Abstract
A study on pH and chemical composition of precipitation was carried out in two Italian sites, one urban (site 1) and one rural (site 2), located approximately 30 km far from Bologna, during a 3-year period. No significative site variation was found. In both locations, bulk deposition pH ranged from slightly acid to slightly alkaline, despite the volume weighted mean concentration of acidic species, NO −3 and SO 2−4 (67.4 and 118.4 μeq l−1 in site 1 and 88.7 and 103.8 μeq l−1 in site 2), that were similar to those of typical acidic rainfall region. This might be ascribed to the neutralization reaction of the Ca2+, attributed to the calcareous soil and the frequent dusty air mass intrusion from the Sahara. The pair correlation matrix and the analysis of the main components suggested also ammonium and other crustal elements as neutralization agents. The depositional rate of SO 2−4 and NO −3 , chemical elements of agricultural interest, amounted to 38 and 28 and 32 and 35 kg ha−1 for site 1 and site 2, respectively. These supplies of nutrient were not negligible and had to be considered on cultivated lands. NH +4 deposition rate on site 2 was 7 kg ha−1, 23% over site 1, probably due to nitrogen fertilization in the fields around the monitoring station. In site 1, SO 2−4 presented a seasonal trend, indicating that its principal source was the residential heating. Results emphasized that the entity of the bulk deposition acidification is linked not only to the ions local emission sources (fossil fuel combustions, heating, and fertilizers) but also to the surrounding territory and the prevalent wind that transports through kilometers air masses which may contain acidic and alkaline species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The wet and dry depositions are recognized as important sources of chemical constituents for many ecosystems. Anthropic activity strongly affects their composition: in particular, land-use and industrial activities are considered the most important driving forces in the ongoing alteration of the atmospheric chemistry (Lara et al. 2001). Areas with high fossil fuels usage, such as central Europe, northeast America, and East Asia, produce large emissions of SO2 and NOx, which are acid rain precursors (Alastuey et al. 1999; Lee et al. 2000). The rainfall pH is, in fact, determined by the presence of acid and basic components. In a clean atmosphere, its value is between 5.0 and 5.6, due to both the dissolution of natural CO2 in rain and cloud droplets and the existence of background SO2 (Vong et al. 1985; Rao et al. 1995), but, likely, this value decreases when there is a large concentration of acidic components, such as SO 2−4 and NO −3 , not neutralized by alkaline ions, such as Ca2+, Mg2+, and NH +4 . In addition, the extent of rainfall acidification and the relative cation/anion neutralization mostly depend on the environment through which the rain drops travel.
At the end of 1990s, in southwestern Europe and circum-Mediterranean area, the concentration of pollutants species in rainwater results to be similar to central and northern Europe (Kaya and Tuncel 1997; Alastuey et al. 1999), while the rainfall pH is slightly more alkaline (Mosello 1993; Balestrini et al. 2000). In several papers dealing with Mediterranean precipitations quality, the neutral pH, in spite of the large concentration of sulfates and nitrates, is attributed to the intrusion of carbonate-rich air mass from Africa (Moulin et al. 1998; Israelevich et al. 2002) or carbonate-rich chemistry of the local soil or proximal areas (Al-Momani et al. 1995). Moreover, Khemani (1992) reported that the rain drops, immediately coming out of the cloud, possess relatively low pH, but when they reach the earth’s surface, the pH is increased.
The main aim of the present investigation was to examine the chemical composition of the bulk deposition at two sites, the first one in an urban area (downtown of Bologna, Italy) and the second one in a rural area (30 km far from Bologna downtown). In the two sites, bulk deposition samples were collected for a 3-year period and analyzed for the major anions (Cl−, HCO −3 , NO −2 , NO −3 , PO 3−4 , SO 2−4 ) and cations (Ca2+, H+, K+, Mg2+, Na+, NH +4 ). On the basis of different data elaboration, inferences on the neutralization reactions, on the origin of the ions, their seasonal trend, and the significative differences in ions content at the sampling sites were presented.
2 Experimental
2.1 Sampling Sites
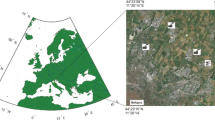
Bulk precipitation samples were collected at two sites in the Emilia Romagna region in a limited area of the southern Po Valley (Fig. 1). According to Köppen–Geiger classification, the climate of the area can be defined as temperate Mediterranean.
The Bologna monitoring station (site 1; 40 m asl; 11°28′ E, 44°30′ N) is located in the center of Bologna city (373,000 inhabitants), 80 km far from the Adriatic Sea. Site 1 is situated in a residential area, 50 m away from the nearest busy road (traffic density ≈ 103 vehicles per day). There is no heavy industrial activity in the surrounding area. The two main sources of domestic energy are gas oil (approximately 60%) and gas methane (approximately 40%). The mean temperature was 13.2°C during the last 30 years. Precipitation accounts for a mean of 74 days with a mean annual precipitation of 705 mm (data are available for a period of 30 years). Most of the rainfalls are concentrated in two rainy seasons: autumn and spring.
