Abstract
This study characterizes the effects of water–soil flooding volume ratio and flooding time on copper (Cu) desorption and toxicity following multiple floodings of field-collected soils from agricultural sites acquired under the Comprehensive Everglades Restoration Plan (CERP) in south Florida. Soils from four field sites were flooded with three water–soil ratios (2, 4, and 6 [water] to 1 [soil]) and held for 14 days to characterize the effects of volume ratio and flooding duration on Cu desorption (volume ratio and flooding duration study). Desorption of Cu was also characterized by flooding soils four times from seven field sites with a volume ratio of 2 (water) to 1 (soil) (multiple flooding study). Acute toxicity tests were also conducted using overlying waters from the first flooding event to characterize the effects of Cu on the survival of fathead minnows (Pimephales promelas), cladocerans (Daphnia magna), amphipods (Hyalella azteca), midges (Chironomus tentans), duckweed (Lemna minor), and Florida apple snails (Pomacea paludosa). Acute tests were also conducted with D. magna exposed to overlying water from the second and third flooding events. Results indicate that dissolved Cu concentrations in overlying water increased with flooding duration and decreased with volume ratio. In the multiple flooding study, initial Cu concentrations in soils ranged from 5 to 223 mg/kg (dw) and were similar to Cu concentration after four flooding events, indicating retention of Cu in soils. Copper desorption was dependent on soil Cu content and soil characteristics. Total Cu concentration in overlying water (Cuw) was a function of dissolved organic carbon (DOC), alkalinity, and soil Cu concentration (Cus): log(Cuw) = 1.2909 + 0.0279 (DOC) + 0.0026 (Cus) − 0.0038 (alkalinity). The model was validated and highly predictive. Most of the desorbed Cu in the water column complexed with organic matter in the soils and accounted for 99% of the total dissolved Cu. Although total dissolved Cu concentrations in overlying water did not significantly decrease with number of flooding events, concentrations of free Cu2+ increased with the number of flooding events, due to a decrease in DOC concentrations. The fraction of bioavailable Cu species (Cu2+, CuOH+, CuCO3) was also less than 1% of the total Cu. Overlying water from the first flooding event was only acutely toxic to the Florida apple snail from one site. However, overlying water from the third flooding of six out of seven soils was acutely toxic to D. magna. The decrease in DOC concentrations and increase in bioavailable Cu2+ species may explain the changes in acute toxicity to D. magna. Results of this study reveal potential for high Cu bioavailability (Cu2+) and toxicity to aquatic biota overtime in inundated agricultural lands acquired under the CERP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Copper (Cu) has been used in Florida citrus agriculture as an algaecide, fungicide and soil amendment for over 50 years (Alva et al. 1995). In 2005, according to the US Department of Agriculture, 469,350 kg of copper hydroxide and 51,550 kg of copper sulfate or basic copper sulfate were applied to grapefruit, orange, tangelo, tangerine and temple crops on 259,563 ha in Florida (USDA 2005). Under implementation of the Comprehensive Everglades Restoration Plan (CERP), thousands of acres of citrus agriculture soils will be flooded for maintaining hydrologic buffer areas and for the creation of storm water treatment areas, water storage reservoirs, and wetlands in South Florida. Reports on these soils indicate Cu concentrations as high as 1,200 mg/kg, dw (SFWMD 2001–2006). Our earlier study found high Cu concentrations in overlying water as a result of Cu desorption from a single flooding of citrus agricultural soils (Hoang et al. 2008a). Desorbed Cu from flooded soils also adversely affected the survival and growth of the Florida apple snail, an important food resource for many species, including the federally endangered Everglades snail kite (Hoang et al. 2008a, b).
In water, Cu can occur bound to particulate and/or colloidal matter or as different dissolved species (Eisler 1998). Copper bioavailability in water is influenced by environmental factors such as pH, alkalinity, hardness, dissolved organic carbon (DOC), and salinity (Arnold 2005; De Schamphelaere et al. 2003; Erickson et al. 1996; Gensemer et al. 2002; Hall et al. 2008; Nriagu 1979; Santore et al. 2001). Free cupric ion (Cu2+) and Cu hydroxide (CuOH+) are the two most bioavailable forms to aquatic organisms (Pagenkopf et al. 1974, 1983, Santore et al. 2001). Recent studies suggest that Cu carbonate (CuCO3) is also bioavailable to invertebrate species (De Schamphelaere and Janssen 2002; Rogevich et al. 2008). Agriculture soils usually contain DOC, carbonate salts, and other minerals which will also complex with Cu and influence Cu speciation and bioavailability (He et al. 2006). Monitoring studies have shown that the quality of receiving waters has been reduced as a result of increased Cu loads in surface runoff from agriculture (Moore et al. 1998; Zhang et al. 2003). It should be noted that in the Clean Water Act, Section 303 (D) special list of impaired waters, the U.S.EPA (Office of Water) ranks metals as the principle reason for water bodies having impaired water quality and places Cu, out of ten metals, in the top tier for producing such changes (Reiley 2007).
