Abstract
The biofilter system containing tire-derived rubber particle (TDRP) filter media was utilized to treat the odorous gas contaminant, hydrogen sulfide, in consideration of the economic advantage of reusing discarded tire materials and the high potential of TDRP media for biofilm attachment. The pilot-scale system having 0.38 m3 of bed volume operated with synthetic hydrogen sulfide gas on continuous basis from a range of 0.34 to 1.15 m3/min. This bioreactor system achieved over 94% removal efficiency at 20–90 ppm of inlet H2S concentration while operating in 20–67 s of empty bed retention time, indicating that overall effective operation was performed at mass loading rates of H2S ranging from 19.6 to 28.5 g H2S/(m3 h). It was apparent by the effectiveness of the system’s performance that this system had the capability to effectively remove hydrogen sulfide with high efficiency over a range of concentrations. A maximum elimination capacity was not found for the biofilter during this study, which tested loading rates between 0 and 30 g H2S/(m3 h).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Waste gases from a number of industrial plants, waste disposals, composing plants, and wastewater treatment plants emit unwanted odors containing sulfur compounds, namely hydrogen sulfide (H2S) and mercaptans as the principal source (Burgess et al. 2001). Odorous gases should be removed for reasons of public health, safety, and the prevention of corrosion (Jensen and Webb 1995). Over 10 ppm of hydrogen sulfide can affect the human health, and fatal problems can be raised at higher than 600 ppm (Droste 1997). Hence, various odor removal systems are used at sites producing odorous gases.
The most common methods for hydrogen sulfide removal are physicochemical processes (Gabriel et al. 2004). Investigation and application of biological processes, however, have been increasing recently due to high operating costs and by-products associated with chemical methods (Hansen and Rindel 2000; Gabriel et al. 2004). Among the biological processes, biofilters, biotrickling filters, rotating biological contactors, bioscrubbers, and suspended cell bioreactors are commonly used for hydrogen sulfide removal treatment (Shareefdeen and Singh 2005). Several biological methods of odor treatment have been developed and used with good results (Morton et al. 2005; Nishimura and Yoda 1997; Wolstenholme and Schafer 2005). In consideration of these biological odor removal systems, filter media is one of the most critical factors by providing biomass surface to attach and contact with contaminants (Shareefdeen and Singh 2005). Fine media or media with a high specific surface area achieve higher levels of treatment efficiency (Luo and Lindsey 2006). Media porosity impacts flow dynamics and mass transfer characteristics, which leads to impacts on biofiltration operation and performance potentially (Yang et al. 2009). The organic matter content of a biofilter media may affect its efficacy in the system (Pagans et al. 2007). Organic media naturally biodegrade, however, and have a limited application lifetime (Saliling et al. 2007). Adsorptive media are considered beneficial for rapid startup and for minimizing transient loading effects (Duan et al. 2006; Oh et al. 2009). Layering of media was found to more evenly distribute gas flow, resulting in more consistent performance (Yang et al. 2003). Overall, a good medium is defined with the following characteristics (Hirai et al. 2001):
-
1.
High water holding capacity
-
2.
High porosity
-
3.
Large specific surface area
-
4.
Less compactive
-
5.
Low pressure drop over a range of conditions
-
6.
Little change in character over long operation periods
-
7.
Low density
-
8.
Low cost
-
9.
Adsorption capability
-
10.
Buffering capacity
Among synthetic filter media materials, rubber material has high potential for application as biofiltration media, owing to its physical and chemical properties. Tire rubber is a thermoset material and is shown to be composed of synthetic and natural rubbers, carbon black and silica, mineral oils, sulfur, zinc oxide, stearic acid, sulfenamide and thiazoles, antioxidants and antiozonants, and softeners (Barbin and Rodgers 1994). Tire rubber is exploited for its constant shape and durability (Seda et al. 2007). One study shows that recycled tire rubber maintains a specific gravity near 1, a water adsorption capacity between 5% and 10%, a hydraulic conductivity between 0.2 and 0.8 cm/s, and variable surface pH (Moo-Young et al. 2003).