The Ozzano monitoring station (site 2; 190 m asl; 11°29′ E, 44°25′ N) is located in a rural area on the hills (calcareous soil) surrounding the town of Ozzano (5,300 inhabitants). Site 2 is situated at a distance of 30 km from Bologna monitoring station. Agricultural fields and natural vegetation surround the Ozzano monitoring station. Wheat and barley are the main crops during the winter, while maize, sunflower, and soybean are the main crops during the summer. The nearest industrial area is 6 km away toward the northeast. The two busy roads “Via Emilia” (103–104 vehicles per day) and “A14 highway” (104–105 vehicles per day) are in plain 2 and 5 km away from site 2.
The dominant wind direction is from northwest, which means that air masses from Bologna town reach the rural area of site 2.
2.2 Sampling Methods
Sampling of bulk deposition was carried out at the two sites by means of standard rain gauge (33.5-cm polyethylene funnel fitted on a 15-l polyethylene bucket) from February 1997 to January 2000. Winter snow samples were collected by bigger buckets (39.4 cm diameter). During the study period, 163 and 123 samples were collected at sites 1 and 2, respectively. The rain gauge was carefully cleaned with ultrapure water after sampling. The samples contained the dry aerosol deposition between precipitation events as well as the net-only deposition for a precipitation event. Bulk samples were collected daily at site 1 and weekly at site 2.
2.3 Analytical Procedures
On the day after collection, conductivity (Crison conductivity meter) and pH (at 20°C; Crison pH meter) of samples were measured. After filtration through 0.4-μm nucleopore membranes, samples were stored at 4°C in the dark before analysis performed typically within a week from the collection. A capillary electrophoresis system (Beckman P/ACE 5500) was used for the quantification of major anions (SO 2−4 , Cl−, NO −3 , NO −2 , PO 3−4 , HCO −3 ) and cations (Ca2+, K+, Mg2+, Na+, NH +4 ), by the external standard calibration method with linear regression, according to the methods proposed by Dinelli et al. (1998). Both cation and anion separations were carried out in conventional untreated fused silica capillaries (Beckman) 50 cm long (from injection point to detector) and 75 μm internal diameter at a constant temperature of 25°C. The indirect detection wavelength was 220 nm. The applied voltage was −20 and 20 kV for anion and cation separation, respectively. The electrolyte buffer employed for the Cl−, NO −2 , NO −3 , and SO 2−4 determination was 1.8 mM potassium dichromate, 34 mM boric acid, 14 mM sodium borate, and 1 mM diethylenetriamine. The electrolyte buffer for PO 3−4 and HCO −3 determination was 10 mM potassium chromate, 0.3 mM cetyltrimethylammonium bromide, and 0.1% Triton X-100 adjusted to pH 8 with boric acid. The electrolyte buffer for Ca2+, Mg2+, and Na+ determination was 40 mM citric acid and 23 mM imidazole. The electrolyte buffer for K+ and NH +4 determination was 20 mM benzylamine adjusted at pH 8.7 with hydrochloric acid. Repeatability was determined by analysis of rain samples from at least five replicate measurements at different concentrations. The results showed that the repeatability for Cl−, NO −2 , NO −3 , SO 2−4 , and Na+ was <3%; for Mg2+, NH +4 , and K+ was 5%; and for PO 3−4 , HCO −3 , and Ca2+ was <6%. In addition, laboratory tests using prepared standards and synthetic rainwater samples indicated that the overall uncertainties (both precision and accuracy) in ion determinations were less than 10% of typical concentrations. Finally, the quality of analytical data was checked by cation–anion balance and by comparison of measured conductivity with the conductivity calculated from the concentration of all measured ions (Balestrini et al. 2000). Ion balance has a very important role in data quality assessment. It reflects the quality of analysis as well as the possibility of any parameter missing for the analysis. For data quality check, ion balance has been performed in bulk samples collected during the study period of 3 years at both site 1 and site 2 (Fig. 2a, b). The acceptable range of ion difference (indicating that all the major ions had been analyzed) in rainwater samples is 10% (Alastuey et al. 1999). The observed difference in anions and cations falls within this acceptable range. The conductivity of a solution is due to the total dissolved solids. If all major ions of the solutions are measured, then the conductivity of the total ions should be approximately equal to the conductivity of the solution measured by a conductivity meter. For data quality check, the difference in measured conductivity and calculated conductivity has been determined for bulk samples collected during the study period of 3 years at both site 1 and site 2. As illustrated in Fig. 2a, b, both curves showed an average deviation of around 2%, which was within the range for quantitative balance (Alastuey et al. 1999).
3 Results and Discussion
3.1 Meteorology
The mean temperature recorded during the study period at site 1 (14.3°C) and site 2 (13.4°C) was approximately 1° higher with respect to the historical (30 years) mean value (Table 1). At both sites in 1997 and 1998, a precipitation deficit was observed, while in 1999, rainfall was higher than the mean value of the last 30 years. The average value of the 3 years was 39 mm lower than the historical data in site 1 and 53 mm lower in site 2. Finally, during the study period, the mean number of raining days at site 1 (54 days) and site 2 (53 days) was considerably lower than the historical mean value. On the basis of these data, the study period was abnormal if compared with the last 30 years.