South Florida receives between 40 and 65 inches in rainfall annually, mostly during the wet season (i.e., June through October) and with a flat topography it is susceptible to multiple floodings events with uncertain water–soil volume ratios. For example, rainfall measurements in two large citrus farming counties (St. Lucie and Martin) in south Florida indicate a monthly rainfall range of 5 to 270 mm, resulting in varying water–soil volume ratios (Zhang et al. 2003). Citrus agricultural lands, inundated under the CERP, will thus be subjected to multiple rainfall events and various soil-water volume ratios.
This study characterizes the effect of water–soil volume ratio, flooding duration (hereafter referred to as the volume ratio and flooding duration study) and multiple flooding events (hereafter referred to as the multiple flooding study) on Cu desorption and the bioavailability of Cu in flooded water (overlying water) from south Florida citrus agricultural soils. A model was formulated to predict dissolved Cu concentrations in water following desorption from flooded agricultural soils. This study also evaluates the acute toxicity of overlying waters to fathead minnows (Pimephales promelas), daphnids (Daphnia magna), amphipods (Hyalella azteca), midges (Chironomus tentans), duckweed (Lemna minor), and Florida apple snails (Pomacea paludosa).
2 Materials and Methods
2.1 Volume Ratio and Flooding Duration Study
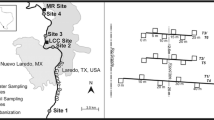
Soils were collected from agriculture sites (n = 11 in four counties [Dade (n = 2), Palm Beach (n = 1), Martin (n = 2), St. Lucie (n = 6)] of south Florida (Fig. 1). At each of the 11 sites, soil was collected at three locations, 2 m apart, to a depth of 6 cm from the surface. In the laboratory, soils were composited. Physical and chemical characteristics of soils are published in our earlier study (Hoang et al. 2008a). Soil Cu concentrations (Cus) are presented in Table 1 and Fig. 2. Pesticide (organochlorine and organophosphate) and metal (Cd, Pb, As, Hg, and Zn) concentrations were at negligible levels.
To determine the effect of water–soil flooding volume ratio and flooding duration on Cu desorption, 0.15 L of soil from four out of the eleven sites were chosen to represent four different soil textures [Aquacalma-A (Sandy), Birdsall (loamy sand), L31N Buffer (silty loam), Agler (sandy loam)]. Each soil was flooded with 0.3, 0.6, and 0.9 L of laboratory freshwater (carbon-filtered and UV-sterilized city water) in 1 L glass vessels to obtain volume ratios of 2, 4, and 6 (water) to 1 (soil), respectively. Vessels were held under static conditions for 14 days. Three replicates were used for each water/soil ratio. During the 14 days, overlying water (i.e., water on top of soils) samples from each replicate were collected on days 1, 4, 7, and 14 for dissolved Cu analysis.
2.2 Multiple Flooding Study
To determine Cu desorption from multiple flooding events, soils from seven sites (Agler, Arcco, Birdsall, McArthur, L31N Buffer, Equus-A, Sunrise Boys) representing four different soil textures (sandy, sandy loam, loamy sand, silty loam) and a control soil were flooded and dried four times. A water–soil volume ratio of 2 (water) to 1 (soil) was chosen for this study based on monthly average rainfall in St. Lucie and Martin Counties, South Florida (approximately 126 mm; Zhang et al. 2003). Six liters of soil from each of the seven sites and a control soil were randomly distributed to 18-L glass tanks, with two replicates per site. Tanks were subsequently flooded with 12 L of laboratory freshwater and held for 14 days. Overlying water was then removed and soil was allowed to air dry for 14 days. Flooding (14 days)/drying (14 days) events were repeated four times for each soil. During the first three flooding events, overlying water samples from each replicate were collected on days 1, 4, 7, and 14, and for the fourth flooding event on days 1, 7, and 14, to measure dissolved Cu, free Cu (Cu2+) and DOC concentrations. On day 14 of the first flooding, water was also collected for anion analysis. Water samples for dissolved Cu, DOC, and anions were filtered through 0.45-μm Gelman Nylon Mesh®. Total water hardness, alkalinity, and pH were measured at each water sampling day. Soils were then air-dried for 14 days at 25 ± 1°C prior to the next flooding event. Soil Cu concentrations were re-measured after the fourth flooding using the method published by Hoang et al. (2008a). On day 14, all overlying water from each replicate soil–water treatment was collected and composited to conduct acute toxicity tests.
