.42 mg l−1. In the porewater, Mn concentrations increase with depth but never reach as high concentrations as at Gültzauudden.
The presence of a solid Fe2O3 maximum at the sediment surface at Skutviken indicates that the redox conditions permit precipitation of Fe(III) hydroxides at the sediment-water interface. The same anoxic conditions that occur at a depth of 3 cm in the sediment at Gültzauudden seem to occur already above the sediment column in Skutviken, with reductive dissolution of Fe hydroxides taking place already at the sediment surface. The decrease of total S in porewater at Skutviken suggests that reduction of SO 2−4 occurs immediately below the sediment-water interface (0–2 cm). The simultaneous increase of solid S indicates precipitation of solid sulphides in the sediment (Fig. 5). The solid S concentration at 0.5–11 cm depth (2,500–4,200 mg kg−1) exceed that at Gültzauudden by a factor of 5–7.
3.1.3 Element/Al Ratios in the Sediment Profiles
Regional element/Al ratios have been found to be relatively constant in sediment, also when sediment grain size changes and sedimentation rates vary (Hirst 1962; Loring 1991; Ebbing et al. 2002). In the sampled sediments the element/Al ratios for the major elements Ti, Ca, Mg, Na and K are similar at Skutviken and Gültzauudden, with only small deviations from local till ratios for Ca/Al, Na/Al and K/Al (Table 1). This indicates that both sediments mainly are composed of local minerogenic matter. In the 1–7 cm section, the Fe/Al and the Mn/Al ratios are higher at Gültzauudden (Table 1), suggesting precipitation of Fe–Mn oxyhydroxides in a more oxic environment (Davison 1993).
Peinerud et al. (2001) used the Si/Al ratio of lake sediments as a measure of the diatom concentration. In the two sampled cores the Si/Al ratio is even lower than that of local till (Öhlander et al. 1991), suggesting a negligible content of diatoms at both sampling sites (Table 1).
3.2 Total Carbon and Nitrogen in Sediments
TC at both sites shows high concentrations in the surface sediment and a decrease with depth (Fig. 6). At Skutviken the concentration in the upper sediment segment (1–7 cm) is 4–5%, which is significantly higher than at Gültzauudden (1–2.5%). At Skutviken TC decreases sharply below 7 cm depth to ca 1% at 10 cm depth, from where on the TC concentration is approximately constant. The content of TN follows a similar pattern as for TC at both sample sites (Fig. 6). The TC/TN molar ratio indicates a change in sediment composition at Skutviken from 7 to 11 cm depth, where the TC/TN ratio decreases from 19 to 11. Below 11 cm depth the TC/TN ratio of both sites are similar. Above 11 cm, the concentration of organic material is enriched at Skutviken which is consistent with low oxygen saturation above the sediment in wintertime. The TC/TN molar ratio is thereby higher than the C/N ratio of 6.6 in the Redfield empirical formula ((CH2O)106(NH3)16(H3PO4)) (Redfield 1958), which indicates an anthropogenic impact.
3.3 Trace Elements in Sediments Compared with Reference Values
For the sediment section 0–2 cm the detected contents of As, Cd, Co, Cr, Cu, Hg, Ni, Pb and Zn can be compared with reference values for coastal sediment from the Swedish Environmental Protection Agency (Swedish EPA 1999) and a deviation value can be determined by dividing the sediment content value with the reference value (Table 2). According to Swedish EPA (1999), the deviation values for Cu (3.63) and Zn (2.98) at Skutviken are classified as “large”, while the deviation at Gültzauudden only shows “slight” difference from the reference value. Cadmium (3.09) and Pb (1.87) appear with a “significant” deviation at Skutviken. Cadmium, Cu, Pb and Zn are of main concern in urban stormwater (Hvitved-Jacobsen and Yousef 1991). Thus, a significant influence of stormwater sediment can be assumed for these four metals in Skutviken, while at Gültzauudden no effect can be seen for any of the studied elements.
3.4 Trace Elements in the Sediment and Porewater
Cadmium, Cu, Pb and Zn concentrations in porewater and sediment are shown in Figs. 7 and 8. At Skutviken, these elements show almost identical sediment profiles with the highest concentrations above 6 cm depth, with exception of the surface sediment. At Gültzauudden, the Cd, Cu, Pb and Zn profiles are different. The contents of Cd and Pb are three times higher and Cu and Zn six times higher in the 0.5 to 6 cm section at Skutviken compared with Gültzauudden.
