Abstract
Heavy metals and some major element concentrations were investigated in overbank sediments and stream bed sediments of the ephemeral Beal wadi creek in the Cartagena-La Union mining district (SE Spain). Two vertical sediment profiles were extracted and the chemical and mineralogical compositions were both investigated by X-ray fluorescence and X-ray diffraction. Geochemical variations in vertical profiles of these two kinds of sediments allow observing noticeable heavy metals pollution (especially Fe, Pb, and Zn) in both kind of sediments but especially in the overbank sediments (reaching values of approximately 13% Fe, 6% Pb, and 6% Zn). A single extraction (DIN 38414-S4 leaching test) was made to observe the transfer of metals from solids to liquid phase. Pb, Zn, Cu, and Cd contents surpass the leaching values established by the DIN 38414-S4 limits established by Spanish legislation to consider these wastes as hazardous wastes. Geochemical comparison between leaching behavior of the two types of sediments reveals a major ability of overbank sediments in transferring heavy metals to the water flow.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
River sediments are a widely used sampling tool for geochemical studies and ore prospection. Likewise they can be used in environmental research for screening purposes or to investigate the mobility of pollutants such as heavy metals in mining affected areas. Concentration of heavy metals and major elements in floodplain sediments deposited during floods is widely related to their content in sediments found in upstream river channel prior to flooding events (Ciszewski 2002; Rothwell et al. 2007; Taylor and Kesterton 2002). Stream and overbank sediments deposited along rivers that drain mining areas are often highly polluted (Macklin 1992; Swennen et al. 1994) due to both the natural geochemical background and also to the industrial mining practices that produce an important quantity of wastes, often transferred to the nearest river channels. However, concentration of pollutants amongst different sedimentary packages in a upstream river catchment is usually inhomogeneous due to different physicochemical processes involved in sediment settling. Secondary mobilization and transport of heavy metals within the catchment will depend on the geochemical features of the overbank and mid-bed sediments that influence the water pollution level in flooding events. This is extremely important in Mediterranean region where the hydrological river regime is highly discontinuous and subjected to sporadic extreme rainy events. Ephemeral creeks (so called wadi) are a common feature along the entire Mediterranean basin. They can remain completely dry for long time and remobilize huge amounts of sediments in short strong storm events not only supplying solid sediments to lowlands but changing the water flow chemistry with the subsequent potential hazard for the surrounding lowlands areas.
The ecological impact of heavy metals in soils is particularly determined by the soluble and easily mobile fraction (Herms and Brümmer 1984). The major changes that occur in redox conditions between oxic waters and anoxic sediments can have profound influences on the speciation and bioavailability of many trace metals (Morse and Luther 1999).
Secondary processes in sediments include metal complexation of organic and inorganic ligands, modifying surface properties of adsorptive particles, and formation and dissolution of metal-bearing precipitates. Post-depositional mobilization of heavy metals is an important process from environmental point of view, owing to the potential affection over surface and ground water quality and its role as secondary pollution source (Swennen et al. 1994). Secondary mobilization of heavy metals in soils and sediments polluted is controlled by pH, redox potential, and salinity changes.
Recent studies on metal mobility along soils and sediment profiles, recognize the role of overbank sediments as a secondary pollution source for long time (Aleksander-Kwaterczak and Helios-Rybicka 2009; Cappuyns and Swennen 2007; Cappuyns et al. 2006; Cappuyns and Swennen 2004; Swennen and Van der Sluys 2002).
Mining extraction and processing of sulfide ore deposits is one of the more important activities causing pollution in topsoils (Cappuyns et al. 2006; Elderling et al. 2002; Monna et al. 2004). Mining activities have a very important environmental impact due to the fact that normally only a 1% of the processed material is recovered as available metal (Field 2003). Wastes were deposited in different forms according to the historical period, geographical location, and the environmental legislation in the exploitation moment.
Cartagena-La Union mining district is one of the oldest mining districts of Iberian Peninsula, located in the Betic mountain range (SE, Spain). This region contains important Pb–Zn massive ore deposits. The richness in Zn and Pb of these deposits made possible its exploitation from Phoenician times to the closure of extractive industry in 1991. Mining activity within this region was discontinuous and only was important during some determined periods. In Roman ages, Pb production reaches peak production of 45,000 Tm/year during second and first century BC (Moreno-Grau et al. 2002). Later, between 1840 and 1930, mining in Cartagena reached maximum extraction levels. Finally, between 1953 and 1991, there was another important extraction period due to the open pit mining and the improvement of hydrometallurgical techniques. The implementation of flotation techniques allowed a bigger recovery rate and maintained Pb and Zn production during the nineteenth and twentieth centuries (Linares-Martinez 2005) thus promoting huge increase of landfill disposal sites along the area.