A regression model was developed (as described in data analysis) to predict dissolved Cu concentrations from flooded soils based on results of the multiple flooding study. Results of the first flooding of Aquacalma-A, Aquacalma-B, Rodriguez Sanchez and Equus-B soils were used to validate the model.
Free Cu+2 was analyzed with a copper selective electrode using a buffering method (Steenbergen et al. 2005). The buffering method allows measurement of free Cu+2 concentrations as low as 10−14 M Cu. Dissolved Cu was analyzed using inductively coupled plasma atomic emission spectrometry (ICPAES; Perkin Elmer Corporation, Toronto, Canada). Concentrations of DOC were measured using a Shimadzu Total Carbon Analyzer (model OC-5050A; Shimadzu Scientific Instruments, Columbia, MD, USA). Anions were measured with Dionex DX500 ion chromatography (IC) (Dionex Corporation, Sunnyvale, CA, USA). Hardness and alkalinity were measured by titrating with 0.01 M ethylenediamine tetraacetic acetate (EDTA) solution and 0.02 N H2SO4, respectively. Concentrations of DO and pH were measured with a YSI Meter (YSI Inc., Yellow Springs, OH, USA) and an Accumet Meter (Fisher Scientific, Fairlawn, NJ, USA), respectively. Results of chemistry of day 14 overlying water for the first flooding event were used to determine the chemical speciation of Cu for each soil using the Visual Minteq Model (http://www.lwr.kth.se/English/OurSoftware/vminteq).
2.3 Toxicity Study
Overlying water (day 14) collected from the first flooding event was used to conduct toxicity tests with D. magna, H. azteca, P. promelas, C. tentans, L. minor, and P. paludosa. The overlying water from all replicates of each flooded soil treatment was composited and aerated for 24 h prior to test initiation. All toxicity tests were conducted based on US EPA Standard Methods (US EPA 2002). Test conditions for each species are described in Table 2. All tests were conducted in polypropylene beakers under standard laboratory conditions (25 ± 1°C, 16 h darkness/8 h light). Water hardness, alkalinity, pH, dissolved oxygen (DO), and ammonia were measured at test initiation and termination for all organisms. Mortality was monitored daily. Water samples were collected at test initiation and termination for analyses of free Cu, dissolved Cu, and DOC. Sampling procedures and analyses were described in the fate study.
To evaluate the toxicity of Cu in overlying water from later flooding events, two additional toxicity tests were conducted with D. magna using overlying water (day 14) from the second and the third flooding events. D. magna was chosen for these two tests because they represent the most sensitive species to Cu in the present study based on acute toxicity test results from the species sensitivity distribution for Cu (Schuler et al. 2008). Test procedures for the D. magna tests were similar to the procedures described above.
2.4 Data Analysis
The effects of water–soil volume ratio and flooding duration on Cu desorption were analyzed using two-way ANOVA analysis. The correlations between soil and water Cu concentrations, water quality parameters, and number of flooding events were determined using the Pearson correlation analysis. Multiple regression analysis was also conducted to determine the relationship of dissolved Cu concentrations in overlying water from the four flooding events and water quality parameters (alkalinity, pH, hardness, DOC) and soil Cu concentrations. Data were log transformed to meet the assumptions of normality and homogeneity of variance before conducting multiple regression analysis.
Principal component and factors analysis (PCA) was conducted for all soils, using the varimax rotation method to characterize desorption of Cu forms (e.g., Cu2+, Cu-DOC, CuCO3, etc.) and other factors in soils (alkalinity, hardness, pH, DOC). Survival data were analyzed using multiple treatment comparisons with Dunnetts-test. An effect with a p value < 0.05 was considered significant. Data from control and Equus-A soils (low Cu concentrations) were not used for the multiple regression and PCA analyses.
All analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) and SPSS (SPSS Inc. Chicago, IL, USA).