Monthly precipitation means at sampling sites were reported in Fig. 3. At both sites, the lowest rainfall was recorded in January, February, and July, while the largest rainfall was observed in November. The pluviometric regime at the two sites was characteristic of the southern Po Valley, with the major proportion of precipitation during the spring and autumn (approximately 60–70% of total rainfall). The most frequent rainfall intensities were 2–10 mm/h in site 1 and 5–10 mm/h in site 2.
3.2 General Features of Precipitation Chemistry
The basic statistics (mean, median, volume weighted mean (VWM), maximum and minimum values) of pH, cations, and anions in bulk deposition samples collected at sites 1 and 2 in the 3-year period are reported in Table 2.
3.2.1 pH
During the study period, in site 2, the mean and median pH values were 5.3 and 5.2, respectively, while in site 1, they were coincident (5.2; Table 2). The VWM pH, calculated from the H+ concentration, was 5.1 in site 1 and 5.3 in site 2. As the pH of the unpolluted natural water is between 5 and 5.6 (Vong et al. 1985; Rao et al. 1995), these pH values indicated in both locations as nonacid bulk depositions. As shown in Table 3, the pH values of both sites were higher than those recorded in typical acid precipitation regions, such as Northern America (4.2; Khawaja and Husain 1990), Singapore (4.5; Balasubramanian et al. 2001), Southern Korea (4.7; Lee et al. 2000), and China (4.4; Tanner 1999). With respect to Mediterranean area countries (Table 3), the mean pH values in both the sites were comparable than the others observed in Italy (4.8 and 5.2; Le Bolloch and Guerzoni 1995; Balestrini et al. 2000), but lower than those detected in other Mediterranean countries such as Morella-Spain (6.7), Ankara-Turkey (6.3), and Eshidiya-Jordan (6.6; Alastuey et al. 1999; Topcu et al. 2002; Al-Khashman 2005).
In both investigated sites, the failure of the Kolmogorov–Smirnov test indicated that the two data sets of H+ concentration did not follow a Gaussian distribution, even if there is not a statistically difference between mean, median, and VWM concentration of H+. In addition, in the 3-year sampling period, the pH values of individual precipitation showed a restricted fluctuation, ranging from 4.5 to 6.0 in site 1 and from 4.4 to 5.5 in site 2 (Fig. 4).
Precipitations with a pH below 5.0 were episodically observed. Only 17% and 12% of the site 1 and site 2 events were characterized by an acid pH (Fig. 4). In other countries, the precipitation pH is typified by a larger fluctuation, such as in south Brazil, where values range between 3.4 and 7.3 (Pelicho et al. 2006) or in the Korean peninsula, where pH values are from 3.4 to 8.0 (Bo Kyoung et al. 2000).
The nonnormal distribution and the limited pH fluctuation suggested a sort of buffer effect, probably due to calcium carbonate (i.e., intrusion of Saharan dust), maintaining the H+ concentrations in bulk depositions within a defined and limited range of values, rather independently from the concentration of acidic species.
3.2.2 Anions and Cations
The bulk deposition dataset showed a wide range of concentrations for each analyte over the sampling period. As evidenced by the minimum and maximum values detected during the study period (Table 2), the concentration of the major ions varied over more than three orders of magnitude.
For all the studied elements, the concentrations showed a log-normal distribution, while the nonnormality of the data was illustrated by the discrepancies between median and mean analyte concentrations (Table 2). At both sites, the median concentration values of all investigated ions were significantly lower than the respective mean concentration values. In addition, except for H+ at site 1 and PO 3−4 at site 2, VWM analyte concentration was smaller than the mean concentration. As suggested by Tanner (1999), the log-normal distribution of ion concentrations indicates lower analyte concentrations on days with more rainfall, resulting from exhaustive scavenging and less importance of evaporation in the humid atmosphere of prolonged rainfall. Similar results were obtained by Beverland and Crowther (1992) and Akkoyunlu and Tayanç (2003).
Despite precipitation amount is one of the physical factors affecting the incorporation of analytes into rainwater, chemical factors are also expected to be important.
The VWM concentrations of the anions can be arranged in a descending order as follows: SO 2−4 (118.4 μeq l−1) > Cl− (105.5 μeq l−1) > NO −3 (67.4 μeq l−1) for site 1 and SO 2−4 (103.8 μeq l−1) > NO −3 (88.7 μeq l−1) > Cl− (87.6 μeq l−1) in site 2 (Table 2). These three ions contributed to the 89% and 81% of total anions detected in sites 1 and 2, respectively. The concentration ratio of the main acid species, SO 2−4 /NO −3 , equal to 1.8 in site 1 and 1.2 in site 2, indicated that sulfates dominated at site 1 precipitation, while in site 2, the contribution of the two anions was similar.
