Abstract
The aim if this study was to assess the distribution of mercury in water, suspended particulate matter, surface sediments and biota from the Sado estuary, which, for the most part, is classified as a natural reserve, so as to evaluate its environmental quality status in terms of mercury contamination. Besides the diffuse sources of mercury coming into the Sado estuary, there are also additional contributions from the northern industrial zone and from the urban areas within the system. Applying national and international guidelines to different environmental matrices, the results obtained show that the system does not seem to be under environmental risk as far as mercury contamination is concerned. These quality guidelines can be used to rank and prioritise sites of concern. Hence, the area at the northern industrialised area deserves particular attention. The concentration of mercury in sediments of this area (0.54 mg kg−1) simultaneously succeeded the European Union Water Framework Directive (EU-WFD) reference value, the OSPAR Convention Ecotoxicological Assessment Criteria (EACs) and is classified as class 2 in degree of contamination by the National legislation, which implies some legal restrictive rules. Considering the stations close to urbanised areas, one exceeded the OSPAR EACs for dissolved mercury, whilst the other exceeded the EU-WFD reference value for mercury concentrations in sediments. No statistical significant relations were found between mercury concentrations in biota (Ulva sp, Hediste diversicolor, Scrobicularia plana, Cerstoderma edule and Carcinus maenas) and in the abiotic matrices (sediment and water column, including mercury in its dissolved form and associated to suspended particulate matter). This paper provides an overview of the guidelines for Hg proposed for a considerable number of coastal systems of the northern hemisphere and highlights the complex interactions of Hg in the different environmental compartments in low contaminated systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The toxicity of mercury (Hg) compounds is well known as being the ecological and toxicological effects dependent on the species of mercury present in the environment. Most anthropogenic mercury enters aquatic systems in its inorganic form via anthropogenic point discharges, mostly related to chlor-alkali plants (e.g. Alonso et al. 2000; Ram et al. 2003; Bloom et al. 2004; Pereira et al. 2009) and mining activities (e.g. Donkor et al. 2006) or through diffuse sources (e.g. De Marco et al. 2006; Rothenberg et al. 2008). The anthropogenic sources of mercury into the aquatic systems have been reduced due to legal restrictive rules (e.g. 50 µg L−1 is the limit value for discharges from chlor-alkali plants, in accordance with the European Union Directive 82/176/EEC). Nevertheless, Hg-contaminated sediments are still a cause for concern due to the potential release of Hg into other environmental matrices, such as the overlying water column and biota. Knowledge of the concentration of mercury in biological tissues is required, particularly in benthic fauna, in order to understand its bioamplification in the food web. In contaminated systems, mercury may be transferred from the abiotic to the biotic compartment (e.g. Baeyers et al. 2003; De Marco et al. 2006; Donkor et al. 2006). Once in biota, other processes may occur, namely the increase of mercury concentrations in fish (e.g. Baeyers et al. 2003; De Marco et al. 2006) or in shellfish (Coelho et al. 2006) with age (bioaccumulation); higher mercury concentration in seston (Baeyers et al. 2003) or in macroalgae (Cairrão et al. 2007) than in the water column (bioconcentration); or the increase of mercury concentrations through the trophic chain (biomagnification/bioamplification; Baeyers et al. 2003; De Marco et al. 2006).
Thus, Hg still is one of the most hazardous contaminants present in the aquatic environment, and it is included in the list of high-priority environmental pollutants within the Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR Convention), the European Union Water Framework Directive (EU-WFD) and the United States Environmental Protection Agency (U.S. EPA). As a result of historical contaminations and due to the deleterious effects of Hg on biota and ecosystem functions, the assessment of Hg contamination has been of increasing concern in aquatic systems all over the world.
Several classification methods have been developed to evaluate the environmental quality status of aquatic systems contaminated by mercury, namely the examples summarised in Table 1, which will be applied to the Sado estuary (Portugal). These criteria were selected because they involve a considerable number of coastal systems of the northern hemisphere, namely the North-East Atlantic countries (OSPAR), the European Union countries (WFD) and the United States (EPA).
According to the Portuguese legislation (Diário da República, II Série, Decreto-Lei 141/95 de 21 de Junho de 1995) and in agreement with the Oslo Convention, for dredging purposes, the sediments are classified within five classes according to their degree of contamination by metals (Cd, Cr, Cu, Hg, Pb, Ni and Zn), metalloids (As) and organic compounds (PCB, PAH and HCB), although in our assessment, only threshold values for Hg will be applied. Taking into account several ecotoxicological studies based on the Hg concentrations in sediments associated to toxic effects, Long (1992) and Long et al. (1995) calculated the Effects Range–Low (ERL) and Effects Range–Median (ERM); the Washington State Department of Ecology developed the criteria of Apparent Effects Threshold (AET) (Beckvar et al. 1996); whilst the OSPAR Convention defined the Ecotoxicological Assessment Criteria (EACs; Bignert et al. 2004). The EU-WFD has established a reference value for the concentration of Hg in sediments (Bignert et al. 2004), whilst (Directive 2008/105/EC) has been set up to define the ecological quality status of a water body (EQS), the annual average value (AA-EQS) and the maximum allowable concentration (MAC-EQS) of Hg and its compounds in the water column. As far as the water column is concerned, the OSPAR Convention has defined the EACs (Bignert et al. 2004), whilst the U.S. EPA established Current National Recommended Water Quality Criteria (WQC) for priority toxic pollutants (U.S. EPA 2006a; Table 1). Concerning biota, we applied the model proposed by the U.S. EPA (2006a, b) and by the European Commission (2006) and followed the examples of other authors in this field (e.g. Coelho et al. 2008a; Lewis and Chancy 2008).