3 Results
3.1 Volume Ratio and Flooding Duration Study
Results of the volume ratio and flooding duration study are illustrated in Fig. 2. In general, dissolved Cu concentrations in overlying water significantly increased with flooding duration and decreased with volume ratio. At each time point, Cu concentrations in overlying water were higher at the lower volume ratio. At each volume ratio, Cu concentrations in overlying water increased with flooding time. There was also a significant effect on Cu desorption as a result of the interaction of water–soil volume ratio and flooding duration.
3.2 Multiple Flooding Study
Results of the multiple flooding study are shown in Fig. 3 and Table 1. Measured concentrations of total dissolved Cu, free Cu2+ and water quality parameters of overlying water on day 14 from each of the four flooding events are shown in Table 3.
In general, soils with higher Cu concentrations resulted in higher dissolved Cu concentrations in overlying water. Similar desorption patterns were found following each of the four flooding events; dissolved Cu concentrations in overlying water increased with flooding duration. Dissolved Cu concentrations in overlying water at day 14 were similar for flooding events one through four (Figs. 3 and 4). However, free Cu2+ significantly increased with number of flooding events while DOC concentrations significantly decreased with number of flooding events (Fig. 4). After the fourth flooding event, total Cu release from the soils was less than 5% of the initial soil Cu content (Table 1).
Results of Pearson correlation analysis are shown in Table 4. Total dissolved Cu concentrations in water were significantly correlated with alkalinity, DOC, soil Cu concentrations, and pH. Free Cu2+ concentrations were significantly correlated with hardness and flooding number. Hardness was significantly correlated with alkalinity and flooding number. Alkalinity was significantly correlated with pH. DOC concentrations were significantly correlated with pH, flooding number, and soil Cu concentration.
Results of the PCA are shown in Table 5. There were four components characterizing desorption of Cu and other constituents from the soils. Component 1 had high loading factors for Cu (0.862) and DOC (0.951), characterizing desorption of total dissolved Cu and DOC. Component 2 had high loading factors for alkalinity (0.698) and pH (0.916), characterizing the desorption of carbonate. Component 3 had a high loading factor for hardness (0.922), characterizing desorption of calcium and magnesium. Component 4 had a high loading factor for Cu2+ (0.975), characterizing desorption of free Cu2+.
The total dissolved Cu concentration in overlying water (Cuw) was a function of the DOC concentration in water, water alkalinity, and soil Cu concentration (Cus): log (Cuw) = 1.2909 + 0.0279 (DOC) + 0.0026 (Cus) − 0.0038 (alkalinity). The variation of DOC, alkalinity, and soil Cu concentration explained 92% of the variation of Cu concentration in overlying water (R 2 = 0.92). Results of model validation are shown in Fig. 5. In general, the measured and predicted total dissolved Cu concentrations were highly correlated (R 2 = 0.81) (Fig. 5).
Results of Cu speciation in overlying water after the first flooding characterized by the Visual Minteq model are illustrated in Fig. 6. The copper and organic matter complex (Cu-DOC) was the dominant species and accounted for 99% of total dissolved Cu in the overlying water. Other Cu species were less than 1% of total dissolved Cu.
3.3 Toxicity Study
Results of toxicity studies for overlying water collected from the first flooding event are illustrated in Fig. 7. There was no significant effect of Cu on survival of P. promelas, D. magna, H. azteca, C. tentans or L. minor. In one soil (McArthur), the Florida apple snail had significantly lower survival than in the control and other treatments. Figure 8 shows the effects of exposure of overlying water collected from the second and third flooding events on the survival D. magna neonates after 72- and 96-h. The survival (%) of D. magna was not significantly affected by exposure to overlying water from the first or second flooding event (64–100% survival), but was significantly affected by exposure to overlying water from the third flooding event (14–54% survival) in six out of the seven soils. Water hardness, alkalinity, DOC, and pH at the beginning and the end of each toxicity test were not significantly different. Measured values of water quality parameters are shown in Table 3. Dissolved oxygen and ammonia concentrations for all tests were 6.0 ± 1 and 0.5 ± 0.2 mg/L, respectively.
4 Discussion
4.1 Volume Ratio and Flooding Duration Study: Dependence of Copper Desorption on Duration, Concentration, and Soil Characteristics
Results of the volume ratio and flooding duration study indicated that Cu desorption from soils was dependent on flooding time and water–soil volume ratio. During a 14 day flooding period, dissolved Cu concentrations in water increased as flooding duration increased and as water–soil volume ratio decreased. He et al. (2006) reported similar results for Cu and Zn desorption. Stemmer et al. (1990) investigated the influence of sediment volume and surface area (SA) on selenium-spiked sediment toxicity to D. magna and found a decrease in sediment-to-water ratio from 1:4 to 1:8 and increased SA decreased survival of test organisms.