Cd, Cu and Pb in sediment (mg kg−1) and Cd, Cu and Pb in porewater (μg l−1) at Skutviken and Gültzauudden. The top value for “porewater” represents the bottom water (3 cm above sediment surface). At Gültzauudden Cd was only detectable in porewater at 0–0.5 cm sediment depth (detection level 0.01 μg l−1)
At Skutviken, low values were detected for Cd, Cu, Pb and Zn in the uppermost layer in the solid sediment (0–0.5 cm). The bottom water contents of these elements are below the porewater contents in the uppermost sediment. Porewater maxima at or below the sediment surface indicate element transfer from the solid sediment to the porewater for Cd, Pb and Zn (Figs. 7 and 8). The porewater minima for the elements from 0.5 to ∼5 cm for the elements indicate a sink in the sediment. From 0.5 to ∼5 cm depth Cd, Cu, Pb and Zn show maxima in the solid sediment, coinciding with maxima for solid S, TC and the TC/TN ratio (Figs. 5 and 6). The change in concentrations of Cd, Cu, Pb and Zn at Skutviken around 6 cm depth accompanies a change in the composition of the sediment. The particle size distribution at Skutviken was similar for the upper two analysed layers (2–3 and 5–6 cm). For both layers, the content of particles >10 μm is about 60%, while for the sample from 10 to 11 cm depth the content >10 μm is 15%. Coarser particles in the upper sediment column and higher TC suggest that elements with higher contents in the upper sediment column may be more related to organic components than mainly to clay minerals. Also the TC/TN ratio indicates a change in sediment composition at Skutviken between 7 and 11 cm depth. The high fraction of TC represents mostly organic compounds which decompose slowly in the upper 7 cm of the sediment at Skutviken, since at this depth, anoxic conditions exist in the sediment column.
The S decline in porewater in the upper sediment at Skutviken signifies sulphate reduction and coeval sulphide formation in the solid sediment (Fig. 5). The enrichment of Cd, Cu, Pb and Zn in the sediment at 0.5 to ∼5 cm depth may thus be related to sulphide formation in the organic rich 1–7 cm section of the sediment. Below 6 cm the sediment contents of Cd, Cu, Pb and Zn decline rapidly, and stabilise at a much lower value than in the 0.5 to ∼5 cm section (Figs. 7 and 8). If organic compounds act as carriers of trace elements, they can also contribute to the enrichment of Cd, Cu, Pb and Zn in the upper 7 cm of the Skutviken sediment (Charlesworth and Lees 1999).
At Gültzauudden, the sediment and porewater profiles of As resemble those of Fe (Fig. 4), and appear to be coupled to the redox cycling of Fe. Porewater concentrations of As are low in the oxidised surface layer (0–2.5 cm), and a solid As maximum of ∼40 mg kg−1 occurs at 3.5 cm depth in the sediment. At Skutviken, where anoxic conditions prevail in the sediment, only a slight increase in porewater As up to 5–8 μg l−1 occurs below 2 cm depth, and no solid maximum of As occurs in the sediment (Fig. 8).
The correlation of the trace elements Cd, Cu, Pb and Zn with S shows a uniform pattern where the trace element content increases with higher S content (excluding two samples from 6 to 11 cm depth the correlation coefficient is 0.98 for Cd, Cu, Pb and Zn) (Fig. 9). Two points with high S concentrations deviate from the main trend. These are situated in the 6–11 cm depth section, where the Cd, Cu, Pb and Zn concentrations change rapidly. The trace elements Cd, Cu, Pb and Zn are also positively correlated with TC (Fig. 10) for the samples from 0.5 cm to 21 cm (correlation coefficient for Cd, 0.99; Cu, 0.98; Pb, 0.97; and Zn, 0.99). Only the 0–0.5-cm layer with the highest TC content does not fit into this pattern. It is unclear whether organic matter is a carrier for Cd, Cu, Pb and Zn, or whether this pattern reflects a coupling between organic matter and sulphide formation in the sediment.