Thus, the objectives of the present study were: (a) to assess the distribution of mercury (reactive and total) in samples of filtered water, suspended particulate matter, surface sediments and biota (green alga—Ulva sp.; bivalves—Scrobicularia plana, Cerstoderma edule; worms—Hediste diversicolor; and crabs—Carcinus maenas) in the Sado estuary; (b) to compare the maximum concentrations of total Hg in sediments collected in 1986 (Quevauviller et al. 1989), in 2000/2001 (Caeiro et al. 2005) and in 2006 (this study); and (c) to evaluate the environmental quality status of mercury using the national and international quality guidelines, which can be used to rank and prioritise sites in need of special attention.
2 Material and Methods
2.1 Study Site
The Sado River estuary is a warm-temperate mesotidal system located on the European Atlantic coast at the transitional zone between temperate and tropical climates (Quevauviller et al. 1989). The system has relevant ecological importance and a considerable economical and social value. The Sado estuary supports a resident population of bottlenose dolphins (Tursiops truncatus) and also supports activities intrinsically associated with the major population in coastal areas, namely industry, aquaculture, salt production, fishing and recreational activities. The estuarine area is 180 km2, with an intertidal area of 78 km2 (Vasconcelos et al. 2007), forming a wide bay and a narrow channel with large areas of salt marshes and intertidal flats. Tides are semidiurnal, with a tidal range of about 1.6 m in spring tides and 0.6 m in neap tides (Martins et al. 2000). The mean river flow is 40 m3 s−1 (Vasconcelos et al. 2007); however, it displays a strong seasonal variability, with freshwater discharges occurring mostly in winter (Rocha 2000). Thus, the system has also been referred to as a lagoon-type estuary (Cabeçadas et al. 1999), with a water volume of 500 × 106 m3 and mean water residence time of 30 days (Vasconcelos et al. 2007), .
The Sado estuary is, for the most part, classified as a natural reserve. However, it supports the industrial zone of the Setubal city located at its northern part, which confers high anthropogenic pressure (e.g. Caeiro et al. 2003; Martins et al. 2008). Furthermore, the system is located 40 km south of the very industrialised and populated metropolitan area of Lisbon (Costa et al. 2002). Moreover, in the Sado drainage basin, history of mining activities and pyrite outcrop erosion have contributed to the input of metals, namely Cd, Zn, Cu and locally Hg and Pb (Quevauviller et al. 1989). Mercury contamination levels in the estuary (lower and middle areas) have been determined in air particulate matter (fine, coarse and total and using lichens as biomonitors; Costa et al. 2002) and in sediments (Quevauviller et al. 1989; Caeiro et al. 2005). These last two authors found relatively high concentrations of metals with anthropogenic origin in the lower estuary. In spite of this, the system has never been assessed for mercury contamination combining the matrices sediment, water column and biota such as macroalga and benthic macrofauna, which are essential for a better understanding of mercury behaviour in aquatic systems.
2.2 Sampling Procedure, Samples Handling and Storing
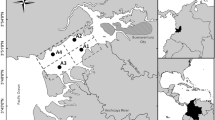
The sampling programme took place on the 16th of May 2006 at 24 sites enumerated from A to W and covering the entire estuarine area (Fig. 1). The sampling was made on two consecutive days, during low tide, and involved two teams of four people who simultaneously took samples from different locations. At each sampling station, water temperature and salinity were measured with the field sets: WTW Cond. 330i/set—Tetracon® 325 probe. In order to characterise the surface sediments (0–5 cm), sediment samples were collected during low tide and homogenised. Subsamples were analysed for organic matter content, as percentage of loss on ignition (%LOI; Dwt-Ash, dry weight minus ashes weight 500°C for 4 h) and for granulometry, according to Shepard (1954) classification method.
Sediment homogenised subsamples were oven-dried to constant weight at 60°C, sieved through an acid-washed 1-mm net sieve and stored until analysis for total mercury. Superficial water samples (1.5 L) for total and reactive dissolved mercury and for total mercury associated to suspended particulate mater (SPM) were collected during low tide with acid-washed poly(ethylene terephthalate) bottles and kept on ice during transportation to the laboratory where they were filtered with 0.45-µm pore size Millipore filters, acidified with concentrated HNO3 “Hg free” to pH < 2 and kept refrigerated (4°C) until analysis. Filters for mercury analysis in SPM were oven-dried at 60°C and digested with HNO3 4 mol L−1 (Coelho et al. 2006). At each sampling station, during low tide, whenever estuarine key species such as green alga (Ulva sp.), bivalves (S. plana and Cerastoderma edule), worms (H. diversicolor) or crabs (C. maenas) were present (species were selected for being abundant, ubiquitous and ecologically relevant), specimens (n = 10–20 to account for within-site variability) were collected from the intertidal mudflats and transported in a cool box. In the laboratory, algae samples were carefully washed with gentle rubbing to remove adherent sediment and epiphytes, dried to constant weight in a forced air oven at 60°C and homogenised. Sediment ingesting specimens were left to depurate overnight for gut cleansing, dissected, freeze-dried at −50°C and 0.06 bars and homogenised with a glass mortar and pestle. All biological samples were stored in acid-washed polypropylene flasks until analysis for total mercury.