The current study also confirmed that copper desorption is dependent on soil characteristics, as previously reported by Gasser et al. (1994); Zhang et al. (2003); Ponizovsky et al. (2006) and Hoang et al. (2008a). For example, Agler soil had lower soil Cu concentrations than L31N Buffer soil; however, overlying water Cu concentrations were similar in both soil-water systems (Fig. 2). The higher cation exchange capacity (CEC = 35 meq/100 g) and lower sand composition (19%) in L31N Buffer soil compared to Agler soil (CEC = 30 meq/100 g and % sand = 72%) may explain the difference in Cu desorption in these two soils (Hoang et al. 2008a).
4.2 Multiple Flooding Study: Copper Speciation and Bioavailability
Copper in natural soils can complex with organic matter (e.g., humic and fulvic acids) and inorganic ligands (e.g., carbonate, mineral oxides; Nriagu 1979). These Cu complexes can desorb into water and may change depending on water quality (e.g., pH). The present study found that the total dissolved Cu concentration did not significantly decrease with the number of flooding events. In contrast, free Cu2+ concentration significantly increased with the number of flooding events. This may be due to the decrease in DOC concentration with increasing flooding events, resulting in decreased concentrations of Cu-DOC.
Pearson correlation analysis indicated that total dissolved Cu concentrations were significantly correlated with DOC concentrations and up to 99% of the total dissolved Cu was complexed with DOC. This may be a result of desorption of Cu-DOC from the soils and/or complexation of Cu and DOC in the overlying water. The significant correlations between total dissolved Cu and DOC in water and soil Cu and DOC suggest that most of Cu in the soils was complexed with DOC.
Results of PCA indicated that desorbed Cu was classified as total dissolved Cu and DOC in component 1 and free Cu in component 4. This suggests that desorbed Cu was in both organic complexes (Cu-DOC) and free (Cu2+) forms. However, concentrations of Cu2+ were minor as compared with those of Cu-DOC. Copper bicarbonate (CuHCO3 +) and copper carbonate (CuCO3) did not contribute to desorbed Cu, as indicated by the low loading factor of total dissolved Cu concentration in component 2, which characterized the desorption of carbonate. These data are similar to findings by Sajwan et al. (2006), who also demonstrated that the fraction of Cu present in carbonate form in soils collected from citrus groves in Lake Alfred, Florida and Savannah, Georgia were small compared to total Cu. Sajwan et al. (2006) reported that the fraction of organic Cu and exchangeable Cu (free Cu2+) in soils from Florida citrus groves and Savannah (Georgia) soils ranged from 32% to 59% and 1% to 4% of total Cu, respectively. McBride and Bouldin (1984) found that up to 99.5% of total Cu in soils was in an organically complexed form. He et al. (2006) also reported up to 70% of total dissolved Cu released from Florida soils was in organic complexes. The fraction of organic Cu complexes varies, depending on soil organic matter content. Saeki et al. (2002) reported that the fraction of organic Cu complexes ranged from 1% to 56% of total Cu in soils that had soluble organic carbon from 1 to 102 mg/L.
Desorption of Cu is also dependent on water pH. The negative correlation between total Cu concentration and pH indicates that Cu desorption decreased with increasing pH. Essington (2004) reported that surface enhanced metal hydrolysis at mineral surfaces (e.g., SiO2, kaolinite, gibbsite, etc.) results in increased metal adsorption and decreased water pH. This may explain the inverse relation between Cu desorption and pH in the present study.
Results of the PCA and Visual Minteq model prediction suggested that copper bicarbonate and copper carbonate complexes did not contribute to Cu desorption; however, our empirical model indicated that total Cu concentration was inversely related to alkalinity. Most of the Cu in the soils was complexed with organic matter; therefore, desorption of Cu would be confounded by desorption of organic matter. In the natural environment, carboxylic acid is the most important functional group of organic matter (Thurman 1986a). The dissociation of carboxyl groups results in organic matter with a negative charge. The presence of carbonate and bicarbonate in overlying water first increased the ionic strength of overlying water and second creates a negative charge repulsion between Cu–organic matter and carbonate and bicarbonate. This would decrease desorption and solubility of Cu–organic matter complexes. The effect of alkalinity on desorption of Cu–organic matter hence, may be called “salting out” effect (Thurman 1986b; Schwarzenbach et al. 1993). This may explain the inverse relation between total Cu concentration and alkalinity in the present study. Similarly, the presence of Ca2+ and Mg2+ in overlying water may decrease desorption of Cu2+. The “salting out” effect may explain the negative relationship between hardness and Cu2+. The relationship between total Cu and DOC concentrations in the model can be explained by desorption of Cu–organic matter complexes.
