Abstract
Lipophilic anthropogenic contaminants enter the environment from different kinds of human activities and corresponding emission sources. In the hydrosphere, they accumulate frequently in specific sedimentary zones, among others, and at coastal areas, forming reservoirs of pollutants. Marine and freshwater sediment samples as well as soil samples from a highly industrialized coastal area in Northern Greece have been analyzed in order to have a detailed view on the state of the particle-associated pollution. Noteworthy, based on extended GC/MS non-target screening analyses, interesting, so far unknown, or rarely documented contaminants have been identified and quantified comprising, e.g., mono- and dichlorocarbazoles, bromocarbazole, 2,6-di-tert-butyl-4-nitrophenol, etc. However, all relevant contaminants are discussed with respect to their spatial concentration profiles, their emission sources, and their pathway. In addition, numerous pollutants are suggested to become selected for environmental monitoring programs. Hence, this study can act as an example for adapting individual monitoring programs to the individual contamination in coastal areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Marine and freshwater sediments are integral and dynamic parts of the aquatic environment that are frequently affected directly or indirectly by anthropogenic discharge of contaminants. Principally, two general emission sources can be differentiated: (1) direct point emissions including industrial discharge, municipal sewage effluents, or harbor activities and (2) activities like production, refining, or usage of petrogenic products, shipping, or agriculture activities, representing more diffuse sources contaminating indirectly the aquatic system (Chen and White 2004; Heim et al. 2004; Schwarzbauer et al. 2000). The organic contaminants from the latter emissions can enter the aquatic environment by different pathways like surface runoff, erosion, or leaching of soils, aerial deposition, spray drifts, volatilization, or direct discharge of wastes.
Due to their hydrophobic nature, many organic contaminants in aquatic systems are dominantly associated with particulate matter (Parks 1975; Olsen et al. 1982). Hence, sediments and suspended particulate matter act as important tools for transport and deposition of associated organic compounds and, with respect to the long-term behavior, tend to support geoaccumulation on partially high levels. Further on, contaminated particulate matter in main aquatic sedimentation zones, e.g., coastal areas, can form natural reservoirs for secondary contamination which can affect the benthic organisms and, consequently, can transfer pollutants to higher trophic level organisms through food chain transfer (Warren et al. 2003; EPA 1996).
The important role of sediment pollution in the environmental assessment is the main motivation for many studies applied on aquatic sediment systems. In particular, nowadays, several international sediment management and monitoring programs have been developed. For example, the European Sediment Network is an activity which provides new aims and methods in sediment management including riverine, estuarine, and marine sediments (Apitz and Power 2002). Furthermore, the European AquaTerra project is a second example which provides a better understanding of the river–sediment–soil–groundwater system (Barth et al. 2007; Gerzabek et al. 2007). On the other hand, many programs have national character like the Alaska Environmental Monitoring and Assessment Program (Long et al. 1995) and the San Francisco Estuary Regional Monitoring Program (Hoenicke et al. 2007). However, most of the monitoring programs investigate the presence of preselected groups of dominantly priority pollutants like polycyclic aromatic hydrocarbons (PAHs; e.g., Gocht and Grathwohl 2004), organochlorine compounds or polychlorinated biphenyls (PCBs; e.g., Peré-Trepat et al. 2006). According to Petty et al. (2004), monitoring programs might be inherently limited to provide a holistic exposure assessment for several reasons, e.g., the site conditions (e.g., water quality) or the insufficiency of the analytical sensitivity and selectivity to detect and quantify trace levels of contaminants.