2.3 Mercury Quantifications
Mercury concentrations in sediments and biota were determined by atomic absorption spectrometry with thermal decomposition of the sample without prior digestion and collection of the mercury vapour on a gold amalgamator (LECO AMA 254; Costley et al. 2000). In order to assess the accuracy and precision of the analytical methodology, analysis of certified reference materials were carried out (MESS-3 estuarine sediments; PACS-2 harbour sediment; BCR-60 Lagarosiphon major; TORT-2 lobster hepatopancreas) in parallel with samples and procedure blanks. Certified and measured values were in agreement with recoveries between 83–97%, 92–99%, 86–88% and 90–104%, respectively.
In the water, dissolved mercury (reactive and total) and SPM mercury analyses were done by cold-vapour atomic fluorescence spectrometry using a PSA model Merlin 10.023 equipped with a detector PSA model 10.003, with tin chloride as reducing agent (2% in 10% HCl). Reactive mercury was analysed directly in the acidified samples, whilst total dissolved mercury concentrations were determined according to the method described by Mucci et al. (1995). This determination is based on the addition of potassium persulphate to the sample and subsequent UV irradiation (1,000 W). The excess of oxidant is reduced with hydroxylamine solution. The accuracy of the method was tested by fortification of samples, with recoveries between 92% and 110%.
2.4 Statistical Analysis
Data were tested for normality (Kolmogorov–Smirnov test), and whenever it failed, data were log(x + 1)-transformed. The relation between abiotic matrices was tested using Pearson’s correlation, whilst the relation between mercury concentration in abiotic and biological matrices (dependent variable) was tested using a linear regression model. Statistical analyses were carried out with SPSS programme version 16.0.
A principal components analysis (PCA) was performed using the PRIMER version 5 software. The PCA is a technique to identify patterns in data and to express similarities and differences by projecting the data in a new coordinate system. In this projection, the greatest variance of the data falls on the first coordinate (called the first principal component), the second greatest variance on the second coordinate and so on. PCA can be used for dimensionality reduction without much loss of information. Due to the missing values concerning the biota, projections considered the principal components 1 and 2 for environmental variables vectors (HgWaterreactive, HgWatertotal, HgSPM, HgSediment, %HgSPM, %LOI, %Silt, %Clay, Salinity, HgHediste, HgUlva, HgCarcinushepatopancreas, HgCarcinusmuscle and HgCarcinusgills and stations (C, E, F, G, N, S, T, U, V and X). All concentration data were log(x + 1)-transformed.
3 Results and Discussion
3.1 The Environmental Conditions and Sediment Characterisation
The water temperature at low tide ranged from 19.1°C to 36.6°C, and the highest value corresponded to an abandoned salt pan (station I, Fig. 2a). At this station, salinity value reached 236, whilst in station W, located more upstream (narrow channel) close to the town of Alcácer do Sal, salinity dropped to 5. All the other stations are within the salinity range of 21–45 (Fig. 2c). These values are within the salinity range cited by other authors (e.g. Quevauviller et al. 1989) and a previous study which looked at the system salt pans and also recorded salinity values up to 249 (Amaral and Costa 1999). The organic matter content (%LOI) of the sediment ranged from 0.3% to 10%. The highest values corresponded to the northern industrialised zone (stations A–F, 2–10% LOI), to stations close to urban areas (G-Zambujal, W-Alcácer do Sal and T-Comporta, 5–9% LOI) and other sheltered areas of the system (stations H-J, O, S, U and X, 7–10% LOI; Fig. 2b). These areas had lower water circulation as they also corresponded to areas with higher percentage of fine particles in the sediments (Fig. 2d). In fact, there is a significant correlation (Pearson’s correlation, significant at the 0.05 level (two-tailed), N = 24) between the sediment organic matter content (%LOI) and the percentage of fine particles (%Silt+clay; r = 0.482, p = 0.017). The percentage of silt and clay in the sediment ranged from 17% to 48% (median 29%) at the northern industrialised zone, from 37% to 39% at the stations close to urban areas and from 19% to 50% (median 43%) at the other sheltered areas of the system. On the other hand, lower percentages of organic matter content corresponded to areas with a higher percentage of sand (Fig. 2d) suggesting that they are subject to higher hydrodynamics. In stations K, L, N, P, Q and R, the percentage of sand ranged from 82% to 99% (median 91%) and the organic matter content (%LOI) from 0.3% to 3%. These results are in agreement with previous studies (e.g. Caeiro et al. 2003, 2005).