Results of the PCA (component 1) also support the relationship between total Cu and DOC concentrations in the model. Among the three significant variables, DOC alone accounted for 68% of the total desorbed Cu concentration. Soil Cu concentrations and alkalinity accounted for 24% of the total desorbed Cu. The dependence of desorbed Cu concentrations on soil Cu and organic matter concentrations in the present study is in agreement with the literature (Sauve et al. 2000; Impellitteri et al. 2003; He et al. 2004).
The relationship between the measured and model predicted total Cu concentrations in the overlying water yielded a slope of 1.02 and an intercept not significantly different from zero. This indicates that the model is accurate in its prediction. Results of the model validation also indicate that the model is highly predictive. The predicted concentrations were within 20% of the measured concentrations. Using this model, the total desorbed Cu concentration in overlying water can be predicted based on soil Cu concentration, DOC, and alkalinity.
Among the aqueous Cu species, Cu2+ and CuOH+ are considered bioavailable to aquatic organisms in acute exposures (Pagenkopf et al. 1974; Pagenkopf 1983; Santore et al. 2001). Recently, CuCO3 was also suggested to be bioavailable to invertebrate species (De Schamphelaere and Janssen 2002). Rogevich et al. (2008) indicate that CuCO3 may be bioavailable to the Florida apple snail. Results of the present study indicated that the fraction of bioavailable Cu species in the overlying water of the first flooding were less than 1% of total Cu. The measured Cu2+ concentrations in the overlying water from the first flooding were less than 0.06 µg/L Cu and were similar to Cu2+ concentration (0.07 µg/L) in our laboratory freshwater, which contained a total dissolved Cu concentration of 4 µg/L and DOC = 0.4 mg/L. The low fraction of bioavailable Cu in the overlying water of the first flooding explains the insignificant effect of Cu on survival of the tested organisms. This is in agreement with the literature (Erickson et al. 1996; Santore et al. 2001; Sciera et al. 2004; Ryan et al. 2004; DeSchamphelaere et al. 2003; DeSchamphelaere and Janssen 2004).
5 Conclusions and Implications in Everglades Restoration
Reports on agricultural properties acquired under the CERP show the presence of Cu in soils with concentrations as high as 1,200 mg/kg (dw). The present study showed that once inundated by rainwater events, Cu will desorb from these flooded agricultural soils [in soil (sediment)–water systems] and overlying water will contain dissolved Cu concentrations which will increase with flooding duration and decrease with soil/water volume ratios. In addition, concentration of the free ion (Cu2+), a readily bioavailable and toxic species, increases with number of flooding events due to a decrease in DOC concentrations. The latter was supported by the significant decrease in survival in the acute toxicity tests to Daphnia magna neonates, as a result of exposure to overlying waters from flooding one (64–100%) to flooding three (14–54% survival). Furthermore, although there is a significant increase in the concentration of Cu2+ in overlying water, total soil Cu concentrations were similar between the initial measurement (before flooding) and after flooding four, which was four months in duration. This indicates that Cu will be retained in agricultural soils.
Recruitment of organisms into these newly managed systems may initially lead to diverse communities which ultimately will be exposed to Cu from several potential environmental sources-via water, sediment and food. At first, these systems will serve as a reservoir for long-term Cu exposures and low-level chronic uptake with few implications for acute exposures because bioavailable, free Cu2+ concentrations will be limited. However, over time these systems will present a challenge to organisms as a result of acute exposures because an increase in natural rainfall events will eventually lower DOC concentrations with concomitant increases in the bioavailable Cu2+, the toxic form. Therefore, as these systems age they will produce high potential for acute and chronic risks to aquatic organisms. Risk will be especially high for planktonic herbivores and herbivorous fish since they will be exposed directly from water and indirectly from consuming phytoplankton and periphyton; two trophic groups which have a high propensity to bioconcentrate Cu. Since Cu will remain in sediment for long periods of time, both infaunal and epibenthic species will also be at risk because of their short life cycles.