In both sites, the NO −3 was considerably higher than in other Mediterranean locations, with the only exception for Jordan, while the SO 2−4 was similar to that observed in Sardinia-Italy, Spain, and Jordan, but it resulted to be almost double with respect to Longone-Italy and Turkey (Table 3). The concentration of these two acidic anions was also higher or similar to that recorded in areas characterized by acid rainfall, such as Northern America, China, Singapore, and Korea (Table 3).
As concerns cations, in both locations, Ca2+ was the most abundant (121.9 μeq l−1 in site 1 and 136.8 μeq l−1 in site 2), and its VWM concentration was approximately constant during the 3 years of bulk deposition sampling. With respect to available literature (Table 3), the Ca2+ was higher than that reported in Mediterranean countries and in acid rainfall regions. Finally, in the present study, the second and the third most abundant cation was Na+ (96.0 and 83.2 μeq l−1, respectively, in sites 1 and 2) and NH +4 (58.4 and 82.2 μeq l−1, respectively, in sites 1 and 2).
3.3 Sources of Ions
Relationships between measured ions were estimated through factor analysis and correlation analysis. The results analyzed by principal component analysis (PCA) followed by varimax rotation to a set of orthogonal axes for 278 precipitation data are presented in Table 4. PCA is a statistical tool for identifying the sources and is applicable whenever the quantities are expressed as linear combination of others (Harman 1968). For a principal component (PC), a physical interpretation of sources is possible by comparing the elements having high correlation in a particular PC with elements associated with known possible sources (Hopke et al. 1976).
The PC1 accounts for 46% of the total variance and has high loading for Na+, Cl−, and Mg2+. This result, together with the pair correlation coefficients obtained with the correlation analysis between Na+ and Cl−, and Mg2+ and Cl−, r = 0.85 and r = 0.69 in site 1 and r = 0.85 and r = 0.53 in site 2, reflects the strong influence of marine aerosols (i.e., Adriatic sea 80 km far from the sampling locations). This hypothesis is supported also by the ions ratios: the mean Cl−/Na+ ratio equal to 0.89 in site 1 and to 0.99 in site 2 is close to that reported for seawater (1.16), confirming their common marine origin. Also for Mg2+ a marine origin is postulated on the basis of mean Cl−/Na++Mg2+ ratio equal to 0.9 in site 1 and to 1.2 in site 2 (i.e., in seawater, a Cl−/Na++Mg2+ ratio of 1 is reported). By contrast, the ratios of Cl− with K+, Ca2+, and SO 2−4 greatly departed from the values reported for seawater, suggesting for these cations an anthropogenic and/or crustal origin.
In PC2, SO 2−4 , NO −3 , NH +4 , and Ca2+ are loaded and account for 20% of the total variance. This is clearly a gas and aged aerosol component. Strong positive correlations among the ions belonging to PC2 are shown in correlation analysis. In site 1, the highest correlations appear in the ion pair NO −3 and Ca2+ (r = 0.90), SO 2−4 and Ca2+ (r = 0.84), and SO 2−4 and NO −3 (r = 0.73), while in site 2, the highest coefficients were for the pairs SO 2−4 and NO −3 (r = 0.92), NH +4 and NO −3 (r = 0.74), NH +4 and SO 2−4 (r = 0.73), Ca2+ and NO −3 (r = 0.70), and Ca2+ and SO 2−4 (r = 0.70). The significant correlation between the two acid species, SO 2−4 and NO −3 , confirmed their plausible common anthropogenic origin, i.e., the co-emission of their precursors (SO2 and NOx) from motor vehicles, fossil fuel combustion, and their behavior in precipitation (Huang et al. 2008), but besides suggesting a common ion origin, these high correlation coefficients also reveal their co-occurrence in precipitation mostly as (NH4)2SO4, (NH4)HSO4, (NH4)NO3, CaSO4, and Ca(NO3)2 (Khawaja and Husain 1990; Lee et al. 2000).
PCA has also evidenced a loading of sulfate on both PC1 (marine aerosol) and PC2 (gas emission), suggesting that SO 2−4 can be differentiated as from marine and anthropogenic origin. The relative contribution of the two sources was calculated by assuming a SO −24 /Na+ ratio equal to 0.12 in seawater (Balestrini et al. 2000). Table 5 indicated that in both sites, sulfates derived prevalently from human activities, probably from fossil fuel combustion and/or urban heating.
PCA and correlation analysis indicated also that SO 2−4 and NO −3 are presented mostly as neutralized forms in the precipitation. No secure conclusions about whether the neutralization occurred in raindrops, in the atmospheric gas phase, or even before the introduction to the atmosphere may be inferred (Lee et al. 2000).
Because of the significant neutralization, H+ was not expected to have a positive correlation with SO 2−4 or NO −3 , and this is actually observed. H+ is not correlated with SO 2−4 and NO −3 and is not loaded in PC2 with a statistically significant level. It is loaded in PC3, which accounts for 8% of total variance. In PC3, also other minor anions, such as NO −2 and PO 3−4 (contributing to less than 1% of total bulk deposition), are loaded. In both sites, no high correlations were found (r < 0.20) between H+ and these anions. Some pollens have a significant content of phosphate, and it was postulated as a possible source of this ion in dry and bulk deposition (Noll and Khalili 1998). Nitrite is mainly derived from nitrate after photolysis: its content in rainfall is probably dependent on the occurrence of the events (day and/or night, summer/springtime, and/or autumn/winter period).