However, a further restriction of monitoring programs is related to a large variety of organic contaminants comprising not only pesticides, pharmaceuticals, industrial chemicals, or personal care products but also numerous so far unknown or rarely reported compounds. These latter substances are usually man-made xenobiotics that are usually not considered in monitoring network programs. The application of a non-target screening approach provides the opportunity to expand the knowledge on low-molecular-weight organic pollutants in a restricted region and reveals a first deeper insight into the state of pollution (Bester et al. 1998; Ricking et al. 2003; Kronimus et al. 2004). Further on, compounds identified by screening analyses should be evaluated based on their molecular structure, their technical usage, and their (eco)toxicity. Additionally, results of non-target screening analyses might allow (1) to identify individual emission pathways, (2) to link contaminants with specific sources, and (3) to investigate in more detail the spatial distribution of specific contaminations (Bester and Theobald 2000; Galassi et al. 2004; Dsikowitzky et al. 2004; Schwarzbauer and Heim 2005; Weigel et al. 2005). However, the most important value of non-target screening analyses is to provide suggestions for including so far unnoticed or new contaminants in optimized monitoring programs.
In this study, detailed gas chromatographic-mass spectrometric (GC/MS) non-target screening analyses have been applied to riverine and marine sediments as well as to soils derived from a highly industrialized coastal area next to Kavala city, in Northeastern Greece. The numerous compounds identified in the area are classified dominantly according to their molecular structure. Furthermore, they are discussed with respect to: (a) their quantitative data, (b) their potential source specificity, and (c) their usage and/or applications if such data are available.
2 Materials and Methods
2.1 Area Description and Sampling
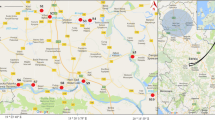
The studied region is a coastal area situated 15 km east of Kavala city (Northeast Greece), which is significantly affected by anthropogenic activities. The main industrial activities are attributed to petrochemical industry (in particular, crude oil treatment facility, sulfur and natural gas liquids extraction facilities, and storage of these products), a phosphoric fertilizer plant (with production facilities of ammonia, phosphoric acid, sulfuric acid, nitric acid, nitrogenous fertilizers, etc. and installations for the storage, bagging, palletizing, in-plant handling, and loading of products on ships and trucks and a harbor), a fishery trade (with production and storage areas, freezing compartments, and tin ware production units), marble quarries, and some enterprises which trade with inert materials. Additionally, in the surrounding area, extended agricultural activities are taking place and the new national highway (Egnatia odos) is passing through, and furthermore, uncontrolled dumping of huge amounts of household wastes contaminates this area (Grigoriadou et al. 2007).
In January 2004, 22 soil samples (S1 to S22) of approximately 150 g were collected from the mainland site next to the costal line. Sampling was performed using a stainless steel shovel with a sampling depth of approximately 10 cm. Additionally, seven aquatic sediment samples (T1 to T7) were taken from swales and canals in the area between the fishery and the petrochemical industry. The collected amount of riverine sediment was approximately 150 g, respectively. Ten marine sediment samples (M1 to M10) of approximately 300 g, respectively, were collected from the bottom of the sea at a distance between 500 and 1,500 m from the coastline (Fig. 1). Both kinds of sediment samples were collected from the top 10 cm by a sediment rabber. Further on, for each sample, the loss-on-ignition was determined after DIN 38414 (Deutsches Institut fur Normung e.V.), and the total organic carbon content (% TOC, s. Table 1) has been calculated using a factor of 1.724 as proposed by Scheffer and Schachtschabel (1998).
2.2 Analysis
Approximately 100 g of soil and terrestrial sediment samples have been treated by Soxhlet extraction. Each sample was placed in a Soxhlet apparature and extracted sequentially with 150 mL of acetone for 24 h and 250 mL of hexane for 24 h. The extracts were combined and carefully concentrated to a volume of approximately 2 mL at room temperature using a rotary evaporator.
Further on, aliquots of 150 g of marine sediments were extracted by a extraction procedure using an overhead-shaking method. The samples were extracted with a mixture of 80 mL of acetone and 120 mL of n-hexane. The extraction was initiated by ultrasonication for 15 min, followed by 6 h overhead-shaking. The water phase was discarded, and the acetone–hexane mixture was concentrated at room temperature by rotary evaporation down to a volume of approximately 2 mL. All samples were dried over 2 g of anhydrous granulated sodium sulfate and re-concentrated to a volume of approximately 1 mL. Finally, the raw extracts were desulferized by addition of pre-cleaned and activated copper powder.