3.2 Mercury Concentration in Sediments and Water Column
Within the 24 stations, the median concentration of mercury in the top 5 cm of the sediment (Fig. 3a) was 0.13 mg kg−1, with a maximum of 0.54 mg kg−1 at station C—the northern industrial zone and a minimum of 0.003 mg kg−1 at site K. The higher concentrations correspond to the northern industrialised zone (stations A–D and F), stations close to urban areas (G-Zambujal, W-Alcácer do Sal and T-Comporta) and other sheltered areas of the system (stations H, J, N, O, S and X). These results may be explained by the higher influence of anthropogenic activity in addition to the higher organic content (%LOI) and lower hydrodynamics of these areas (higher percentage of sediment fine particles). Quevauviller et al. (1989) and Caeiro et al. (2005) also found higher mercury concentrations (maximum concentration of 0.9 and 0.7 mg kg−1, respectively) in the system of the northern industrial zone. In addition, Quevauviller et al. (1989) concluded that the anthropogenic sources of metals in the lower estuary, which were identified as urban and industrial, were found containing high sediment particulate organic matter (POC). Furthermore, in our study, the lower mercury concentrations were found in stations referred to as being exposed to higher hydrodynamics that is with low organic content (%LOI) and coarser texture.
The median concentration of total dissolved mercury was 12.5 ng L−1, with a maximum of 79 ng L−1 and a minimum of 4.0 ng L−1, whilst the maximum concentration of reactive mercury was 7.0 ng L−1 (Fig. 3c). The higher dissolved mercury concentrations were found in stations with lower direct influence of anthropogenic activity (outside the industrial and urban areas, except for station T which is close to Comporta village). In the Sado estuary, the higher dissolved reactive mercury concentrations were restricted to the central area of the system (stations K, L, N and O) and to the more downstream station Q, although values were quite close to the analytical limit of detection (2 ng L−1).
The median concentration of total mercury in SPM was 0.30 mg kg−1, with a maximum of 0.70 mg kg−1 and a minimum of 0.072 mg kg−1 (Fig. 3b). Concentrations of mercury were higher in SPM comparative to the sediment pool (based on a mass relation) due to the association of mercury with the very fine particles in suspension. To better understand the effect of the particulate fraction (SPM) on the transport of Hg in the water column, the percentage of mercury associated to the SPM (particulate fraction) was related to the concentration of total dissolved Hg (dissolved fraction), calculated as:
The percentage of mercury associated to the SPM ranged from 4% to 93%, with a median value of 49%. Results show a higher percentage of mercury associated to the SPM in the northern industrialised zone (stations A–F), stations close to urban areas (G-Zambujal and W-Alcácer do Sal) and other sheltered areas of the system (stations H, R, S, U, V and X; Fig. 3d). No influence of salinity in the distribution of the metal between the dissolved and the particulate fractions were observed, i.e. neither SPM (particulate fraction) nor total dissolved Hg (dissolved fraction) were correlated to salinity (Pearson’s correlation, significant at the 0.05 level (two-tailed), N = 24). The percentage of mercury associated to the SPM was significantly correlated to mercury concentrations in sediments (Hg%SPM and Hgsediment, r = 0.507, p = 0.012), together with the organic matter content (Hg%SPM and %LOI, r = 0.410, p = 0.047), but not to the percentage of the sediments’ finer fractions (Hg%SPM and %silt, r = 0.255, p = 0.228, and Hg%SPM and %clay, r = 0.330, p = 0.115). The concentration of mercury in sediments was also significantly correlated to the sediment organic matter content (Hgsediment and %LOI, r = 0.493, p = 0.014), which is in agrement with results from Quevauviller et al. (1989). However, the concentration of mercury in sediments was not correlated with the percentage of the sediments’ finer fractions (Hgsediment and %Silt, r = 0.298, p = 0.157, and Hg%SPM and %Clay, r = 0.336, p = 0.108). These results show that association of mercury to organic matter is more important than the increase in surface area; other important bearing phases for mercury like iron and manganese oxyhydroxides can also disturb the expected relationship with sediment fine fraction. However, in estuaries, sediment organic matter can be considerably more important than Fe–Mn oxides in mercury sorption (Turner et al. 2004). Although most of the sampling stations are located in areas with low hydrodynamics, they have, as most depositional areas do, a higher percentage of sediment fine fractions. These areas are also subjected to tidal action that is responsible for the resuspension of the suspended particulate matter, particularly in intertidal areas. In addition, bioturbation causes the redistribution of particles at the sediment surface and increases the surface/area available for sediment–water interface exchange.
The higher concentrations of total dissolved mercury corresponded mostly to stations with lower concentration of mercury in the sediment and lower percentage of mercury in SPM. However, no significant correlations (Pearson’s correlation) could be found between Hgdissolved_fraction and Hgsediment (r = −0.384, p = 0.064). Yet, Hgdissolved_fraction and Hg%SPM were significantly negatively correlated (r = −0.673, p < 0.001). Overall, the concentrations of total dissolved mercury are low; even so, these results suggest that dissolved mercury at the central part of the system has its source in other estuarine areas and that mercury is transported within the system. The coarser granulometry at the system central areas also suggests a higher hydrodynamics that at this time of the year (May) is mostly due to tidal influence. These results pointed out the complex interactions within and beteween the environmental matrices, suggesting that the variability of metal partition along the system potentially influences the exposure to mercury in organisms with different bioaccumulative pathways.