Tire-derived rubber particles (TDRP) from Envirotech Systems, Inc., seem to be a valuable option for odor removal system biotechnology since they can be easily produced from the reuse market at low cost. Moreover, several bioreactors with TDRP, trickling filters, hybrid SGBR with TDRP, and anoxic TDRP filters have successful performances, owing to the applicability of TDRP to the biofilter media (Park et al. 2006), which contain a high surface area, provides high porosity, adsorbs hydrogen sulfide over a range of conditions, and resists biodegradation. The use of rubber particles in the odor removal system seems to be a viable option because of the economic advantage of reusing discarded tire materials and the high potential of TDRP media for biofilm attachment. The objective of this research was, therefore, to evaluate the characteristics of TDRP to ensure that it would be a good media for use as a biofilter and to develop and test a biofilter system utilizing TDRP filter media for growth of a biofilm capable of biologically oxidizing hydrogen sulfide.
2 Methodology
2.1 TDRP Evaluation
The TDRP material was obtained from Envirotech, Inc. Size distribution analysis was carried out using sieve analysis and microscopy. A wet sieve was used to characterize the fine shredded tire rubber. The sieve test was performed using a wet sieve machine with about 100-g samples. Nine sieves were arranged from the largest opening to the smallest and allowed all possible materials to pass through each sieve to distribute by particle size. The surface characterization and element analyses were conducted, using a scanning electron microscope with an energy-dispersive X-ray spectroscope (Hitachi, S-2460 N). The samples were applied to a carbon-based disk and inserted under the electron stream. The elements were identified in spots or over a larger area of the sample-based refracted waves from the material.
2.2 Overview of the Pilot-Scale Biofilter System
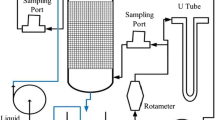
A biofilter system filled with TDRP treated a synthetic odorous gas stream supplied by a premixed hydrogen sulfide gas cylinder. A schematic of the laboratory’s pilot biofilter is shown in Fig. 1. The biofilter consisted of a 1.24-m-high and 1.17-m-diameter polyethylene tank, a 100-mm polyvinyl chloride (PVC) inlet pipe, and a 76-mm PVC outlet pipe. This reactor was filled with 100-mm perforated, corrugated high-density polyethylene (HDPE) pipes on the bottom of the reactor for sustaining the media bed and venting the treated air. Above the HDPE pipes, three different types of TDRP media were used as the filter bed for system stability—0.10 m3 of chunk rubber, 0.12 m3 of shredded rubber, and 0.16 m3of fine rubber—located from the bottom bed to the top bed, respectively. Total empty bed volume of this reactor was 0.38 m3. The filter media were seeded with 0.06 m3 of biomass (e.g., biomass concentration = 2,140 g VSS/m3, total seeded biomass = 128 g VSS) from the Boone Water Pollution Control Plant located in Boone, IA, USA.
2.3 System Operation and Analysis
Synthetic hydrogen sulfide was supplied to the main air stream inlet pipeline at the target concentration, simulating contaminated air to the pilot reactor. Two spray mist nozzles, a fogging nozzle, and a high-volume clog-resistant misting nozzle were located below the top of the reactor to provide effective moisture content and nutrients to the biomass. A nutrient solution was fed to the reactor by spray mist nozzles at an average flow rate of 1.2 L/h. The constituents of concentrated nutrient solution and trace element solution are shown in Tables 1 and 2. The concentrated nutrient solution was diluted with tap water by a factor of 10 after the addition of nonfat dry milk concentrated at 0.5 g/L.
Inlet and outlet H2S concentrations were measured using a BW Defender multigas detector (BW Technology, Pantego, TX) at concentration ranging from 0 to 150 ppm. Air flow was measured using a Model 9880 air velocity meter (Terra Universal, Inc., Anaheim, CA). Two water-filled manometers were installed at each inlet and outlet line to monitor the pressure change in the system.
3 Results
The size distribution and chemical tests are very important to the potential uses of a material. It is important to know the sizes and distribution of particles in order to select for the optimum characteristics of the media. A chemical analysis can further help to predict characteristics and problems that may occur. Scanning electron micrographs reveal the high surface area and porous character of the TDRP (Fig. 2). The fine TDRP used for these tests presented similar fractions for each size distribution as evidenced by the consistent slope of the line representing the size of particles as shown in Fig. 3.