The long-term persistence of copper in these managed systems and consequent biological effects as a result of such exposures must be considered in the future acquisition of agricultural lands under the CERP. Based on the results of copper studies with flooded agricultural soils and freshwater organisms from this laboratory, it appears that, in some cases, the creation of these wetland systems under the CERP may not be consistent with the goal of improving the functional quality of habitats or increasing the abundance and diversity of native plant and animal species in south Florida. Therefore, with respect to the remedial actions and environmental management decisions taken on agricultural lands acquired under the CERP, the “precautionary principle” should be applied. In essence, although cause-and-effect relationships and scientific evidence are not completely established for the biological consequences of flooded, copper-contaminated agricultural soils, we have an obligation to adopt a conservative approach and try to avoid increasing risks to the ecosystem that we are trying to restore.
References
Alva, A. K., Graham, J. H., & Anderson, C. A. (1995). Soil-pH and copper effects on young Hamlin orange trees. Soil Science Society of America Journal, 59, 481–487.
Arnold, W. R. (2005). Effects of dissolved organic carbon on copper toxicity: Implications for saltwater copper criteria. Integrated Environmental Assessment and Management, 1, 34–39. doi:10.1897/IEAM_2004a-002b.1.
De Schamphelaere, K. A. C., & Janssen, C. R. (2002). A biotic ligand model predicting acute copper toxicity for Daphnia magna: The effects of calcium, magnesium, sodium, potassium, and pH. Environmental Science & Technology, 36, 48–54. doi:10.1021/es000253s.
De Schamphelaere, K. A. C., & Janssen, C. R. (2004). Effects of dissolved organic carbon concentration and source, pH, and water hardness on chronic toxicity of copper to Daphnia magna. Environmental Toxicology and Chemistry, 23, 1115–1122. doi:10.1897/02-593.
De Schamphelaere, K. A. C., Vasconcelos, F. M., Heijerick, D. G., Tack, F. M. G., Delbeke, K., Allen, H. E., et al. (2003). Development and field validation of predictive copper toxicity model for the green alga Pseudokirchneriella subcapitata. Environmental Toxicology and Chemistry, 22, 2454–2465. doi:10.1897/02-499.
Eisler, R. (1998). Copper hazards to fish, wildlife, and invertebrates: A synoptic review. Biological Science Report USGS/BRD/BSR-1997–0002, Contaminant Hazard Reviews Report No. 33, US Geological Survey, Washington, DC, pp. 1–101.
Erickson, R. J., Benoit, D. A., Mattson, V. R., Nelson, H. P., & Leonard, E. N. (1996). The effects of water chemistry on the toxicity of copper to fathead minnows. Environmental Toxicology and Chemistry, 15, 181–193. doi:10.1897/1551-5028(1996)015<0181:TEOWCO>2.3.CO;2.
Essington, M. E. (2004). Soil and water chemistry pp. 358–397. Boca Raton, FL, USA: CRC.
Gasser, U. G., Juchler, S. J., & Sticher, H. (1994). Chemistry and speciation of soil water from serpentinic soils: Importance of colloids in the transport of Cr, Fe, Mg, and Ni. Soil Science, 158, 314–322.
Gensemer, R. W., Naddy, R. B., Stubblefield, W. A., Hockett, J. R., Santore, R., & Paquin, P. (2002). Evaluating the role of ion composition on the toxicity of copper to Ceriodaphnia dubia in very hard waters. Comparative Biochemistry and Physiology, 133c, 87–97.
Hall Jr, L. W., Lewis, A. R. D., & Arnold, W. R. (2008). The influence of salinity and dissolved organic carbon on the toxicity of copper to the estuarine copepod, Eurytemora affinis. Archives of Environmental Contamination and Toxicology, 54, 44–56.
He, Z. L., Zhang, M. K., Calvert, D. V., Stoffella, P. J., Yang, X. E., & Yu, S. (2004). Division S-6-soil & water management & conservation. Transport of heavy metals in surface runoff from vegetable and citrus fields. Soil Science Society of America Journal, 68, 1662–1669.
He, Z. L., Zhang, M. K., Yang, X. E., & Stoffella, P. J. (2006). Release behavior of copper and zinc from sandy soils. Soil Science Society of America Journal, 70, 1699–1707. doi:10.2136/sssaj2005.0255.
Hoang, T. C., Rogevich, E. C., Rand, G. M., Gardinali, P. R., Frakes, R. A., & Bargar, T. A. (2008a). Copper desorption in flooded agricultural soils and toxicity to the Florida apple snail (Pomacea Paludosa): implications in Everglades restoration. Environmental Pollution, 154, 338–347. doi:10.1016/j.envpol.2007.09.024.