3.3 Mercury Concentration in Biota
Even though benthic macrofauna and green macroalga tolerate a wide range of physicochemical environmental conditions, in two stations, none of the selected biota were found: one corresponded to an extremely high salinity value (station I—the abandoned salt pan) and the other to the lowest salinity value (station W—located more upstream (narrow channel) close to the town of Alcácer do Sal).
Mercury concentrations in Ulva sp. ranged from 0.006 to 0.050 mg kg−1 wet weight (wwt), with a median concentration of 0.018 mg kg−1 wwt (Fig. 4a). Macroalgae have been studied as biomonitors of dissolved mercury (e.g. Coelho et al. 2005; Cairrão et al. 2007), yet free-floating algal species like the green macroalgae Ulva sp. will have higher mobility and may not reflect environment contamination where collected (Coelho et al. 2005). In the Sado estuary, no significant relations (linear regression model) were found between the concentrations of mercury in Ulva sp. and the concentration of mercury in the abiotic matrices (sediment and water column, including mercury in its dissolved form and associated to the suspended particulate matter).
Regarding fauna, the worm H. diversicolor had a median mercury concentration of 0.03 mg kg−1, wwt, in a range of 0.015–0.073 mg kg−1, wwt (Fig. 4b), whilst in bivalves, mercury concentrations (S. plana and C. edule) ranged from 0.016 to 0.041 mg kg−1, wwt (Fig. 4c). In the crab C. maenas, the concentration of mercury was analysed in three different tissues since accumulation may occur through specific pathways, namely from water column, sediments or diet (e.g. Coelho et al. 2008b). Results show that the higher accumulation of mercury occurred in the muscle tissue (median 0.082; maximum 0.17; minimum 0.015 mg kg−1, wwt) followed by the hepatopancreas (median 0.055; maximum 0.14; minimum 0.007 mg kg−1, wwt; Fig. 4d). The gills presented the lowest levels of mercury (median 0.021; maximum 0.058; minimum 0.008 mg kg−1, wwt). This tissue mercury distribution is in agreement with the pattern described for C. maenas living in low contaminated areas where diet was considered the main source of mercury (Coelho et al. 2008b). That pathway is reflected in higher levels on internal organs (muscle and hepatopancreas) as opposed to gills (Coelho et al. 2008b). Our results did not show any statistical significant relations (linear regression model) between mercury concentrations in biota and the amount of the metal in sediment or in the water column, including mercury in the dissolved form or associated to the suspended particulate matter.
The PCA showed that principal components 1 and 2 only explained 50% of the variance. Figure 5 shows that principal component 1, which explains 29% of the variance, separates on the right side of the axis (positive values) stations G, C, T, U and V characterised by Hg in sediment and in SPM, total dissolved Hg and Hg in the green macroalgae Ulva sp. The left side of the principal components 1 (axis negative values) includes stations S, X, F and E. Principal component 2, which explains 21% of the variance, separates station V, which was characterised by a higher total of dissolved Hg and Hg in the green macroalgae Ulva sp.; station E, which is characterised by reactive dissolved Hg and Hg in H. diversicolor and in C. maenas gills; and stations S, X and F, which are characterised by per cent of Hg in SPM and Hg in C. maenas hepatopancreas and in the muscle. However, the PCA analysis must be interpreted with caution since it only explains 50% of the variance, even though Hg in the sediment was not related with Hg in biota. As pointed out by Beckvar et al. (1996), the difficulty in correlating mercury in sediment with mercury in organisms reflects the complexity of variables that affect both the methylation of mercury in surface sediments and the transfer of mercury between trophic levels. Thus, the concentration of total mercury in sediments or water column alone may not provide information on the potential exposure of biota since mercury bioavailability can be affected by numerous variables such as sediment geochemistry, particle size or organic matter content, as well as the physical factors that affect the rate of methylation and demethylation (Beckvar et al. 1996). Ideally, all trophic levels should be included, from primary producers to top predators, including fish. However, it was not possible to include icthiofauna in the present assessment.
Results from the PCA showing the principal components 1 and 2 for environmental variables vectors (HgWaterreactive, HgWatertotal, HgSPM, HgSediment, %HgSPM, %LOI, %Silt, %Clay, Salinity, HgHediste, HgUlva, HgCarcinushepatopancreas, HgCarcinusmuscle and HgCarcinusgills and stations (C, E, F, G, N, S, T, U, V and X)
3.4 Application of National and International Guidelines to the Studied Matrices
Applying the Portuguese legislation (Diário da República, II Série, Decreto-Lei 141/95 de 21 de Junho de 1995) criteria concerning only the concentration of Hg in sediments, all of the studied stations fall within class 1, except station C at the northern industrial zone which corresponds to class 2 (implying some legal restrictive rules; Table 2). Taking into account the Effects Range–Low concentration and Effects Range–Median concentration guidelines, proposed by Long (1992), none of the study sites exceeds the ERM concentration, yet 11 stations (Table 2) are above the ERL. Most of these stations are located at the northern industrial zone, and the others are close to Zambujal and Comporta villages and the town of Alcácer do Sal, proving the anthropogenic sources of mercury. Applying the AET concentrations (Beckvar et al. 1996), all studied sites at Sado estuary are far below this threshold. Considering the EACs (OSPAR Convention; Bignert et al. 2004), only station C, at the northern industrial zone, is above the defined range. According to the EU-WFD criteria (Bignert et al. 2004), four stations in the northern industrial zone and the station close to the town of Alcácer do Sal town exceed the reference value (0.360 mg kg−1).