Using SEM, it was possible to detect elements present in the TDRP. Chlorine was present in both small and large areas tested. Sulfur was also found, which was expected, since sulfur is used to manufacture tires. In some of the areas tested, sulfur spiked to many times that of the other chemicals. Silicon, calcium, and oxygen were also observed, but at lower amounts than many of the other elements. Zinc and magnesium were also detected in concentrations greater than 1,000 mg/kg. Scanning electron micrographs show the development of various cocci with some filaments on TDRP in Fig. 2, supporting biological transformation as the mechanism.
A gas cylinder having 0.5–5% H2S and 5% methane was used with a two-stage gas regulator to give the biofilter system consistent H2S gas concentrations under varying flow conditions. Figure 4 shows the performance of the biofilter supplied with H2S from the gas cylinder. After initiating the H2S supply system, the biofilter was operated with an empty bed retention time (EBRT) of greater than 60 s to evaluate initial system stability and provide a maintenance check. The initial airflow rate was 0.34 m3/min, corresponding to a 67 s EBRT. There was no H2S detected in the outflow during this operating condition. A majority of the reported biofilter systems treating H2S gas are operated below 60 s of EBRT, since the biodegradability of sulfide is rapid (Subletle and Sylvester 1987; Potivichayanon et al. 2006; Wolstenholme and Schafer 2005). Due to this, the airflow rate of this system was gradually increased to 1.15 m3/min (EBRT of 20 s) to determine the optimum operating capacity of the TDRP biofilter. The inflow H2S gas concentration was varied from 20 to 60 ppm at each flow rate in this study. During the various operating periods, there was no H2S detected (e.g., minimum detection limit = 1 ppm) in the outflow, indicative of effective H2S removal performed by the TDRP biofilter system.
A large fraction of the nutrient water dosed to the system was lost as vapor with the outlet gas flow. Although a small fraction of the nutrient water dosed was recovered, the media were saturated based on drainage from the system. The drain water was tested for sulfate, which was below the detection limit. Additionally, the scale of the system made recovery of outlet gas and drain water challenging. The sulfur mass balance, therefore, could not be completed. One possible fate of the sulfur is as elemental sulfur based on the sulfur mass load. Production of elemental sulfur would be consistent with the work of Chung et al. (1996) who found that the sulfur product(s) produced depend on the species of Thiobacillus and on the mass loading rate of sulfur. In their study, elemental sulfur was the dominant product at a loading rate of 25.0 g S/(m3 h).
Based on hydrogen sulfide removal from the gas phase, it is estimated that between 2 and 4 kg S was converted from hydrogen sulfide to oxidized product during the course of the study. Based on the density of elemental sulfur, this translates to between 1 and 3 L of volume that would have been occupied by the sulfur. Consequently, solid-phase elemental sulfur would displace between 0.2% and 0.8% of the empty bed volume, resulting in little effect on system operation.
The gas cylinder with 8% of hydrogen sulfide was replaced for the application before adding higher concentrations of odorous compounds to this system. However, there was a delay in replacement of the gas cylinder, halting hydrogen sulfide supply for 2 months. After replacement, the system was operated at 20–90 ppm of H2S in inflow with 20–25 s of EBRT as shown in Fig. 5. H2S was detected in the outlet at a concentration ranging from 1 to 5 ppm while the inlet H2S concentration was above 70 ppm. The removal efficiency decreased at increased H2S loading rates although over 94% H2S removal efficiency was sustained during the study, which included inlet concentrations greater than 90 ppm H2S.
Inlet mass load was calculated using the equation below:
Where
-
Inlet mass load, g/m3/h
-
Q, flow rate of inlet (m3/h)
-
Cin, pollutant concentration in inflow (g/m3)
-
Vr, reactor volume (m3)
Inlet mass loads were varied from 1.6 to 28.5 g H2S/(m3 h) during the operation. A majority of the loaded H2S to the system was removed below 19.6 g H2S/(m3 h) (Fig. 6). The H2S removal efficiency decreased with the mass load above 19.6 g H2S/(m3 h). However, the overall system performance was maintained over 94% H2S removal efficiency at the mass loading rates ranging from 19.6 to 28.5 g H2S/(m3 h).