Following, the extracts were separated into six fractions by column chromatography on silica gel using mixtures of pentane, dichloromethane, and methanol as eluates with increasing polarity according to Schwarzbauer et al. (2000). After fractionation, 50 μL of a surrogate standard solution containing 6 ng/μL of n-hexadecane was added to fractions 1 to 5 and 200 μL of surrogate standard solution containing 5.8 ng/μL of fluoroacetophenone was added to the sixth fraction. Acidic compounds in the sixth fraction were methylated using a methanolic diazomethane solution. Finally, prior to gas chromatographic and gas chromatographic-mass spectrometric analyses, all extracts were reduced to a volume of approximately 50 μL by rotary evaporation at room temperature.
2.2.1 GC and GC/MS Analyses
The GC analysis was carried out on a GC6000 gas chromatograph (Carlo Erba, Vega Series 2, Milan, Italy) equipped with a 30 m × 0.25 mm id × 0.25 μm film ZB5 fused silica capillary column (Zebron, Germany) and a flame ionization detector. Chromatographic conditions were as follows: injection volume 1 μL; split/splitless injection at 270°C; splitless time 60 s; temperature program, 60οC oven temperature, 3 min isothermal time, temperature raising rate of 3°C/min up to 300°C hold for 15 min. The hydrogen carrier gas velocity was 40 cm/s.
GC/MS analyses were performed on a Finnigan MAT 8222 mass spectrometer (Finnigan, Germany) linked to a HP 5890 gas chromatograph (Hewlett Packard, USA) equipped with a 30 m × 0.25 mm id × 0.25 μm film BPX5 fused silica capillary column (SGE, Germany). Chromatographic conditions were the same as described above. The mass spectrometer was operated in full-scan mode at a resolution of 1,000 in EI+ mode (70 eV), source temperature of 200οC, scanning from 35–700 amu at a rate of 1 s/decade and an inter-scan time of 0.1 s.
2.2.2 Quality and Quantity Results
Identification of individual compounds was based on the comparison of EI+—mass spectra and gas chromatographic retention times with those of reference compounds. First evidence of identification derived from comparison of mass spectra with mass spectral data bases (NIST/EPA/NIH Mass Spectral Library NIST02, Wiley/NBS Registry of Mass Spectral Data, 7th Ed., electronic versions). Retention time inaccuracies were corrected by the retention time of the surrogate standard.
Quantitative data of selected target compounds were obtained by integration of specific ion chromatograms extracted from the TIC. Injection volume and sample volume inaccuracies were corrected by using d34-hexadecane as a surrogate standard. An external four-point-calibration generated from a mixture of reference compounds was used for quantification, and all data were normalized to dry matter. The limit of quantification was determined to be approximately 0.1 ng/g, but no attempts were made to quantify components at concentrations less than 0.5 ng/g.
Finally, blank experiments of the two applied extraction methods (Soxhlet and overhead-shaking) have been carried out in order to consider artifacts as the result of laboratory contamination, e.g., by phthalates, phosphates, etc. The amount of each contaminant calculated in the blank sample, which, in general, was lower than 2 % of the sample concentration, has been subtracted from the amount of the same contaminant in the environmental samples.
3 Results and Discussion
GC/MS non-target screening analyses of the 39 samples collected from the coastal industrial area of Kavala city revealed numerous compounds which belong to several groups of well-known contaminants as well as of compounds which are rarely documented or unknown. Six main groups of contaminants regarding their chemical structure or technical application have been distinguished: (1) halogenated compounds, (2) nitrogen-containing compounds, (3) sulfur-containing compounds, (4) technical additives and plasticizers, (5) polycyclic aromatic compounds (PACs), and (6) oxygen-containing compounds. In the following, selected contaminants that might be harmful to the environment are discussed with respect to their concentrations, spatial distribution, and possibility to be used as source indicators for specific emissions, and finally their applications and pathways, if this kind of information is available.