Concerning the water column (total dissolved Hg) and applying the U.S. EPA WQC (U.S. EPA 2006a), all studied sites at the Sado estuary are far below the established thresholds (Table 2). However, applying the EACs recommended by OSPAR Convention, three stations (close to Zambujal village and two located at the southern part) were below the defined range (below 5 ng L−1), whilst three stations (close to Comporta village and two located at the northern part) exceeded the EAC range. All other stations fall within the defined interval (Table 2). Concerning the maximum allowed concentration of (MAC-EQS) established by the EU-WFD for surface waters (other than inland waters), only one station exceeded the threshold (Table 2). Concerning biota, green macroalga may be harvested for direct or indirect human uses, and benthic macrofauna species such as S. plana, C. edule and C. maenas may be captured for human consumption. The worm H. diversicolor is not consumed by humans, yet is one of the most widely distributed marine polychaetes and like the aforementioned species constitutes an important prey for higher trophic levels, namely fish and birds. None of the analysed fauna from the Sado estuary exceeded the threshold values. The application of this international regulatory limits is important due to the possibility of bioaccumulation (the increase of mercury concentrations with age; e.g. Baeyers et al. 2003; Coelho et al. 2006), bioconcentration (higher concentration in biota than in the abiotic matrix; e.g. Baeyers et al. 2003; Cairrão et al. 2007) and biomagnification/bioamplification (the increase of mercury concentrations through the trophic web; e.g. Baeyers et al. 2003; De Marco et al. 2006).
Considering the mercury levels in different environmental matrices and the quality guidelines applied, summarised in Table 1, the Sado estuary does not seem to be under environmental risk. Caeiro et al. (2005), by assessing heavy metal contamination in Sado estuary sediment using an index analysis approach, also found that concerning Hg the estuary showed only trace contamination. However, further research should include the mercury levels in fish tissues, namely methylmercury, since it has been shown in other systems that a water body may meet the water quality guidelines, yet have high fish tissues level (U.S. EPA 2006b). A good target to assess the contamination of methylmercury seems to be the flatfish (Solea senegalensis Kaup, 1858) since it is a common benthic fish in the Sado estuary and is a valuable bycatch for local fisheries (Costa et al. 2009).
As these quality guidelines can be used to rank and prioritise sites of concern, the northern industrialised area (study site C) justifies special attention concerning mercury contamination. This is reinforced by previous studies, namely the assessment of metals contamination in Sado estuary (Quevauviller et al. 1989; Caeiro et al. 2005). The area located at the northern industrial zone, which also corresponds to a management unit defined by high organic load (Caeiro et al. 2003), was classified, based on the sediment contamination by Cd, Cu, Pb, Cr, Hg, Al, Zn and As, from lightly to highly contaminated, in bad condition and with high impact potential according to the applied classification indices (Caeiro et al. 2005). Unfortunately, our results cannot be compared with the previous ones as the analysed sediment fractions are not comparable. The fact is that in 1986, the highest concentrations of Hg in the sediment at the northern industrial zone was 0.9 mg kg−1 (sediment fraction <63 µm; Quevauviller et al. 1989); in 2000/2001, it was 0.7 mg kg−1 (entire sample; Caeiro et al. 2005), whilst in this study (2006), the highest concentration was 0.54 mg kg−1 (sediment fraction <1 mm). Thus, it is not possible to establish a temporal variation of Hg concentration on the sediment surface at the northern industrial area. As far as the atmospheric compartment is concerned, Costa et al. (2002) also found higher mercury levels in air particulate matter (fine, coarse and total) in the northern part of the region. Authors related these mercury levels to an important chemical industry at Barreiro industrial complex located in the southern Tagus estuary. Comparatively, and in the same study, the southern part of the system (Troia, Fig. 1) showed lower mercury levels, which are in agreement with our results.
4 Conclusions
In the Sado estuary, mercury contamination seems to result from diffuse sources in addition to the contribution of the northern industrial zone, together with Alcácer do Sal and the Comporta urban areas. Station C, situated in the northern industrial area, was the only one with mercury concentration in sediments exceeding simultaneously the EU-WFD reference value, the OSPAR Convention Ecotoxicological Assessment Criteria (EACs) and classified as class 2 in degree of contamination by the national legislation, which implies some legal restrictive rules. Looking at the stations close to urbanised areas, the one close to Comporta village exceeded the OSPAR EACs for dissolved mercury, whilst the one close to Alcácer do Sal town exceeded the EU-WFD reference value for mercury concentrations in sediments. However, none of the analysed biota exceeded international regulatory limit for mercury concentration. No significant statistical correlations were found between the contamination by mercury in abiotic (sediment and water column) and the biological matrices (algae and estuarine key fauna species). From the present results, we may conclude that the Sado estuary does not seem to be under environmental risk from mercury contamination. However, further research should include the mercury levels in fish tissues to ensure that there is no human health risk through fish consumption.