3.5 PAH Content in the Sediment
In general the most abundant PAHs in stormwater are phenantrene, anthracene, fluoranthene and pyrene (Gonzalez et al. 2000; Brown 2002), which are classified as priority pollutants by the US Environmental Protection Agency (US EPA) (ATSDR 1995). All of them are found in high to very high concentrations in the 0–2 cm sediment layer at Skutviken. In the 14–16 cm, only pyrene shows high contents. At Gültzauudden the PAH contents do not exceed moderately high contents (Tables 3). As found by Marsalek (1997) and Gonzalez et al. (2000), PAHs are correlated to suspended solids and according to Krein and Schorer (2000), heavy PAHs (four to six benzo rings) are enriched in the fine and fine-middle silt phase of road runoff and light PAHs correlated with fine sand.
At Skutviken the particle size analysis for the 2–3 and 5–6 cm layers showed a range from fine to coarse silt, offering conditions for light and heavy PAHs to be associated with the sediment particles. In the upper 7 cm sediment section at Skutviken the TC content is permanently high around 5% suggesting a possible coupling to the presence of PAHs (Menzie et al. 2002).
3.6 Stormwater Impact and Possible Sources of Contamination
Characteristic metals in stormwater like Cu, Cd, Pb and Zn (Hvitved-Jacobsen and Yousef 1991) are significantly enriched at Skutviken compared with the reference sampling site at Gültzauudden. The mean concentrations of Cu, Pb and Zn are with 60, 67 and 287 mg kg−1, respectively, in the uppermost 6 cm of the sediment at Skutviken in the range of the metal concentrations reported in street sediment on the road bank that separates Skutviken from the Lule River (Viklander 1998) while the metal concentrations reported in the gully pots are lower than in the Skutviken bay (Karlsson and Viklander 2008b). A reason for this might be that most metals, with concentrations higher in the Skutviken sediment than in the gully pots, are attached to smaller particles. Gully pots are relatively poor in retaining small particles (Sartor and Boyd 1972). Compared with the Swedish EPA (2000), the Skutviken sediment is classified as class 3 for Cd, and Pb (biological effects can be found), and class 4 for Cu and Zn (enhanced risk for biological effects). The concentration of metals in the sediment at Skutviken was higher than found by Schiff and Bay (2003), in Santa Monica bay, USA, while it was in the same range as in an urban stream in Denmark, where Munch Christensen et al. (2006) found that sediment and porewater were toxic to algae. Assuming that the sediment above a depth of 6–7 cm represents the time period after construction of the road bank, stormwater impact appears to have increased the concentrations of Cd, Cu, Pb and Zn by a factor of 3–4 (Figs. 7 and 8). However, these metals are probably present as relatively immobile metal sulphides.
The use of trace element ratios can help to identify the potential sources of these contaminants. The ratios for Pb/Zn, Hg/Zn, Cd/Zn, Cu/Zn, Ni/Zn and As/Zn in the Skutviken sediment are comparatively constant with depth from 0.5 to 5 cm. Except for Hg, all ratios change below 5 cm sediment depth (Fig. 11). In the upper 5 cm the Pb/Zn ratio follows the ratio for gully pot sediment from a road. For the Cr/Zn ratio a change below 5 cm depth to higher Cr impact for the Skutviken sediment can be noticed, while the 0.5 to 5.5 cm section has a ratio close to both gully pot ratios. Even though the gully pot sediment contains more coarse particles than the Skutviken sediment, similarities for the trace element ratios are evident in Fig. 11. If gully pots are an interim storage also for clay and silt (Morrison et al. 1988), similar ratios can indicate the stormwater particle transport chain. The pollutants that are linked to the clay and silt fraction pass through gully pots and eventually reach the bay. These particle fractions also offer surfaces for PAHs to bind to (Evans et al. 1990).
Trace element/Zn ratios of Skutviken sediment compared with gully pot sediment (<2,000 μm) from a housing area and road in Luleå (Karlsson and Viklander 2008b)
In floodplain sediments from the Rhine Valley deposited over the last 170 years, the vertical distribution profiles of PAHs are similar to those of the heavy metals Cr, Cu, Pb and Zn (Gocht et al. 2001). Even though the analysed sediment at Skutviken was accumulated over a shorter time period, the PAH profiles resemble in this case those of Cd, Cu, Pb and Zn, with high concentrations in the upper sediment and lower beneath. This suggests a common stormwater origin for PAHs and trace metals. The anoxic conditions in the Skutviken sediment hamper biological activity and reduce the degradation of organic matter, which results in accumulation of organic matter (Canfield et al. 1993). Dissipation of PAHs is less efficient (and limited to three-ring PAHs) in anoxic sediments when oxidation of organic matter is coupled with the microbial reduction of manganese, iron and sulphur (Quantin et al. 2005). As a consequence, PAHs can accumulate with organic matter. PAH affinity to fine particles is known from other studies (Budzinski et al. 1997; Krein and Schorer 2000) and seems certain for this study where the sediments are mostly covering the silt fraction at Skutviken.