At the Cartagena-La Union mining district, mining wastes were deposited in three different ways: initially, they were thrown to wadis. Later, they were deposited in mining dams. Finally, wastes were conducted by a pipe into the sea and settled in a bay zone (Martínez-Sánchez et al. 2008). These wastes were characterized in a previous work and very high heavy metals concentrations have been found (Gonzalez-Fernandez et al. 2007).
Some previous work about environmental risk of the Cartagena-La Union mining wastes was made by the study of inhalable particles (Moreno-Grau et al. 2002). However, there are not many studies evaluating leaching of metals to hydrous media. In this study we selected overbank sediments and mid-course sediments from the Beal wadi at Cartagena-La Union mining district to evaluate sediment metal content and metal leaching to the nearest aquatic medium.
2 Materials and Methods
2.1 Sampling
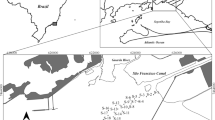
Samples for the study were collected in the upstream course of the El Beal wadi downhill from an ancient mining concentration plant (coordinates: 37°38′13″ N; 0°50′14″ W). Two discontinuous drill sediment core samples were obtained by using an Eijkelkamp hand auger probe. This hand-held probe allows obtaining discrete samples at incremental depths. One core was extracted in the middle of the wadi course and another one was extracted from the overbank sediments (Fig. 1). The mid-course core reached 100 cm depth and it was divided into 12 subsamples representing different in-depth layer beds. The overbank core reached 92 cm depth and was divided into 15 subsamples. Samples were transported in ZipBlock® sealed bags to the laboratory.
2.2 Chemical Analysis
Samples were dried at 60°C and subsequently a fraction of sediments were sieved to pass a 2-mm sieve. This fraction was used to measure pH by employing the potentiometric method (NRCS 2004) from a 1:2.5 soil–water ratio after 15 min of mixing. Conductivity and redox potential (Eh) were measured after 15 min of mixing the sediment with milli-Q water using a relationship 1:5 (NRCS 2004).
The elemental chemical analysis was performed by employing a wavelength dispersive X-ray fluorescence spectrometer (WDXRF). The sediment samples were analyzed using a commercial WDXRF spectrometer (Bruker S4 Explorer) equipped with a Rh anticathode X-ray tube (0–50 kV, 0–50 mA, and 1 kW maximum); four analyzer crystals (OVO-B, OVO-55, LiF 200, and PET) and both a flow proportional counter for light element detection and a scintillation counter for heavy elements detection. This equipment allows the simultaneous multielemental determination of a wide analytical range (from Be to U) with a typical measuring time of 20–60 s per element. Energy resolution and efficiency for each analytical line were determined both by collimator aperture and analyzer crystal used (i.e., resolution combining the two effects for Cu Kα line was 40 eV). Analysis was made in a vacuum atmosphere allowing the detection of low Z elements.
Sample specimens for the analysis were obtained by performing the classical pressed powder-pellets using 5 g of sample and 0.4 g of binder (Margui et al. 2009). Spectral data was evaluated by the software provided with the equipment (Software Spectra Plus® 1998). Quantitative evaluation procedure was carried out by fundamental parameters standardless method, owing to the lack of adequate certified reference materials of soils or sediments with metal concentrations in the same range of the mining sediments analyzed in this study. The validity of standardless procedures by the fundamental parameters methodology has been pointed out by Rousseau (2001). Accuracy and precision of the method was checked by the analysis of several international certified reference materials (Table 1).
Leaching of metals was assessed by employing the normalized test DIN 38414-S4 (1984). This test was done by mixing 10 g of sediment having grain size less than 2000 μm with Milli-Q water using a ratio 1:10, then it was shaken for 24 h in a rotating device. Such kinds of batch studies using simple leaching tests have been successfully applied for these objectives (Fic and Isenbeck-Schröter 1989; Robles-Arenas et al. 2006).
The test allows evaluating the easily soluble fraction of metals. The pH and the conductivity of the suspension were measured at 15 min from the start and at the end (24 h). Afterwards, sediment batches were filtered by 45 μm filters and the elemental analyses of the extracts were carried out by an ICP-MS (Liberty, RL sequential, Varian) and an ICP-AES (Liberty AX2, Varian).