A common way to evaluate the system performance is by analysis of elimination capacity, calculated as follows:
Where
-
Elimination capacity, g H2S/(m3 h)
-
Cout, pollutant concentration in outflow (g/m3)
In the odor control system, one of the most important observations is the relationship between the H2S inlet mass load to the system and the elimination capacity of system, owing to its effective indication of system capacity. Normally, increasing the loading rates increases the elimination capacity (Shareefdeen and Singh 2005). However, this increase of elimination capacity does not continue above certain values of load rates. This is reflected as the maximum elimination capacity (Koe and Yang 2000). The maximum elimination capacity can be determined at the start of the flat line on the elimination capacity curve, corresponding to the mass load rates (Koe and Yang 2000). This study shows the increase of elimination capacity along with an increase of mass load rates (Fig. 7). The maximum elimination capacity was not determined because this plot had no flat line; however, the data between 20 and 25 g H2S/(m3 h) show some scatter. Therefore, the maximum elimination capacity appears to be greater than or equal to 20 g H2S/(m3 h), which indicates that the system could treat H2S efficiently at loading rates of 20 g H2S/(m3 h) or higher. This result is consistent with other studies; one study found a biofiltration removal capacity between 10 and 15 g H2S/(m3 h) (Shanchayan et al. 2006); another study found a removal capacity between 120 and 245 g H2S/(m3 h) (Qiao et al. 2008), Shareefdeen et al. (2003) achieved a removal between 5 and 10 g H2S/(m3 h) with departure from 100% removal at a loading above 6 g H2S/(m3 h). Removals up to 200 g H2S/(m3 h) were achieved with activated carbon as a biofilter media (Duan et al. 2006).
4 Conclusions
A biofilter system filled with TDRP media was applied to hydrogen sulfide treatment to evaluate efficiency of the system. During the study, synthetic hydrogen sulfide from the gas cylinder was provided to the TDRP biofilter with 20–90 ppm of H2S in 20–67 s of EBRT. The bioreactor system achieved over 94% removal efficiency of H2S, which indicates the effective performance of the TDRP bioreactor. This was true as long as EBRTs were not shorter than 20 s at less than 100 ppm of H2S in inflow. Moreover, the maximum elimination capacity of this system was equal to or greater than 20 g H2S/(m3 h). Therefore, this study showed the high potential of this system for hydrogen sulfide removal, owing to system stability and effective removal efficiency.
References
Barbin, W. W., & Rodgers, M. B. (1994). The science of rubber compounding. In J. E. Mark, B. Erman, & F. R. Eirich (Eds.), Science and technology of rubber (pp. 419–469). San Diego: Academic.
Burgess, J. E., Parsons, A. A., & Stuetz, R. M. (2001). Developments in odour control and waste gas treatment biotechnology: A review. Biotechnology Advances, 19(35), 36–63.
Chung, Y.-C., Huang, C., & Tseng, C.-P. (1996). Operation optimization of Thiobacillus thioparus CH11 biofilter for hydrogen sulfide removal. Journal of Biotechnology, 52(1), 31–38.
Droste, R. L. (1997). Theory and practice of water and wastewater treatment. New York: Wiley.
Duan, H., Koe, L. C. C., et al. (2006). Biological treatment of H2S using pellet activated carbon as a carrier of microorganisms in a biofilter. Water Research, 40(14), 2629–2636.
Gabriel, D., Cox, H. H. J., & Deshusses, M. A. (2004). Conversion of full-scale wet scrubbers to biotrickling filters for H2S control at publicly owned treatment works. Journal of Environmental Engineering, 130(10), 1110.
Hansen, N. G., & Rindel, K. (2000). Bioscrubbing, an effective and economic solution to odour control at wastewater treatment plants. Water Science and Technology, 41(6), 155.
Hirai, M., Kamamoto, M., et al. (2001). Comparison of the biological H2S removal characteristics among four inorganic packing materials. Journal of Bioscience and Bioengineering, 91(4), 396–402.
Jensen, A. B., & Webb, C. (1995). Treatment of H2S-containing gases: A review of microbiological alternatives. Enzyme and Microbial Technology, 17, 2–10.