This paper provides an overview of the guidelines for Hg proposed for a considerable number of coastal systems in the north hemisphere. These conventions and directives have harmonised rules not only to protect the aquatic environment against the discharge of priority substances but also to monitor and evaluate the effects of these substances at the biological community level. This study also highlights the complex interactions of Hg in the biotic and abiotic environmental compartments of low contaminated systems.
References
Alonso, D., Pineda, P., Olivero, J., Gonzaález, H., & Campos, N. (2000). Mercury levels in muscle of two fish species and sediments from the Cartagena Bay and the Cie naga Grande de Santa Marta, Colombia. Environmental Pollution, 109, 157–163.
Amaral, M. J., & Costa, M. H. (1999). Macrobenthic communities of saltpans from the Sado estuary (Portugal). Acta Oecologica, 20, 327–332.
Baeyers, W., Leermakers, M., Papina, T., Saprykin, A., Brion, N., Noyen, J., et al. (2003). Bioconcentration and biomagnification of mercury and methylmercury in North Sea and Scheldt Estuary Fish. Archives of Environmental Contamination and Toxicology, 45, 498–508.
Beckvar, N., Field, J., Salazar, S., & Hoff, R. (1996). Contaminants in aquatic habitats at hazardous waste sites: Mercury. NOAA Technical Memorandum NOS ORCA 100. Seattle: Hazardous Materials Response and Assessment Division, National Oceanic and Atmospheric Administration. 74 pp. Retrieved 20 October 2009 from http://response.restoration.noaa.gov/book_shelf/89_rpt_mercury_aquatic_habitats.pdf.
Bignert, A., Cossa, D., Emmerson, R., Fryer, R., Füll, C., Fumega, J., et al. (2004). OSPAR/ICES Workshop on the evaluation and update of background reference concentrations (B/RCs) and ecotoxicological assessment criteria (EACs) and how these assessment tools should be used in assessing contaminants in water, sediments and biota. Final report to OSPAR Commission, The Hague, pp. 92–96. Retrieved 20 October 2009 from http://www.ospar.org/documents/dbase/publications/p00214_BRC%20EAC%20Workshop.pdf.
Bloom, N. S., Moretto, L. M., Scopece, P., & Ugo, P. (2004). Seasonal cycling of mercury and monomethyl mercury in the Venice Lagoon (Italy). Marine Chemistry, 91, 85–99.
Cabeçadas, G., Nogueira, M., & Brogueira, M. J. (1999). Nutrient dynamics and productivity in three European estuaries. Marine Pollution Bulletin, 12, 1092–1096.
Caeiro, S., Goovaerts, P., Painho, M., & Costa, M. H. (2003). Delineation of estuarine management areas using multivariate geostatistics: The case of Sado estuary. Environmental Science & Technology, 37, 4052–4059.
Caeiro, S., Costa, M. H., Ramos, T. B., Fernandes, F., Silveira, N., Coimbra, A., et al. (2005). Assessing heavy metal contamination in Sado estuary sediment: An index analysis approach. Ecological Indicators, 5, 151–169.
Cairrão, E., Pereira, M. J., Pastorinho, M. R., Morgado, F., Soares, A. M. V. M., & Guilhermino, L. (2007). Fucus spp. as a mercury contamination bioindicator in costal areas (Northwestern Portugal). Bulletin of Environmental Contamination and Toxicology, 79, 388–395.
Coelho, J. P., Pereira, M. E., Duarte, A., & Pardal, M. A. (2005). Macroalgae response to a mercury contamination gradient in a temperate coastal lagoon (Ria de Aveiro, Portugal). Estuarine, Coastal and Shelf Science, 65, 492–500.
Coelho, J. P., Rosa, M., Pereira, M. E., Duarte, A., & Pardal, M. A. (2006). Pattern and annual rates of Scrobicularia plana mercury bioaccumulation in a human induced mercury gradient (Ria de Aveiro, Portugal). Estuarine, Coastal and Shelf Science, 69, 629–635.
Coelho, J. P., Nunes, M., Dolbeth, M., Pereira, M. E., Duarte, A., & Pardal, M. A. (2008a). The role of two sediment-dwelling invertebrates on the mercury transfer from sediments to the estuarine trophic web. Estuarine, Coastal and Shelf Science, 78, 505–512.
Coelho, J. P., Reis, A. T., Ventura, S., Pereira, M. E., Duarte, A., & Pardal, M. A. (2008b). Pattern and pathways for mercury lifespan bioaccumulation in Carcinus maenas. Marine Pollution Bulletin, 56, 1104–1110.
Commission Regulation (EC). (2006). No 1881/2006 of 19 December (2006). Setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union. L364/20.
Costa, C. J., Marques, A. P., Freitas, M. C., Reis, M. A., & Oliveira, O. R. (2002). A comparative study for results obtained using biomonitors and PM10 collectors in Sado estuary. Environmental Pollution, 120, 97–106.
Costa, P. M., Diniz, M. S., Caeiro, S., Lobo, J., Martins, M., Ferreira, A. M., et al. (2009). Histological biomarkers in liver and gills of juvenile Solea senegalensis exposed to contaminated estuarine sediments: A weighted indices approach. Aquatic Toxicology, 92, 202–212.