3.1 Halogenated Compounds
Data on all halogenated compounds identified in the investigated area are presented in Table 2. Numerous pollutants belong to the class of persistent organic pollutants which are important objects in monitoring programs. Notably, two different groups were distinguished, locally restricted compounds and more widespread distributed ones. To the first group belonged the widespread used PCBs with five to seven chlorine substituents which have been identified solely in sample S17 with low concentrations. Also, DDT-related compounds appeared spatially restricted. In detail, the two main metabolites of the pesticide DDT (2-bis(4-chlorophenyl)-1,1,1-trichloroethane), namely the o,p'- and p,p'-isomers of DDE and DDD, were detected with low concentrations only in the soil samples S4, S6, S7, S12, S13, S14, and S19. Noteworthy, although PCBs and DDT-related compounds are frequently used parameters in monitoring approaches, the restricted appearance of these compounds on low concentration levels, points out the limited usefulness of considering these compounds in monitoring programs applied to this area.
Further on, trichloro- and pentachlorobenzenes, partially known as widely used agents in the technosphere, and the well-known flame retardant dibromophenol (Hassenkloever and Bickmeyer 2006) have been identified exclusively in sample S7 with only 1 to 27 ng/g. In the same sample (S7) as well as in a group of aquatic sediment samples from swales and canals around the fishery (T3, T4, and T5), trichlorobutanol was detected with relative high concentrations up to 320 ng/g. Trichlorobutanol is used widespread as bactericide, e.g., in pharmaceuticals and cosmetics. Based on the application fields of the chlorinated monoaromatics and the halogenated alcohol, these contaminants might likely be linked with the uncontrolled discharge of household rubbish in this area.
Finally, a very interesting group of halogenated carbazoles was identified in the investigated area. In detail, 3,6-dichlorocarbazole, which has been rarely reported as environmental pollutant, and the so far unknown contaminants 3-chlorocarbazole and dibromocarbazole have been detected with quite different levels of concentrations. 3,6-Dichlorocarbazole and 3-chlorocarbazole showed a widespread distribution with highest concentrations in soil samples S16 (3500 ng/g), S18 (1600 ng/g), and S19 (2000 ng/g) for dichlorocarbazole and S16 (110 ng/g) and S18 (71 ng/g) for monochlorocarbazole. According to Reischl et al. (2005) information on the industrial usage of carbazole derivatives is limited, though small amounts of chlorocarbazole might be formed naturally. However, the high concentrations of 3,6-dichlorocarbazole in this study are not explainable as the result of natural source emissions, and, consequently, our data give first evidence for a xenobiotic formation of these contaminants in particular by comparison with the concentration levels detected in former studies. The environmental occurrence of 3,6-dichlorocarbazole has been reported in the past only in two studies in Germany: in Lippe river sediments with low concentrations of 50 ng/g (Kronimus et al. 2004) and in soil samples from Bavaria with concentrations around 10 ng/g (Reischl et al. 2005).
Further on, dibromocarbazole was identified solely in sample M1. According to our knowledge, this compound has not been reported formerly as environmental contaminant and, consequently, no information on its emission pathway and environmental behavior is available. It is noteworthy that none of the halogenated compounds has been detected in marine sediment samples. This might be the result of dilution effects that reduce the environmental amounts below the detection limit.
3.2 Sulfur-Containing Compounds
A second group of identified contaminants comprises the sulfur-containing compounds. These compounds can be separated into two groups from a chemical point of view: (a) the heterocyclic polysulfur compounds and (b) the aliphatic sulfur compounds (s. Table 3).
Sulfur-containing heterocyclic compounds are known constituents of sulfur-rich petroleum as well as of process water from petrochemical facilities, generated easily in contact of petroleum-related matter with water phases enriched in reduced forms of sulfur (Witter and Jones 1999). Consequently, the group of polysulfur heterocycles appeared in our study with extraordinary high concentrations exclusively in the sample derived from the canal where the petrochemical industry discharges liquid waste (sample T2). The m/z = 59 ion chromatogram of the semipolar fractions of sample T2 illustrates the elevated appearance of several peaks representing the sulfur-containing compounds (Fig. 2). Since this group of compounds appeared only in a very restricted area, it can be considered as a molecular marker for the emissions of the petrochemical industry.