4 Conclusions
Skutviken has functioned as a large stormwater pond since the road bank was constructed in 1962, with calm conditions within the bay and a limited water exchange with the Lule River. This has resulted in a spatial arrangement of the sediment supply, with coarse sand near the stormwater channels and in particular silt and clay in the deeper central parts of the bay.
The stormwater contaminations have resulted in increased concentrations of Cd, Cu, Pb and Zn in the upper 7 cm of the sediment. Also the PAH concentrations are very high for pyrene and high for phenanthrene, anthracene, fluoranthene, benzo(a)anthracene, chrysene, benzo(k)fluoranthene and benzo(a)pyrene in the surface sediment at Skutviken.
An increased settling of particulate matter and seasonal occurrence of anoxic bottom waters leading to sulphate reduction appear to be the main effects of the road bank. Sedimentation of pollutant carriers and the sulphate reduction result in an increased fixation of metals and PAHs in the sediment. Skutviken appears to be an efficient trap for stormwater contamination since the sediment at Gültzauudden is almost unpolluted.
The analysis of the trace element and PAH concentrations in the sediment of a stormwater-receiving bay and a reference sampling site compared with road runoff sediment enabled to identify the stormwater as an impact factor on the bay. The sediment shows increased contamination of pollutants which most likely originate from stormwater. Fixation of pollutants in the sediment occurred for the last ∼50 years after the building of a road bank.
This study suggests that enclosed bays with restricted water circulation may be efficient traps for urban pollutants. As a consequence, the present-day input of pollutants to the sea are reduced. In areas with postglacial land uplift, where such bays are common, bay sediments are a potential future source of pollutants when uplift results in erosion and oxidation of the sediments.
References
Appleby, P. (2002). Chronostratigraphic Techniques in Recent Sediments. In W. M. Last & J. P. Smol (Eds.), Tracking environmental change using lake sediments. Vol. 1, Basin analysis, coring, and chronological techniques (pp. 171–203). Dordrecht: Kluwer.
ATSDR (1995). Toxicology profiles for polycyclic aromatic hydrocarbons. Agency for Toxic Substances and Disease Registry, US Departement of Health and Human Services, Atlanta, GA
Brown, J. N. (2002). Partitioning of chemical contaminants in urban stormwater. Dunedin, New Zealand: University of Otago.
Brown, J. N., & Peake, B. M. (2006). Sources of heavy metals and polycyclic aromatic hydrocarbons in urban stormwater runoff. The Science of the Total Environment, 359, 145–155.
Budzinski, H., Jones, I., Bellocq, J., Piérard, C., & Garrigues, P. (1997). Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Marine Chemistry, 58, 85–97.
Canfield, D. E., Thamdrup, B., & Hansen, J. W. (1993). The anaerobic degradation of organic matter in Danish coastal sediments: Iron reduction, manganese reduction, and sulfate reduction. Geochimica et Cosmochimica Acta, 57, 3867–3883.
Charlesworth, S. M., & Lees, J. A. (1999). The Transport of particulate-associated heavy metals from source to deposit in the urban environment, Coventry, UK. The Science of the Total Environment, 235, 351–353.
Davison, W. (1993). Iron and manganese in lakes. Earth Science Reviews, 34, 119–163.
Ebbing, J., Zachowicz, J., Uscynowicz, S., & Laban, C. (2002). Normalization as a tool for environmental impact studies: the Gulf of Gdansk as a case study. Baltica, 15, 49–62.
Endlicher, W. (2004). Die Stadt als natürliches System. Berliner Geografische Arbeiten, 97, 33–38.
Endlicher, W., & Simon, U. (2005). Editorial: Perspectives of Urban Ecology—The Metropolis of Berlin as a Natural and Socioeconomic System. Die Erde, 136, 97–102.