Koe, L. C. C., & Yang, F. (2000). A bioscrubber for hydrogen sulfide removal. Water Science and Technology, 41(6), 141.
Luo, J., & Lindsey, S. (2006). The use of pine bark and natural zeolite as biofilter media to remove animal rendering process odours. Bioresource Technology, 97(13), 1461–1469.
Moo-Young, H., Sellasie, K., et al. (2003). Physical and chemical properties of recycled tire shreds for use in construction. Journal of Environmental Engineering, 129(10), 921–929.
Morton, R., Lee, A., Tang, C. C., Horvath, R., & Stahl J. (2005) A two-stage biotrickling filter system for odors and volatile organic compounds removal. In Proceedings of the Water Environment Federation 73rd Annual Conference and Exposition, Washington, DC.
Nishimura, S., & Yoda, M. (1997). Removal of hydrogen sulfide from an anaerobic biogas using a bio-scrubber. Water Science and Technology, 36(6–7), 349.
Oh, D. I., Song, J., et al. (2009). Effects of adsorptive properties of biofilter packing materials on toluene removal. Journal of Hazardous Materials, 170(1), 144–150.
Pagans, E., Font, X., et al. (2007). Adsorption, absorption, and biological degradation of ammonia in different biofilter organic media. Biotechnology and Bioengineering, 97(3), 515–525.
Park, J., Ellis T. G., Lally, M. (2006). Evaluation of tire derived rubber particles for biofiltration media. In Proceedings of the Water Environment Federation 74rd Annual Conference and Exposition, Dallas, TX.
Potivichayanon, S., Pokethitiyook, P., & Kruarachue, M. (2006). Hydrogen sulfide removal by a novel fixed-film bioscrubber system. Process Biochemistry, 41, 708.
Qiao, S., & Fu, L. et al. (2008). Removal characteristics of hydrogen sulfide in biofilters with fibrous peat and resin. In 2nd International Conference on Bioinformatics and Biomedical Engineering, iCBBE.
Saliling, W. J. B., Westerman, P. W., et al. (2007). Wood chips and wheat straw as alternative biofilter media for denitrification reactors treating aquaculture and other wastewaters with high nitrate concentrations. Aquacultural Engineering, 37(3), 222–233.
Seda, J. H., Lee, J. C., et al. (2007). Beneficial use of waste tire rubber for swelling potential mitigation in expansive soils. Soil improvement (GSP 172). Denver: ASCE.
Shanchayan, B., Parker, W., et al. (2006). Dynamic analysis of a biofilter treating autothermal thermophilic aerobic digestion gas. Journal of Environmental Engineering and Science, 5(3), 263–272.
Shareefdeen, Z., Herner B., et al. (2003). Hydrogen sulfide (H2S) removal in synthetic media biofilters. Environmental progress, 22(3), 207–213.
Shareefdeen, Z., & Singh, A. (2005). Biotechnology for odor and air pollution control. New York: Springer.
Subletle, K. L., & Sylvester, N. D. (1987). Oxidation of hydrogen sulfide by Thiobacillus denitrificans: Desulfurization of natural gas. Biotechnology and Bioengineering, 29, 249.
Wolstenholme, P., & Schafer, P. (2005) Odor control bioscrubbers. A 20 year history of successful applications. In Proceedings of the Water Environment Federation 73rd Annual Conference and Exposition, Washington, DC
Yang, C., Chen, H., et al. (2009). Modeling variations of medium porosity in rotating drum biofilter. Chemosphere, 74(2), 245–249.
Yang, C., Suidan, M. T., et al. (2003). Comparison of single-layer and multi-layer rotating drum biofilters for VOC removal. Environmental Progress, 22(2), 87–94.
Acknowledgments
The authors would like to thank to Envirotech Systems, Inc., for providing TDRP media, financing this research, and otherwise supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J., Evans, E.A. & Ellis, T.G. Development of a Biofilter with Tire-Derived Rubber Particle Media for Hydrogen Sulfide Odor Removal. Water Air Soil Pollut 215, 145–153 (2011). https://doi.org/10.1007/s11270-010-0466-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0466-1