2.3 Mineralogy
Mineralogical composition of the different sediment levels of the two cores were determined by X-ray diffraction by using powder diffraction methods. An X-ray diffractometer Bruker D-5005, with a Cu target tube, operating at 40 kV and 30 mA was employed. Diffractograms were recorded at steps of 0.01° (2θ angle) with an acquisition time of 6 s per step. Samples were analyzed within 4–70° of the 2θ angle. The software package EVA (Bruker AXS, Germany) and the PDF2 database (ICDD, Denver, USA) were employed for mineral phase identification.
3 Results
3.1 Chemical Analysis
The main physicochemical properties of sediment samples are shown in Fig. 2. The pH measurements reveal acidic behavior for the sample batches, with very homogeneous level of pH around 4. Similar trend is observed for redox potential with a very weak increase in depth, especially for mid-bed sediments, while overbank sediments exhibit a little larger heterogeneity. On other hand, electrical conductivity (EC) exhibits completely different values from overbank to mid-bed sediment. The mid-bed sediment exhibit the lowest values of EC, being almost constant (around 2,000 μS) in all the samples. However, the overbank sediments exhibit variable values of EC, ranging from 3,000 to 8,000 μS.
The results for the elemental chemistry of sediments are summarized in Tables 2 and 3.
As main distinctive features, overbank sediments exhibit a noticeable increase of mean values for S, Ca, Cu, Zn, Sn and Pb respect to mid-bed sediments. These elements have mean content values in overbank sediments reaching levels as much as twice than those measured for mid-bed sediments. In opposition, mid-bed sediments have a pronounced higher content of Fe, Ti, and Si. The observation of the coefficients of variation (COVs) reveal that always they are higher in overbank materials, thus confirming the heterogeneity in these sediments also pointed out by electrical conductivity.
This heterogeneity can be also assessed by the vertical profiles of elemental contents as shown in Fig. 3, where the data for six major elements (Al, Si, Ca, S, Mn, and Fe) and three minor elements (Pb, Zn, and Cu) are plotted. As it can be seen in the figure differential trend is noticed from both types of sediments.
The results for sediment leachates are shown in Fig. 4. For the main part of elements we consider, the leachability of overbank sediments is higher than for mid-bed sediments, noticeable for heavy metals such as Pb, Zn, Cu, and Mn and also for S. An especial case is the presence of elevated Cd (reaching up to 18 mg L−1) concentration in the extracts of overbank sediments thus confirming the abundance of this element as indicated in the bulk sediments analysis.
3.2 Mineralogy
The mineralogical composition of samples exhibits as a main primary crystalline phases some clay minerals (muscovite, paragonite, chlorite, and kaolinite), quartz, and minor quantities of feldspars. It is possible to identify in selected samples sulfides (pyrite and sphalerite), sulfates (gypsum, bassanite, anglesite, plumbojarosite, and hydronium jarosite), and also carbonates (cerussite). The distribution of the main phases potentially containing heavy metals is presented in Table 4.
An example of XRD spectra for the two samples (one for each of the cores) can be observed in Fig. 5.
X-ray diffraction spectra of soil samples from overbank sediments (AR) and mid-course sediments (RCM). Intensity is in arbitrary units for comparison. (Mus: Muscovite; Clo: Chlorite; Kao: Kaolinite; Pb-Jar: Lead Jarosite; Hid-Jar: Hydronium Jarosite; Qz: Quartz; Cer: Cerussite; Bass: Bassanite; Ang: Anglesite; Pyr: Pyrite)
4 Discussion
Following the Wilding and Drees (1983) criteria for geochemical systems such as soils and sediments, we can classify chemical variables according its coefficient of variation. When COV is larger than 35%, reflect significative changes in the system. As it is shown in the present study, mid-bed course sediments of El Beal wadi only present two elements with a COV > 35% which are Ca and Mn. Zn is also near these values. However, overbank sediments present more pronounced variability. COVs larger than 35% are surpassed by Mg, Al, Si, K, Ca, Mn, Fe, Cu, Zn, Rb, Zr, and Pb. Also Cd, Ti, and S are near to this variability. The presence of Pb and Zn—which are the main mined metals—is ubiquitous in all the samples and Cd, Cu, and Sn are mainly found in overbank materials. Arsenic is found in both types of sediments in slightly large values in overbank sediments.