3.4 Neutralization
In southern Europe and Mediterranean area, carbonate particles are the most dominant neutralizing agents (Al-Momani et al. 1995; Tuncer et al. 2001). Especially in the regions, where the composition of the bulk deposition is strongly affected by high calcite content of Saharan dust, the prevalent role of the calcium has been widely illustrated (Loye-Pilot et al. 1986; Avila et al. 1998). In Italy, the deposition of the atmospheric acidity is mitigated by the supply of dust of Saharan origin (Balestrini et al. 2000): even if not constantly, carbonate particles actually reach areas in the Po Valley, as also suggested by the brownish-red coloration of several bulk deposition samples. Available literature on the prevalent wind direction in the Mediterranean region (Moulin et al. 1998; Israelevich et al. 2002; Prospero et al. 2002) indicated that alkaline elements, especially the calcite powder, could reach northern Italy (i.e., location of the monitored sites) due to dust intrusion from northwest and North Central Africa. Especially, the transport occurs from Sahara desert, in spectacular storm-like events (Israelevich et al. 2002). In order to confirm what found in literature, the events with peaks of Ca2+ concentrations were investigated through back trajectories analysis, by means of the NOAA HYSPLIT 4 Model (Draxel and Rolph 2003). The results obtained at two different heights (50 and 500 m), for a duration of 90 h, demonstrate a marked air mass movement from Sahara desert to our region (Fig. 5).
The two elements presumed to be the responsible of the main basicity, Ca2+ and NH +4 , were correlated with the three anions responsible of the main acidity, NO −3 , SO 2−4 , and NO −2 . If these anions were the only species involved in the acidity of the precipitation, one would expect a linear relationship between SO 2−4 + NO −3 + NO −2 and Ca2+ + NH +4 . Figure 6 shows that the hypothesis is confirmed: the correlation was close to the unity (R 2 = 0.9) for the two sites. The bulk deposition acidity was neutralized prevalently by Ca2+ and NH +4 , while other cations contributed for about 8%.
The neutralization role of each basic ion could be evaluated also by calculating the neutralization factor (NF; Kulshrestha et al. 1995), which computes the ratio of individual cation over acidic anions, NO −3 + SO 2−4 . When the result is close to 1, the rainfall is not acid, despite the high concentration of acidic pollutant in the air.
As in this study the rainwater presented significant concentrations of NO −3 , SO 2−4 , and Ca2+, for each rainwater sample, the Ca2+/SO 2−4 + NO −3 ratio was calculated. For the studied areas, the average values, listed in Table 3, were 0.66 in site 1 and 0.71 in site 2, meaning that calcium alone was able to neutralize most of the total acidity. Probably, its dominant role was due to its large concentration and the high scavenging efficiency of precipitation on the coarse mode particles.
A comparison with literature results (Table 3) evidenced that in Italy and in the other countries close to sandy desert or easily reachable by dusty wind, such in Turkey, Spain, and Jordan, NF ranged between 0.48 and 1.24 and no problems linked to acid rain existed. In other part of the world, such as in Northern America, Singapore, Southern Korea, China, and Brazil, the ratio was notably lower than in Mediterranean countries, with values between 0.04 and 0.39, indicating that Ca2+ is not able to totally neutralize the acidic species. In fact, in these areas, rainfall pHs are subacid or acid and in any case lower than 4.9.
3.5 Deposition Rate and Seasonal Variation
The mean annual deposition rates of chemical species, expressed in kilograms per hectare, are reported in Table 6. In particular, the main acid species, as sulfur and nitrogen, are chemical elements of agricultural interest, with a peculiar importance in plant nutrition. Several studies indicate that in Italy, the sulfur input from atmospheric depositions is sufficient for the nutritional requirement of many crops, while the nitrogen input can be effective for meadows, pastures, and, in general, when soils are not fertilized (Francaviglia et al. 1995). The nutrient supply of nitrogen and sulfur from bulk deposition is not negligible and has to be considered in different agroecosystems to tune the management practices. Its contribution for soil fertilization is also stressed in the Italian Code of Good Agricultural Practice, drawn up according to the EC Directive 91/676 by the Ministry of Agricultural Food and Forestry Resources.
In site 1, the mean annual SO 2−4 and NO −3 deposition was 37.9 and 27.8 kg ha−1 year−1, respectively, and similar supplies were observed in site 2 (31.8 kg SO 2−4 ha−1 year−1 and 35.0 kg NO −3 ha−1 year−1). The small differences observed between the two sites were unexpected, considering the diverse land-use and locations of the two sites. However, the rural site (site 2) is placed near two traffic routes (A14 Highway, Emilia Road) and an industrial area few kilometers far from the monitoring station. In addition, in the area, the prevalent wind direction is from Bologna to Ozzano (DiSTA Servizio Agrometeorologico 2008), transporting air masses from urban site to the rural one. Thus, even if the local emissions from the two sites were presumably different, other factors, especially linked with the air masses movement, contributed in determining the final deposition.