The second group of sulfur compounds contained the following aliphatic compounds: dimethyltrisulphide, di-iso-propyldisulphide, hexanethiol and bis(methylthio)ethylene. The two sulfides (dimethyltri- and di-iso-propyldisulphide) appeared only in subaquatic freshwater samples. It is known that oligosulfides are frequently produced under anoxic conditions and/or in hypertrophic aquatic systems and are connected with algae growth (Ginzburg et al. 1998). According to Witter and Jones (1999), sulfides are by-products of elemental sulfur reduction. Hence, the occurrence of these sulfides indicates more anoxic conditions in selected samples and might act as indicators for the environmental conditions in the affected aquatic systems.
3.3 N-containing Compounds
Beside sulfur-containing compounds, a group of nitrogen-containing compounds was also identified (s. Table 4). Considering the spatial distribution, several of these N-compounds showed a restricted distribution in marine sediment samples. In detail, caffeine, dimethyl- and trimethylpyridine, 2,2,6,6-tetramethyl-4-piperidinone (an intermediate for UV-stabilizers), and indole [an indicator for putrefication processes but also known as constituent in fragrances and flavors (Higashio and Shoji 2004)], and methyl-5-hydroxynicotinate, a so far unreported contaminant, appeared not only exclusively in marine sediment samples but also with low concentrations. However, since natural sources cannot be excluded for several of these compounds (Pinsky and Bose 1988; Higashio and Shoji 2004) and regarding the low concentration levels, they seemed not to be relevant for the environmental assessment of the area investigated.
A more interesting anthropogenic compound, which has been reported rarely as a contaminant in environmental samples (Schwarzbauer and Littke 2004), is 2,6-di-tert-butyl-4-nitrophenol. This compound has been reported as a compound identified in the internal surfaces of a submarine formed when lubricating oil mist containing 2,6-di-tert-butylphenol, an antioxidant additive in many synthetic lubricating oils and hydraulic fluids, passes through an electrostatic precipitator and became nitrated (Alexander et al. 2001). The restricted appearance in the samples S1, M7, and M9 around the petrochemical industry 2,6-di-tert-butyl-4-nitrophenol might indicate a source specificity of this molecule in aquatic systems with respect to petrochemical industry emissions.
Finally, cyclohexylpiperidine appeared solely in the sample S18 with a concentration of 6 ng/g. This sample was collected next to the fertilizer industry and the old national road Kavala-Xanthi. However, according to our knowledge for this compound, there is no environmental information available.
3.4 Technical Additives—Plasticizers
A group of xenobiotics, which are widespread used as technical additives and plasticizers, was detected (s. Table 5). Common plasticizers, e.g., di-iso-butylphthalate, di-n-butylphthalate, bis(2-ethylhexyl)phthalate, NBBS, etc., are widespread distributed and, consequently, detected frequently in the aquatic environment (Larsson et al. 1986; Martinez-Carballo et al. 2007). As it can be noticed from the quantitative results in Table 5, these compounds were determined in almost all the samples investigated on different concentration levels and with different hot spot areas.
With respect to the spatial distribution, several of these anthropogenic substances showed the highest concentrations in the sample S7, likely as the result of huge amounts of household rubbish discharged in this area. In addition, compounds used in daily life, e.g., personal care products, were identified here, including 4-oxoisophorone which is used in fragrance and cosmetic industry (maximum concentration, 210 ng/g), as well as the UV-protector 2-ethylhexyl-4-methoxycinnamate (maximum concentration, 930 ng/g). Triphenylphosphate which is used as flame retardant as well as in lubricants was identified in soil sample S5. This sample was collected near the petrochemical industry and next to the national road of Kavala-Xanthi which is probably the reason of the appearance of this compound.
A further compound, dibutylhydroxytoluene, which is also widespread used, e.g., in chemical, medicinal, and food industry, was detected in almost all the samples of the area showing widespread distribution. The highest concentration was observed in sample T3 (840 ng/g). A similar investigation in China exhibited contamination on a similar level of 390 ng/g (Wang et al. 2003).