The physicochemical parameters of the sediments from El Beal wadi, coming from Pb–Zn mining areas indicate an acid mine drainage with a mean value around 4, which is characteristic of many mine sediments elsewhere (Förstner 2008; Gäbler 1997). One of the most distinctive parameter differentiating overbank and mid-bed sediments is the electrical conductivity. The large values of this parameter in overbank sediments indicate the potential presence of salty minerals, confirmed by the chemistry of the leachates, that exhibit larger content of Ca and S, thus indicating a secondary precipitation of salts in the sedimentary processes, not found in the primary rocks and ores (García 2004). The redissolution of these secondary sulfates can be responsible for heavy metal mobility as evidenced by the batch assays in this study.
The use of easy one-step batch test (DIN 38414-S4) is revealed as a useful tool for the indication of potential harmful effects in the surrounding environment. If some ephemeral flooding event develops resuspension of the sediments in the El Beal wadi the effects on water chemistry and the associated heavy metals water content can be different depending on the ability to detach overbank sediments or mid-bed sediments. As it is shown, from DIN tests, sulfur, zinc, or cadmium in solution can be several times higher if the overbank sediments are supplied to the flooding waters, mainly linked to the presence of soluble sulfates formed from the alteration (principally oxidation) of the sulfide mineralization (Garcia et al. 2008; Plumlee and Logsdon 1999). Despite their lower content in overbank sediments, Mn and Fe exhibit noticeable leaching values, thus implying the presence of immobile Fe–Mn bearing mineral phases in mid-bed sediments. This large leaching contents in overbank sediments can be attributed the presence of soluble sulfates, mainly jarosites, and sulfides which are present in the overbank sediments.
In the studied sediments Pb and Zn show values higher than the threshold value (0.5 and 2 mg L−1, respectively) established for classification as a class II waste (residues that have to be placed in a landfill, RD 1481/2001) according to the Spanish regulations for industrial wastes. In this case there is no difference in Pb leaching among the two sediment types. However, for Zn the leaching values are some orders of magnitude higher for overbank sediments.
Among other leachable elements, Cd is an especial case due to its huge leaching results. Several samples exhibit leaching values reaching levels around 5,000 times greater than recommended for irrigation, implying very high potential poisonous values for plants and biota around the studied area.
The mineralogical data obtained by XRD allows us to identify the metal-bearing phases. The difference among both types of sediments is the presence of sphalerite (ZnS) and pyrite (FeS2) in overbank sediments. Regarding Pb-bearing phases is remarkable the presence of Pb as anglesite (PbSO4), cerussite (PbCO3), and Pb–jarosites. For Pb behavior it was observed that levels with anglesite have higher leaching contents than the others, due to the solubility product constants of these species (Kps PbSO4 = 1.3·10−8 and Kps PbCO3 = 1.5·10−13).
5 Conclusions and Recommendations
The importance of overbank sediments as sources of pollution processes has been pointed out (Langedal and Ottesen 1998; Peh and Miko 2001) becoming of great interest in polluted areas of semiarid climate regions. Geochemical comparison of mid-bed and overbank sediment of El Beal wadi indicates a larger chemical heterogeneity in overbank sediments than those found in mid-bed sediments.
These differential features indicate: (a) distinct processes of accumulation, (b) the differential chemical transport along sedimentary events and the subsequent post-depositional migration, (c) the role of overbank sediments as a sink of heavy metals, and (d) the potential harmful effects from an overbank sediments remobilization to lowlands soils and sediments.
Regarding the elemental contents in sediments the main part of heavy metals (Pb, Zn, Cd, As, and Cu) exceed the threshold values established in Spanish legislation (RD 1310/1990) for industrial wastes and consequently should be disposed in adequate dump sites.
From the leaching tests we can conclude a major environmental risk for heavy metals pollution from overbank sediments. In rainy events producing low water flow regime in the wadi, only mid-bed sediments should be transported, and the transfer of heavy metals to streamwater will be relatively scarce. However, storm events producing remobilization of overbank sediments can dramatically change the situation and S, Mn, Zn, Cu, and Cd can be released to waterflow and remobilized in dissolved form at levels of some orders of magnitude higher than those found for mid-bed sediments. Some preliminary work was undertaken to prevent dispersal of metals in the Cartagena-La Union district, by the use of marble cutting sludges, as a reactive barrier, to promote the pH increase, restricting heavy metals mobilization processes (Martinez-Sanchez et al. 2008). However, these methods do not prevent the transport of some elements such as Zn and Cd, which are mobilized even at basic pH. Therefore, alternative methods to “in situ” fix the overbank sediments should be checked.