Erixon, P. (1996). Luleå innerfjärdar: rapport A: Vattenkvalitet, bottenkvalitet, vegetation. Luleå, Högskolan i Luleå. Avdelningen för ekologi och miljövård.
Evans, K. M., Gill, R. A., & Robotham, P. W. J. (1990). The PAH and organic content of sediment particle size fractions. Water, Air, and Soil Pollution, 51, 13–31.
Gocht, T., Moldenhauer, K.-M., & Püttmann, W. (2001). Historical record of polycyclic aromatic hydrocarbons (PAH) and heavy metals in floodplain sediments from the Rhine River (Hessisches Ried, Germany). Applied Geochemistry, 16, 1707–1721.
Gonzalez, A., Moilleron, R., Chebbo, G., & Thévenot, D. R. (2000). Determination of polycyclic aromatic hydrocarbons in urban runoff samples from the “Le Maraisâ” experimental catchment in Paris centre. Polycyclic Aromatic Compounds, 20, 1–19.
Hauer, F. R., & Lamberti, G. A. (2006). Methods in stream ecology. Amsterdam: Elsevier.
Hernebring, C. (1996). Snösmältningspåverkan på avloppssystem inom urbana områden (Snowmelt Induced Runoff in Sewer Systems). Stockholm, Sweden: VA-Forsk, Swedish Water and Wastewater Association (VAV).
Hirst, D. M. (1962). The geochemistry of modern sediments from the Gulf of Paria–I The relationship between the mineralogy and the distribution of major elements. Geochimica et Cosmochimica Acta, 26, 309–334.
Hvitved-Jacobsen, T., & Yousef, Y. A. (1991). Highway Runoff Quality, Environmental Impacts and control. In R. S. Hamilton & R. M. Harrison (Eds.), Highway pollution. Netherlands: Elsevier.
Hübinette, H. (1998). Närsaltläckage från Björsbyfjärdens avrinningsområde. Luleå, Sweden: Luleå University of Technology.
Ilus, E., & Saxén, R. (2005). Accumulation of Chernobyl-derived 137Cs in bottom sediments of some Finish lakes. Journal of Environmental Radioactivity, 82, 199–221.
Karlsson, K., & Viklander, M. (2008a). Polycyclic aromatic hydrocarbons (PAH) in water and sediment from gully pots. Water, Air, and Soil Pollution, 188, 271–282.
Karlsson, K., & Viklander, M. (2008b). Trace metal composition in water and sediment from catch basins. Journal of Environmental Engineering, 134, 870–878.
Kayhanian, M., Stransky, C., Bay, S., Lau, S. L., & Stenstrom, M. K. (2008). Toxicity of urban highway runoff with respect to storm duration. Science of The Total Environment, 389, 386–406.
Krein, A., & Schorer, M. (2000). Road runoff pollution by polycyclic aromatic hydrocarbons and its contribution to river sediments. Water Research, 34, 4110–4115.
Lindén, M., Möller, P., Björck, S., & Sandgren, P. (2006). Holocene shore displacement and deglaciation chronology in Norrbotten, Sweden. Boreas, 35, 1–22.
Loring, D. H. (1991). Normalization of heavy-metal data from estuarine and coastal sediments. ICES Journal of Marine Science, 48, 101–115.
Luleå Kommun (2007). Trafikmängder för Luleå Kommun—2007. Luleå, Sweden.
Marsalek, J. (1997). Heavy metals and PAHs in stormwater runoff from the Skyway Bridge, Burlington, Ontario. Water Quality Research Journal of Canada, 32, 815–827.
McKenzie, E. R., Wong, C. R., Green, P. G., Kayhanian, M., & Young, T. M. (2008). Size dependent elemental composition of road-associated particles. Science of The Total Environment, 398, 145–153.
Menzie, C., Hoeppner, S., Cura, J., Freshman, J., & Lafrey, E. (2002). Urban and suburban storm water runoff as a source of polycyclic aromatic hydrocarbons (PAHs) to Massachusetts estuarine and coastal environments. Estuaries and Coasts, 25, 165–176.
Morrison, G. M., Revitt, D. M., Ellis, J. B., Svensson, G., & Balmer, P. (1988). Transport mechanisms and processes for metal species in a gullypot system. Water Research, 22, 1417–1427.