The NH +4 deposition in the rural site was 23% over the urban site (7.0 kg ha−1 year−1 in site 1 and 9.5 kg ha−1 year−1 in site 2). This difference is probably due to the repeated nitrogen fertilizations in the rural site. Nevertheless, also in the urban area, the ammonium deposition was not negligible. Bologna is placed in the Padana Plain, southern Po Valley, one of the most cultivated area of Italy (approximately 60% of Italian agriculture production is concentrated in the Po Valley; INEA 2004).
Considering the 3-year period of monitoring, the mean annual N deposition was 11.7 and 15.3 kg ha−1 year−1 for sites 1 and 2, respectively.
These values are in agreement with available literature on nitrogen deposition in the Po Valley. On the basis of a monitoring study carried out in northern Po Valley in the period 1987–1992, Francaviglia et al. (1995) reported that nitrogen in wet and dry depositions amounted to 16.1 kg N ha−1 year−1 as a mean value (range 9–18 kg N ha−1 year−1). In 1997, the nitrogen flux in wet deposition in Lago Maggiore catchment (northern Po Valley) ranged between 6.3 and 21.7 kg N ha−1 year−1 (Mosello et al. 2001). In addition, studies on the Los Angeles (USA) metropolitan and surrounding costal mountain in California have documented high N deposition rate (15–45 kg N ha−1 year−1; Fenn et al. 1996; Fenn and Poth 1999; Hughes et al. 2002). Fenn et al. (2003) reported modeled N deposition loads of 7–18 kg N ha−1 year−1 for urbanized region of Central Arizona around Phoenix (USA).
During the study period, the mean annual S deposition was 12.6 and 10.6 kg ha−1 year−1 in sites 1 and 2, respectively. In 1997, no difference was observed for S deposition loads in sites 1 and 2, while in 1998 and 1999, the significantly (P < 0.05) highest S deposition rate in urban site with respect to the rural one was observed (Table 6). The S fluxes of the present paper are in general agreement with literature. Francaviglia et al. (1995) described for northern Po Valley an average sulfur content of 12.6 kg ha−1 year−1 in bulk depositions, while loads ranging between 5.6 and 12.0 kg S ha−1 year−1 were reported by Mosello et al. (2001).
Sulfur and nitrogen depositions are recognized agents potentially perturbing ecosystems and agronomic environments, i.e., crop growth and yield reduction (ENEL 1991), plant yellowing, and necrosis (Francaviglia et al. 1995). Sequi (1991) stated that in Mediterranean agroecosystems, sulfur and nitrogen depositions do not have appreciable negative effects on agricultural soil. The buffer capacity of the calcareous soils, typical of the monitored areas and of the southern Po Valley, and the continuous chemical recasting deriving from agricultural activity, which is quantitatively more important than changes induced by the atmospheric pollutants, probably limited negative effects on plants. In addition, bulk deposition can have even a significative fertilizing effect on soils lacking of sulfur (i.e., very common condition of Italian soils). The presented values are in fact comparable to the deposition rates observed in some other Italian zones, where benefits on crops have been reported (Francaviglia et al. 1995). However, in order to forecast long-term effects on ecological systems, further investigations are needed. Sensitivity to the effect of bulk depositions varies greatly from place to place, depending on the local geology and the soil type among other factors (Francaviglia et al. 1995). At present, no critical load values of the main acid species are available for the areas investigated in the present paper.
In Fig. 7, the seasonal trend of ion deposition in both sites is described. The ion deposition was divided in two semesters: autumn–winter period, which includes all the rainfall events occurred from October to March, and spring–summer period, which comprises the events occurred from April to September. Except for SO 2−4 , no seasonal trend was observed for investigated ions. In site 1, a significant increase of SO 2−4 deposition (p < 0.05) was observed during the autumn–winter period with respect to the spring–summer semester, probably due to the residential heating, which constitutes, during the cold period, a relevant local sulfur dioxide source. A similar trend was not observed in site 2.
4 Conclusions
The study of pH and chemical composition of precipitation was carried out in two Italian sites, one urban (site 1) and one rural (site 2), during a 3-year period.The major conclusions are summarized as follows:
-
1.
Despite the high annual mean VWM concentration of acidic pollutants, SO 2−4 and NO −3 , in both the sites, bulk deposition pH ranged from slightly acid to slightly alkaline.
-
2.
Ca2+, deriving prevalently from the calcareous soil and the dust–wind from Sahara desert, played the main role in the neutralization process.
-
3.
There is no significant site variations, emphasizing that the composition of the bulk deposition is not linked only to the ion local emission sources (fossil fuel combustions, heating, and fertilizers) but also to the surrounding territory (agricultural lands, coastal area, factories, railways, etc.) and to the prevalent wind that transports through kilometers air masses which may contain acidic and alkaline species.
-
4.
There is a significant seasonal trend for the SO 2−4 deposition in the urban site, due to the residential heating.