3.5 Polycyclic Aromatic Compounds, PACs
PACs are not only well-known but also well-investigated pollutants derived from two major sources: (a) the petrogenic origin (fossil fuels) and (b) the pyrogenic origin (basically from incomplete combustion processes). A very detailed description of the contamination in this area by a subgroup of these compound class, the PAH, has been formerly described (Grigoriadou et al. 2007). However, quantitative results of PACs containing sulfur, nitrogen, or oxygen (S-PAC, N-PAC, and O-PAC) detected in the investigated area are presented in Table 6, whereas the summarized concentration profiles illustrating the spatial distribution are given in Fig. 3.
Maximum values for S-PACs were determined in sample T2 (240000 ng/g) followed by somewhat lower concentrations in the samples S3, S5, S7, S19, T1, M1, and M4. These samples were collected from three different areas, (1) around the petrochemical industry (samples T1, T2, S3, and S7), (2) around the two national roads (S19 and S5), and (3) from two marine sediments (M1 and M4). The very high concentrations of S-PACs in the samples around the petrochemical industry, in particular in sample T2, might be a reason of environmental concern. The elevated concentrations around the national road are tending to be the result of traffic exhaust, especially by cars with insufficient combustion of the fuel. However, interesting is the appearance of elevated concentrations of PACs in the marine sediments which were collected next to each other in front of the fertilizer industry. This hot spot might be interpreted as the result of oil spill from shipping or harbor activities. Interestingly, almost the same hot spots were detected for N-PACs and S-PACS with the exception of the sample T2. This indicates different emission sources for soil-related PAC contamination around the petrochemical plant as compared with the affected riverine and marine sediment samples.
3.6 Oxygen-Containing Compounds
Also, numerous oxygen-containing compounds have been detected in this study including carboxylic acids, alcohols, ketones, and aldehydes (s. Table 7), but only one compound, bis(2-ethylhexyl)adipate DEHA, seemed to be of environmental interest. DEHA is used mainly as a plasticizer for synthetic resins such as PVC films. Leaching procedures from solid polymer matrices lead to DEHA release into the environment. This compound has also been identified in sediments from an urban area in Canada with highest concentration of 4,400 μg/kg (Horn et al. 2004), while, in our investigation, the concentrations peaked in a marine sediment sample (M6) with 7,700 ng/g.
4 Conclusion
GC/MS non-target screening analysis applied to marine and riverine sediment, as well as to soil samples from the highly industrialized coastal area of Kavala city, revealed a detailed description of the contaminants within this area. Contaminants, which have been routinely and intensively investigated in the past like PCBs and PACs, do not have high importance for the state of pollution in the particulate matter. However, rarely noticed or quite new environmental contaminants, e.g., halogenated carbazoles have been identified and determined. According to our knowledge, for many of these interesting compounds, no or limited environmental information was available. Regarding the environmental transport of the anthropogenic contaminants discussed, different emission sources as well as concentration hot spots were revealed. Further on, many of these compounds showed a diffuse distribution while others exhibited a restricted appearance indicating specific point emission sources mainly derived from industrial activities.
Most of the identified compounds in this industrial coastal area are not considered in monitoring network programs, although they exhibit partially toxic and harmful potential for the environment, e.g., by halogenated moieties. Hence, this study using a non-target screening analysis demonstrated the principal usefulness of such approach as base for generating individual monitoring programs, in particular, for aquatic systems that are affected by multiple emission sources. Further on, the still unnoticed contaminants should be subject for further ecotoxicological and toxicological investigations in order to estimate their harmfulness in the environment.
As an overall conclusion, this study can act as an example for adapting individual monitoring programs to the individual contamination in coastal areas.
References
Alexander, W. K., Briggs, G. B., Still, K. R., Jederberg, W. W., MacMahon, K., Baker, W. H., et al. (2001). Toxicity of 2, 6-di-tert-butyl-4-nitrophenol (DBNP). Applied Occupational and Environmental Hygiene, 16, 487–495.