Costley, C., Mossop, K., Dean, J., Garden, L., Marshall, J., & Carroll, J. (2000). Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Chimica Acta, 405, 179–183.
De Marco, S. G., Botté, S. E., & Marcovecchio, J. E. (2006). Mercury distribution in abiotic and biological compartments within several estuarine systems from Argentina: 1980–2005 period. Chemosphere, 65, 213–223.
Diário da República, II Série, Decreto-Lei 141/95 de 21 de Junho de (1995). Classificação de materiais dragados. Despacho Conjunto dos Ministérios do Ambiente e Recursos Naturais e do Mar. Portugal.
Directive 2008/105/EC of the European Parliament and of the Council, of 16 December (2008). Official Journal of the European Union. L 348/93.
Donkor, A. K., Bonzongo, J. C., Nartey, V. K., & Adotey, D. K. (2006). Mercury in different environmental compartments of the Pra River Bay, Ghana. Science of Total Environment, 368, 164–176.
Lewis, M., & Chancy, C. (2008). A summary of total mercury concentrations in flora and fauna near common contaminant sources in the Gulf of Mexico. Chemosphere, 70, 2016–2024.
Long, E. R. (1992). Ranges in chemical concentrations in sediments associated with adverse biological effects. Marine Pollution Bulletin, 24, 38–45.
Long, E. R., Macdonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effect within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97.
Martins, F., Leitão, P., Silva, A., & Neves, R. (2000). 3D modelling in the Sado estuary using a new generic vertical discretization approach. Oceanologica Acta, 24, S51–S62.
Martins, M., Ferreira, A. M., & Vale, C. (2008). The influence of Sarcocornia fruticosa on retention of PAHs in salt marsh sediments (Sado estuary, Portugal). Chemosphere, 71, 1599–1606.
Mucci, A., Lucotte, M., Montgomery, S., Plourde, Y., Pichet, P., & Van Tra, H. (1995). Mercury remobilization from flooded soils in a hydroelectric reservoir of Northern Quebec, La Grande-2: Results of a soil resuspension experiment. Canadian Journal of Fisheries and Aquatic Sciences, 52, 2507–2517.
Pereira, M. E., Lillebø, A. I., Pato, P., Válega, M., Coelho, J. P., Lopes, C., et al. (2009). Mercury pollution in Ria de Aveiro (Portugal): A review of the system assessment. Environmental Monitoring and Assessment, 155, 39–49.
Quevauviller, F., Lavigne, R., & Cortez, L. (1989). Impact of industrial and mine drainage wastes on the heavy metal distribution in the drainage basin and estuary of the Sado River (Portugal). Environmental Pollution, 59, 267–286.
Ram, A., Rokade, M. A., Borole, D. V., & Zingde, M. D. (2003). Mercury in sediments of Ulhas estuary. Marine Pollution Bulletin, 46, 846–857.
Rocha, C. (2000). Density-driven convection during flooding of warm, permeable intertidal sediments: The ecological importance of the convective turnover pump. Journal of Sea Research, 43, 1–14.
Rothenberg, S. E., Ambrose, R. F., & Jay, J. A. (2008). Mercury cycling in surface water, pore water and sediments of Mugu Lagoon, CA, USA. Environmental Pollution, 154, 32–45.
Shepard, F. P. (1954). Nomenclature based on sand–silt–clay ratios. Journal of Sediment Petrology, 24, 151–158.
Turner, A., Millward, G. E., & Le Roux, S. M. (2004). Significance of oxides and particulate organic matter in controlling trace metal partitioning in a contaminated estuary. Marine Chemistry, 88(3–4), 179–192.
U.S. EPA (2006a). National Recommended Water Quality Criteria. (nrwqc-2006.pdf). U.S. Environmental Protection Agency, Office of Water, Washington, DC. Retrieved 17 February 2009 from http://www.epa.gov/waterscience/criteria/wqctable/.
U.S. EPA (2006b). Draft Guidance for Implementing the January 2001 Methylmercury Water Quality Criterion. EPA 823-B-04-001. U.S. Environmental Protection Agency, Office of Water, Washington, DC. Retrieved 17 February 2009 from http://www.epa.gov/waterscience/criteria/methylmercury.
Vasconcelos, R. P., Reis-Santos, P., Fonseca, V., Maia, A., Ruano, M., França, S., et al. (2007). Assessing anthropogenic pressures on estuarine fish nurseries along the Portuguese coast: A multi-metric index and conceptual approach. Science of Total Environment, 374, 199–215.
Acknowledgements
Authors are grateful to FCT (Fundação para a Ciência e Tecnologia) for funding given to the Associated Laboratory CESAM (Centro de Estudos do Ambiente e do Mar). The Post-doc grants SFRH/BPD/48449/2008 (P. Coelho), SFRH/BPD/35068/2007 (P. Pato) and SFRH/BPD/48134/2008 (M. Válega) funded by FCT are acknowledged. Authors also acknowledge the anonymous referees for the careful revision and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lillebø, A.I., Coelho, P.J., Pato, P. et al. Assessment of Mercury in Water, Sediments and Biota of a Southern European Estuary (Sado Estuary, Portugal). Water Air Soil Pollut 214, 667–680 (2011). https://doi.org/10.1007/s11270-010-0457-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0457-2