References
Aleksander-Kwaterczak, U., & Helios-Rybicka, E. (2009). Contaminated sediments as a potential source of Zn, Pb, and Cd for a river system in the historical metalliferous ore mining and smelting industry area of South Poland. Journal of Soils & Sediments, 9, 13–22.
Cappuyns, V., & Swennen, R. (2004). Secondary mobilisation of heavy metals in overbank sediments. Journal of Environmental Monitoring, 6, 434–440.
Cappuyns, V., & Swennen, R. (2007). Classification of alluvial soils according to their potential environmental risk: A case study for Belgian catchments. Journal of Environmental Monitoring, 9, 319–328.
Cappuyns, V., Swennen, R., Vandamme, A., & Niclaes, M. (2006). Environmental impact of the former Pb-Zn mining and smelting in East Belgium. Journal of Geochemical Exploration, 88, 6–9.
Ciszewski, D. (2002). Heavy metals in vertical profiles of the middle odra river overbank sediments: evidence for pollution sediments. Water, Air, and Soil Pollution, 143, 81–98.
DIN 38414-S4. (1984). Schlamm und Sedimente, Bestimmung der Eluierbarkeit mit Wasser. Berlin: DIN Deutsches Institut für Normung.
Elderling, B., Asmund, G., Kunzendorf, H., & Krogstad, E. J. (2002). Geochemical trends in metal-contaminated fiord sediments near a former lead-zinc mine in West Greenland. Applied Geochemistry, 17, 493–502.
Fic, M., & Isenbeck-Schröter, M. (1989). Batch studies for the investigation of the mobility of the heavy metals Cd, Cr, Cu and Zn. Journal of Contaminant Hydrology, 4, 69–78.
Field, S. (2003). The earth’s open wounds: Abandoned and orphaned mine. Environmental Health Perspectives, 111, A-154–A-161.
Förstner, U. (2008). Sediments—Resource or waste? Journal of Soils & Sediments, 4(1), 3.
Gäbler, H. E. (1997). Mobility of heavy metals as a function of pH of samples from an overbank sediment profile contaminated by mining activities. Journal of Geochemical Exploration, 58, 185–194.
García, C. (2004). Impacto y riesgo ambiental de los residuos minero-metalúrgicos de la sierra minera de Cartagena-La Unión. PhD Thesis. Universidad Politécnica de Cartagena. 424pp.
Garcia, G., Peñas, J. M., & Manteca, J. I. (2008). Zn mobility and geochemistry in surface sulfide mining soils from SE Spain. Environmental Research, 106, 333–339.
Gonzalez-Fernandez, O., Queralt, I., Carvalho, M. L., & Garcia, G. (2007). Elemental analysis of mining wastes by energy dispersive X-ray fluorescence (EDXRF). Nuclear Instruments and Methods in Physics Research B, 262(1), 81–86.
Herms, U., & Brümmer, G. (1984). Einflussgröβen der Schwermetallöslichkeit und–bindung in Böden. Z Pflanzenernaehr Bodenk, 147, 400–424.
Langedal, M., & Ottesen, R. T. (1998). Airborne pollution in five drainage basins in eastern Finnmark, Norway: An evaluation of overbank sediments as sampling medium for environmental studies and geochemical mapping. Water, Air, and Soil Pollution, 101(1–4), 377–398.
Linares Martínez, F. (2005). Juegos de estrategia y consecuencias inintencionadas: Un modelo con resultados perversos de la crisis de la minería de Cartagena-La Unión. Papers Journal, 75, 36–61.
Macklin, M. G. (1992). Metal pollution of soils and sediments: A geographical perspective. In M. D. Newson (Ed.), Managing the human impact on the natural environment: Patterns and processes (pp. 172–195). London: Belhaven Press.
Margui, E., Queralt, I., Van Grieken, R. (2009). X-ray fluorescence analysis, sample preparation for. In: R. A. Meyers (Ed.) Encyclopedia of analytical chemistry: Applications, theory, and instrumentation. New York: Wiley Interscience 20pp.
Martínez-Sánchez, M. J., García-Lorenzo, M. L., Pérez-Sirvent, C., & Marimón, J. (2008). Use of marble cutting sludges to immobilize heavy metals and decrease toxicity of contaminated soils. Fresenius' Environmental Bulletin, 17(10B), 1672–1678.