Apitz, S. E., & Power, E. A. (2002). From risk assessment to sediment management: an international perspective. Journal of Soils and Sediments, 2, 61–66.
Barth, J. A. C., Grathwohl, P., & Jones, K. C. (2007). Introduction to AquaTerra special Issue “AquaTerra: pollutant behavior in the soil, sediment, ground, and surface water system”. Environmental Pollution, 148, 693–694.
Bester, K., Gatermann, R., Huehnerfuss, H., Lange, W., & Theobald, N. (1998). Results of non target screening of lipophilic organic pollutants in the German Bight. IV: identification and quantification of chloronitrobenzenes and dichloronitrobenzenes. Environmental Pollution, 102, 163–169.
Bester, K., & Theobald, N. (2000). Results of non-target screening of lipophilic organic pollutants in the German Bight V: xanthen-9-one. Water Research, 34, 2277–2282.
Chen, G., & White, P. A. (2004). The mutagenic hazards of aquatic sediments: a review. Mutation Research, 567, 151–225.
Dsikowitzky, L., Schwarzbauer, J., Kronimus, A., & Littke, R. (2004). The anthropogenic contribution to the organic load of the Lipper River (Germany). Part I: qualitative characterisation of low-molecular organic compounds. Chemosphere, 57, 1275–1288.
EPA (Environmental Protection Agency). (1996). The national sediment quality survey: a report to congress on the extent and severity of sediment contamination in surface waters of the united states. Draft. Washington, D.C.: US Environmental Protection Agency.
Galassi, S., Guzzella, L., & Croce, V. (2004). Screening organic micropollutants in surface waters by SPE extraction and ecotoxicological testing. Chemosphere, 54, 1619–1624.
Gerzabek, M. H., Barceló, D., Bellin, A., Rijnaarts, H. H. M., Slob, A., Darmendrail, D., et al. (2007). The integrated project AquaTerra of the EU sixth framework lays foundations for better understanding of river–sediment–soil–groundwater systems. Journal of Environmental Management, 84, 237–243.
Ginzburg, B., Chalifa, I., Zohary, T., Hadas, O., Dor, I., & Lev, O. (1998). Identification of oligosulphide odorous compounds and their source in the Lake of Galilee. Water Research, 32, 1789–1800.
Gocht, T., & Grathwohl, P. (2004). Diffuse input of polycyclic aromatic hydrocarbons: atmospheric deposition and enrichment in soils of rural areas. Umweltwissenschaften und schadstoff-forschung, 4, 245–254.
Grigoriadou, A., Schwarzbauer, J., & Georgakopoulos, A. (2007). Molecular indicators for pollution source identification in marine and terrestrial water of the industrial area of Kavala city, North Greece. Environmental Pollution, 151, 231–242.
Hassenkloever, T., & Bickmeyer, U. (2006). The marine secondary metabolites 2, 4-dibromophenol and 2, 4, 6-tribromophenol differentially modulate voltage dependent ion currents in neuroendocrine (PC12) cells. Aquatic Toxicology, 79, 384–390.
Heim, S., Schwarzbauer, J., Kronimus, A., Littke, R., Woda, C., & Mangini, A. (2004). Geochronology of anthropogenic pollutants in riparian wetland sediments of the Lippe River (Germany). Organic Geochemistry, 35, 1409–1425.
Higashio, Y., & Shoji, T. (2004). Heterocyclic compounds such as pyrrole, pyridines, pyrrolidine, piperidine, indole, imidazol and pyrazines. Applied Catalysis A, 260, 251–259.
Hoenicke, R., Oros, D. R., Oram, J. J., & Taberski, K. M. (2007). Adapting an ambient monitoring program to the challenge of managing emerging pollutants in the San Francisco Estuary. Environmental Research, 105, 132–144.
Horn, O., Nalli, S., Cooper, D., & Nicell, J. (2004). Plasticizer metabolites in the environment. Water Research, 38, 3693–3698.
Kronimus, A., Schwarzbauer, J., Dsikowitzky, L., Heim, S., & Littke, R. (2004). Anthropogenic organic contaminants in sediments of the Lippe river, Germany. Water Research, 38, 3473–3484.