Munch Christensen, A., Nakajima, F., & Baun, A. (2006). Toxicity of water and sediment in a small urban river (Store Vejlea, Denmark). Environmental Pollution, 144, 621–625.
Öhlander, B., Ingri, J., Pontér, C. (1991). Geochemistry of till weathering in the Kalix River watershed, northern Sweden. In: Rosén, K. (Ed.) Chemical weathering under field conditions. Reports in forest ecology and forest soils. Swedish University of Agricultural Sciences
Olofsson, I. (2002). Kartläggning och provtagning av Lövskataviken och Inre Skurholmsfjärden. 2002:299. Avd. för Tillämpad Geologi. Luleå, Luleå University of Technology.
Peinerud, E. K., Ingri, J., & Pontér, C. (2001). Non-detrital Si concentrations as an estimate of diatom concentrations in lake sediments and suspended material. Chemical Geology, 177, 229–239.
Quantin, C., Joner, E. J., Portal, J. M., & Berthelin, J. (2005). PAH dissipation in a contaminated river sediment under oxic and anoxic conditions. Environmental Pollution, 134, 315–322.
Raab, B., & Vedin, H. (1995). Climate, lakes and rivers. Stockholm, Sweden: SNA Publishing.
Redfield, A. C. (1958). The biological control of chemical factors in the environment. American Journal of Science, 46, 206–226.
Rudnick, R. L., & Gao, S. (2003). Composition of the Continental Crust. In H. D. Holland & K. K. Turekian (Eds.), Treatise on Geochemistry. Oxford: Pergamon.
Sartor, J. D., & Boyd, G. B. (1972). Water pollution aspects of street surface contaminants. Washington, DC: US EPA.
Schiff, K., & Bay, S. (2003). Impacts of stormwater discharges on the nearshore benthic environment of Santa Monica Bay. Marine Environmental Research, 56, 225–243.
Simmons, I. G. (1991). Earth, air, and water: resources and environment in the late 20th century. London, UK: Edward Arnold.
Statistics Sweden (2006). Tätorter 2005. MI 38 SM 0601, SCB, Stockholm, Sweden
Swedish EPA (1999). Bedömningsgrunder för miljökvalitet. Kust och hav. Rapport 4914. Stockholm, Sweden: Swedish Environment Protection Agency
Swedish EPA (2000). Bedömningsgrunder för sjöar och vattendrag. Stockholm, Sweden: Swedish Environmental Protection Agency
UNFPA (2007). State of world population. Unleashing the potential of urban growth. United Nations Population Fund, Geneva, Switzerland
Viklander, M. (1998). Particle size distribution and metal content in street sediments. Journal of Environmental Engineering, 124(8), 761–766. PBD: Aug 1998.
Wehrli, B. (1991). Geochemische Prozesse in Seen. In O. Kandler (Ed.), Die Ökologie der Oberbayerischen Seen. München: Pfeil.
Westerlund, C. (2007). Road Runoff Quality in Cold Climates. 2007:37. Department of Civil, Mining and Environmental Engineering. Luleå University of Technology, Luleå, Sweden
Wildi, W., Dominik, J., Loizeau, J., Thomas, R. L., Favarger, P., Haller, L., et al. (2004). River, reservoir and lake sediment contamination by heavy metals downstream from urban areas of Switzerland. Lakes & Reservoirs: Research and Management, 9, 75–87.
Acknowledgements
This study was financed by Luleå University of Technology (LTU) and the Swedish Research Council for Environment, Agriculture Sciences and Spatial Planning (FORMAS). This support is gratefully acknowledged. For their help with analytical work we like to thank Per Roos at the Radiation Research Division at Risø National Laboratory for Sustainable Energy, Technical University of Danmark (DTU), Erik Lundberg at Umeå Marine Sciences Centre and Bertil Pålsson at the Division of Mineral Processing at LTU. We also thank Kristin Karlsson, Fredrik Nordblad and Magnus Westerstrand for assistance during the field work and contributing with their knowledge in discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rentz, R., Widerlund, A., Viklander, M. et al. Impact of Urban Stormwater on Sediment Quality in an Enclosed Bay of the Lule River, Northern Sweden. Water Air Soil Pollut 218, 651–666 (2011). https://doi.org/10.1007/s11270-010-0675-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0675-7