-
5.
The atmospheric deposition on agricultural area does not seem to be negative: on the contrary, on arable land, some elements in solution in rainfall water may reach sufficient supply to satisfy crops needs (30–45 kg ha−1 SO 2−4 ). However, in order to forecast long-term effects on ecological systems, further investigations are needed.
References
Akkoyunlu, B. O., & Tayanç, M. (2003). Analyses of wet and bulk deposition in four different regions of Istanbul, Turkey. Atmospheric Environment, 37, 3571–3579.
Alastuey, A., Querol, X., Chaves, A., Ruiz, C. R., Carratala, A., & Lopez-Soler, A. (1999). Bulk deposition in a rural area located around a large coal fired power station, northeast Spain. Environmental Pollution, 106, 359–367.
Al-Khashman, O. A. (2005). Study of chemical composition in wet atmospheric precipitation in Eshidiya area, Jordan. Atmospheric Environment, 39, 6175–6183.
Al-Momani, I. F., Ataman, O. Y., Anwari, M. A., Tuncel, S., Köse, C., & Tuncel, G. (1995). Chemical composition of precipitation near an industrial area at Izmir, Tukey. Atmospheric Environment, 29, 1131–1143.
Avila, A., Alarcon, M., & Queralt, I. (1998). The chemical composition of dust transported in red rain—its contribution to the biogeochemical cycle of a Holm oak forest in Catalonia (Spain). Atmospheric Environment, 32, 179–191.
Balasubramanian, R., Victor, T., & Chun, N. (2001). Chemical and statistical analysis of precipitation in Singapore. Water, Air, and Soil Pollution, 130, 451–456.
Balestrini, R., Galli, L., & Tartari, G. (2000). Wet and dry atmospheric deposition at prealpine and alpine sites in northern Italy. Atmospheric Environment, 34, 1455–1470.
Beverland, I. J., & Crowther, J. M. (1992). On the interpretation of event and sub-event rainfall chemistry. Environmental Pollution, 75, 163–174.
Bo Kyoung, L., Seung, H. H., & Dong, S. L. (2000). Chemical composition of precipitation and wet deposition of major ions on the Korean peninsula. Atmospheric Environment, 34, 563–575.
Dinelli, G., Di Martino, E., & Vicari, A. (1998). Multi-determination of mineral ions in environmental water samples by capillary electrophoresis. Recent Research Developments in Agricultural & Food Chemistry, 2, 435–442.
DiSTA Servizio Agrometeorologico Università di Bologna. (2008) http://www.dista.unibo.it/meteo/index.php.
Draxel, R., & Rolph, G. (2003). HYSLIP model (Hybrid Single-Particle Lagrangian Integrated Trajectory). Silver Spring: NOAA Air Resources Laboratory. http://www.arl.noaa.gov/ready/hyslpit4.html.
ENEL (1991). Le piogge acide. I quaderni dell’energia, 19.
Fenn, M. E., & Poth, M. A. (1999). Temporal and spatial trends in streamwater nitrate concentrations in the San Bernardino Mountains, southern California. Journal of Environmental Quality, 28, 822–836.
Fenn, M. E., Poth, M. A., & Johnson, D. W. (1996). Evidence for nitrogen saturation in the San Bernardino Mountains in southern California. Forest Ecology and Management, 82, 211–230.
Fenn, M. E., Haebuer, R., Tonnesen, G. S., Baron, J. S., Grossman-Clarke, S., Hope, D., et al. (2003). Nitrogen emissions, deposition and monitoring in the western United States. BioScience, 53, 391–403.
Flues, M., Hama, P., Lemes, M. J. L., Dantas, E. S. K., & Fornaro, A. (2002). Evaluation of the rainwater acidity of a rural region due to a coal-fired power plant in Brazil. Atmospheric Environment, 36(14), 2397–2404.
Francaviglia, R., Costantini, A., & Morselli, L. (1995). Long term monitoring of atmospheric depositions in a Po valley station. Evaluation of environmental effect. Chemosphere, 30(8), 1513–1525.
Harman, H. H. (1968). Modern factor analysis, 2nd edition (revised). Chicago: The University of Chicago Press.
Hopke, P. K., Glandney, E. S., Gordon, G. E., Zoller, W. H., & Jones, A. G. (1976). The use of multivariate analysis to identify sources of selected elements in the Boston urban aerosol. Atmospheric Environment, 10, 1015–1025.
Huang, K., Zhuang, G., Xu, C., Wang, Y., & Tang, A. (2008). The chemistry of the severe acidic precipitation in Shanghai, China. J Atmos Res, 89(1–2), 149–160.
Hughes, L., Allen, J. O., Salmon, L. G., Mayo, P. R., Johnson, R. J., & Cass, G. R. (2002). Evolution of nitrogen species air pollutants along trajectories crossing the Los Angeles area. Environmental Science and Technology, 36, 3928–3935.
INEA (2004). L’agricoltura italiana conta (Report on Italian agriculture). Roma: Istituto Nazionale di Economia Agraria. http://www.inea.it/pubbl/itaco.cfm. Accessed Jan 2009.