Larsson, P., Thuren, A., & Gahnstroem, G. (1986). Phthalate esters inhibit microbial activity in aquatic sediments. Environmental Pollution Series A, Ecological and Biological, 42, 223–231.
Long, E. R., MacDonal, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects with ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97.
Martinez-Carballo, E., González-Barreiro, C., Sitka, A., Scharf, S., & Gans, O. (2007). Determination of selected organophosphate esters in the aquatic environment of Austria. Science of the Total Environment, 388, 209–299.
Olsen, C. R., Cutshall, N. H., & Larsen, I. L. (1982). Pollutant-particle associations and dynamics in coastal marine environments: a review. Marine Chemistry, 11, 501–533.
Parks, G. A. (1975). Adsorption in the marine environment. Chemical oceanography I (pp. 241–308). New York: Academic.
Peré-Trepat, E., Olivella, L., Ginebreda, A., Caixach, J., & Tauler, R. (2006). Chemometrics modelling of organic contaminants in fish and sediment river samples. Science of the Total Environment, 371, 223–237.
Petty, J. D., Huckins, J. N., Alvarez, D. A., Brumbaugh, W. G., Cranor, W. L., Gale, R. W., et al. (2004). A holistic passive integrative sampling approach for assessing the presence and potential impacts of waterborne environmental contaminants. Chemosphere, 54, 695–705.
Pinsky, C., & Bose, R. (1988). Pyridine and other coal tar constituents as free radical-generating environmental neurotoxicants. Molecular and Cellular Biochemistry, 84, 217–222.
Reischl, A., Joneck, M., & Dumler-Gradl, R. (2005). Chlorocarbazoles in soils. Umweltwissenschaften and Schadstoff-Forschung, 4, 197–200.
Ricking, M., Schwarzbauer, J., & Franke, S. (2003). Molecular markers of anthropogenic activity in sediments of the Havel and Spree rivers (Germany). Water Research, 37, 2607–2617.
Scheffer, F., & Schachtschabel, P. (1998). Lehrbuch der Bodenkunde (14th Edition). Stuttgart: Enke Verlag.
Schwarzbauer, J., Littke, R., & Weigelt, V. (2000). Identification of specific organic contaminants for estimating the contribution of the Elber river to the pollution of the German Bight. Organic Geochemistry, 31, 1713–1731.
Schwarzbauer, J., & Littke, R. (2004). Quantitative evaluation of Elbe river-derived organic marker compounds in sediment samples of the German Bight. Journal of Soils and Sediments, 4, 177–183.
Schwarzbauer, J., & Heim, S. (2005). Lipophilic organic contaminants in the Rhine River, Germany. Water Research, 39, 4735–4748.
Wang, H., Wang, C., Wu, W., Mo, Z., & Wang, Z. (2003). Persistent organic pollutants in water and surface sediments of Taihu Lake, China and risk assessment. Chemosphere, 50, 557–562.
Warren, N., Allan, I. J., Carter, J. E., House, W. A., & Parker, A. (2003). Pesticides and other micro-organic contaminants in freshwater sedimentary environments—a review. Apllied Geochemistry, 18, 159–194.
Weigel, S., Bester, K., & Hühnerfuss, H. (2005). Identification and quantification of pesticides, industrial chemicals, and organobromine compounds of medium to high polarity in the North Sea. Marine Pollution Bulletin, 50, 252–263.
Witter, A. E., & Jones, A. D. (1999). Chemical characterization of organic constituents from sulfide-rich produced water using gas chromatography/mass spectrometry. Environmental Toxicology and Chemistry, 18, 1920–1926.
Acknowledgments
The authors would like to express their gratitude for the financial support provided by the Greek State Scholarships Foundation (I.K.Y.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grigoriadou, A., Schwarzbauer, J. Non-target Screening of Organic Contaminants in Sediments from the Industrial Coastal Area of Kavala City (NE Greece). Water Air Soil Pollut 214, 623–643 (2011). https://doi.org/10.1007/s11270-010-0451-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0451-8