Monna, F., Petit, C., Guillaumet, J. P., Jouffroy-Bapicot, I., Blanchot, C., Dominik, J., et al. (2004). History and environmental impact of mining activity in Celtic aeduan territory recorded in a peat bog (Morvan, France). Environmental Science & Technology, 38(3), 665–673.
Moreno-Grau, S., Cascales-Pujalte, J. A., Martinez-Garcia, M. J., Angosto, J. M., Moreno, J., Bayo, J., et al. (2002). Relationships between levels of lead, cadmium, zinc, and copper in soil and settleable particulate matter in Cartagena (Spain). Water, Air, and Soil Pollution, 137(1–4), 365–383.
Morse, J. W., & Luther, G. W. (1999). Chemical influences on trace metal–sulfide interactions in anoxic sediments. Geochimica et Cosmochimia Acta, 63(19/20), 3373–3378.
NRCS. Natural Resources Conservation Services. (2004). Soil Survey Laboratory Methods Manual. Version 4.0. Soil Survey Investigations report Nº 42. 735pp. <http://soils.usda.gov/technical/lmm/>.
Peh, Z., & Miko, S. (2001). Geochemical comparison of stream and overbank sediments: A case study from the Zumberak Region, Croatia. Geologia Croatica, 54(1), 119–130.
Plumlee, G. S., & Logsdon, M. J. (1999). The environmental geochemistry of mineral deposits, part A: Processes, techniques, and health issues. Society of Economic Geologists, Reviews in Economic Geology, 6A, 371.
Real Decreto (RD) 1310/1990 de 29 de octubre, por el cual se regula la utilización de los lodos de depuración en el sector agrario, 1990, Gobierno de España.
Real Decreto (RD) 1481/2001, de 27 de diciembre, por el que se regula la eliminación de residuos mediante depósito en vertedero, 2001, Gobierno de España.
Robles-Arenas, V. M., Rodríguez, R., García, C., Manteca, J. I., & Pascual, L. (2006). Sulphide-mining impacts in the physical environment: Sierra de Cartagena–La Unión (SE Spain) case study. Environmental Geology, 51(1), 47–64.
Rothwell, J. J., Evans, M. G., & Allott, T. E. H. (2007). Lead contamination of fluvial sediments in an eroding blanket peat cachment. Applied Geochemistry, 22, 446–459.
Rousseau, R. M. (2001). Detection limit and estimate of uncertainty of analytical XRF results. Rigaku Journal, 18(2), 33–47.
Software SPECTRAplus, (1998). Bruker AXS, Oestliche Rheinbrueckenstr. 50, D-76187 Karlsruhe, Germany
Swennen, R., & Van der Sluys, J. (2002). Anthropogenic impact on sediment composition and geochemistry in vertical overbank profiles of river alluvium from Belgium and Luxembourg. Journal of Geochemical Exploration, 75, 93–105.
Swennen, R., Van Keer, I., & Vos, D. (1994). Heavy metal contamination in overbank sediments of the Geul river (East Belgium): Its relation to former Pb-Zn mining activities. Environmental Geology, 24, 12–21.
Taylor, M. P., & Kesterton, R. G. H. (2002). Heavy metal contamination of an arid river environment: Gruben River, Namibia. Geomorphology, 42, 311–327.
Wilding, L. P., & Drees, L. R. (1983). Spatial variability and pedology. In L. P. Wilding, N. E. Smeck, & G. F. Hall (Eds.), Pedogenesis and soil Taxonomy: I. Concepts and interactions (pp. 83–116). New York: Elsevier Science.
Acknowledgments
This study was financed by the Spanish National Research Programme (CGL2007-66861-C04) and by CONSOLIDER Research Programme (CSD2006-00044). O. Gonzalez-Fernandez gratefully acknowledges a grant from the Spanish Ministry of Science and Education (Ref. BES2005-6810).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonzalez-Fernandez, O., Jurado-Roldan, A.M. & Queralt, I. Geochemical and Mineralogical Features of Overbank and Stream Sediments of the Beal Wadi (Cartagena-La Union Mining District, SE Spain): Relation to Former Lead–Zinc Mining Activities and Its Environmental Risk. Water Air Soil Pollut 215, 55–65 (2011). https://doi.org/10.1007/s11270-010-0458-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0458-1