Israelevich, P. L., Levin, Z., Joseph, J. H., & Ganor, E. (2002). Desert aereosol transport in the Mediterranean region as inferred from the TOMS aereosol index. Journal of Geophysical Research, 107(D21), 4572.
Kaya, G., & Tuncel, G. (1997). Trace elements and major ion composition of wet and dry deposition in Ankara, Turkey. Atmospheric Environment, 31, 3958–3998.
Khawaja, H. A., & Husain, L. (1990). Chemical characterization of acid precipitation in Albany, New York. Atmospheric Environment, 24A, 1869–1882.
Khemani, L. T. (1992). The role of alkaline particulates on pH of rain water and implications of control of acid rain. In H. W. Ellasesser (Ed.), Global 2000 revisited: mankinds impact on spaceship earth (pp. 87–123). New York: Pargon.
Kulshrestha, U. C., Sarkar, A. K., Srivastava, S. S., & Parashar, D. C. (1995). Wet-only and bulk deposition studies at New Delhi (India). Water, Air, and Soil Pollution, 85, 2143–2148.
Lara, L. B., Artaxo, L. S., Martinelli, L. A., Victoria, R. L., Camargo, P. B., Krusche, A., et al. (2001). Chemical composition of rainwater and anthropogenic influences in the piracicaba river basin, southeast Brazil. Atmospheric Environment, 38, 4937–4945.
Le Bolloch, O., & Guerzoni, S. (1995). Acid and alkaline deposition in precipitation on the western coast of Sardinia, central Mediterranean (40° N, 8° E). Water, Air, and Soil Pollution, 85, 2155–2160.
Lee, B. K., Hong, S. H., & Lee, D. S. (2000). Chemical composition of precipitation and wet deposition of major ions on the Korean peninsula. Atmospheric Environment, 34, 563–575.
Loye-Pilot, M. D., Martin, J. M., & Morelli, J. (1986). Influence of Saharan dust on the rain acidity and atmospheric input to the Mediterranean. Nature, 321, 427–428.
Mosello, R. (1993) Rapporto sull’attività della rete RIDEP nel quinquennio 1988–1992. Documenta Istituto Italiano Idrobiologia, pp. 44–118.
Mosello, R., Barbieri, A., Brizzio, M. C., Calderoni, A., Marchetto, A., Passera, S., et al. (2001). Nitrogen budget of Lago Maggiore: The relative importance of atmospheric deposition and catchment sources. Journal of Limnology, 60, 27–40.
Moulin, C., Lambert, C. E., Dayan, U., Masson, V., Ramonet, M., Bousquet, P., et al. (1998). Satellite climatology of African dust transport in the Mediterranean atmosphere. Journal of Geophysical Research, 103(D11), 13,138–13,144.
Noll, K. E., & Khalili, E. (1998). Dry deposition of sulfate associated with pollen. Atmospheric Environment, 22(3), 601–604.
Pelicho, A. F., Martins, L. D., Nomi, S. N., & Solci, M. C. (2006). Integrated and sequential bulk and wet only samplings of atmospheric precipitation in Londrina, south Brazil (1998–2002). Atmospheric Environment, 40, 6827–6835.
Prospero, J. M., Ginoux, P., Torres, O., Nicholson, S. E., & Gill, T. E. (2002). Environmental characterization of global sources of atmospheric soil dust identified with the NINBUS 7 Total ozone mapping Spectrometer (TOMS) absorbing aerosol product. Reviews of Geophysics, 40, 1002. doi:10.1029/2000RG00095.
Rao, G., Momin, P., Safai, A., & Khemani, L. (1995). Rain water and through fall chemistry in the silent valley forest in the south India. Atmospheric Environment, 29, 2025–2029.
Sequi, P. (1991). Chimica del suolo. Bologna: Patron Ed.
Tanner, P. A. (1999). Analysis of Hong Kong daily bulk and wet deposition data from 1994 to 1995. Atmospheric Environment, 33, 1757–1766.
Topcu, S., Incecik, S., & Atimtay, A. (2002). Chemical composition of rainwater at EMEP station in Ankara, Turkey. Atmospheric Research, 65(1–2), 77–92.
Tuncer, B., Bayer, B., Yesilyurt, C., & Tuncel, G. (2001). Ionic composition of precipitation at the central Anatolia, Turkey. Atmospheric Environment, 35, 5989–6002.
Vong, R. J., Larson, T. V., Covert, A. P., & Waggoner, A. P. (1985). Measurement and modelling of western Washington precipitation chemistry. Water, Air, and Soil Pollution, 26, 71–84.
Acknowledgments
The study was conducted thanks to the financial support of Progetto Strategico di Ateneo FINqUER and Ecosearch S.r.e.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pieri, L., Matzneller, P., Gaspari, N. et al. Bulk Atmospheric Deposition in the Southern Po Valley (Northern Italy). Water Air Soil Pollut 210, 155–169 (2010). https://doi.org/10.1007/s11270-009-0238-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0238-y