Hoang, T. C., Rogevich, E. C., Rand, G. M., Frakes, R. A., & Bargar, T. A. (2008b). Copper uptake and depuration by juvenile and adult Florida apple snails (Pomacea paludosa). Ecotoxicology (London, England). doi:10.1007/s10646-008-0243-8.
Impellitteri, C. A., Saxe, J. K., Cochran, M., Janssen, G., & Allen, H. E. (2003). Predicting the bioavailability of copper and zinc in soils: modeling the partitioning of potentially bioavailable copper and zinc from soil solid to soil solution. Environmental Toxicology and Chemistry, 22, 1380–1386. doi:10.1897/1551-5028(2003)022<1380:PTBOCA>2.0.CO;2.
McBride, M. B., & Bouldin, D. R. (1984). Long-term reactions of copper (II) in a contaminated calcareous soil. Soil Science Society of America Journal, 48, 56–59.
Moore Jr, P. A., Daniel, T. C., Gilmour, J. T., Shreve, B. R., Edwards, D. R., & Wood, B. H. (1998). Decreasing metal runoff from poultry litter with aluminum sulfate. Journal of Environmental Quality, 27, 92–99.
Nriagu, J. O. (1979). Copper in the atmosphere and precipitation. In J. O. Nriagu (Ed.), Copper in the environment (pp. 44–77). Wiley: New York, NY, USA.
Pagenkopf, G. K. (1983). Gill surface interaction model for trace metal toxicity to fishes: Role of complexation, pH, and water hardness. Environmental Science & Technology, 17, 342–347. doi:10.1021/es00112a007.
Pagenkopf, G. K., Russo, R. C., & Thurston, R. V. (1974). Effects of complexation on toxicity of copper to fishes. Journal of the Fisheries Research Board of Canada, 31, 462–465.
Ponizovsky, A. A., Thakali, S., Allen, H. E., DiToro, D. M., & Ackerman, A. J. (2006). Effect of soil properties on copper release in soil solutions at low moisture content. Environmental Toxicology and Chemistry, 25, 671–682. doi:10.1897/04-621R.1.
Reiley, M. C. (2007). Science, policy, and trends of metals risk assessment at EPA: How understanding metals bioavailability has changed metals risk assessment at U.S.EPA. Aquatic Toxicology (Amsterdam, Netherlands), 84, 292–298. doi:10.1016/j.aquatox.2007.05.014.
Rogevich, E. C., Hoang, T. C., & Rand, G. M. (2008). The effects of water quality and age on the acute toxicity of copper to the Florida apple snail. Pomacea paludosa. Archives of Environmental Contamination and Toxicology, 54, 690–696. doi:10.1007/s00244-007-9106-1.
Ryan, C. A., Van Genderen, E. J., Tomasso, J. R., & Klaine, S. J. (2004). Influence of natural organic matter source on copper toxicity to larval fathead minnows (Pimephales promelas): Implications for the biotic ligand model. Environmental Toxicology and Chemistry, 23, 1567–1574. doi:10.1897/02-476.
Saeki, K., Kunito, T., Oyaizu, H., & Matsumoto, S. (2002). Relationship between bacterial tolerance levels and forms of copper and zinc in soils. Journal of Environmental Quality, 31, 1570–1575.
Sajwan, K. S., Paramasivam, S., Alva, A. K., & Afolabi, J. (2006). Chemical association of trace elements in soils amended with biosolids: comparison of two biosolids. In M. N. V. Prasad, K. S. Sajwan, & R. Naidu (Eds.), Trace elements in the environment (pp. 155–166). Boca Raton, FL, USA: Taylor & Francis.
Santore, R. C., DiToro, D. M., Paquin, P. R., Allen, H. E., & Meyer, J. S. (2001). Biotic ligand model of the acute toxicity of metals. 2. Application to acute copper toxicity in freshwater fish and Daphnia. Environmental Toxicology and Chemistry, 20, 2397–2402. doi:10.1897/1551-5028(2001)020<2397:BLMOTA>2.0.CO;2.
Sauve, S., Hendershot, W., & Allen, H. (2000). Solid-solution partitioning of metals in contaminated soils: dependence on pH, total metal burden, and organic matter. Environmental Science & Technology, 34, 1125–1131. doi:10.1021/es9907764.
Schuler, L. J., Hoang, T. C., Rand, G. M. (2008). Aquatic risk assessment of copper in freshwater and saltwater ecosystems of south Florida. Ecotoxicology (London, England). doi:10.1007/s10646-008-0236-7.
Schwarzenbach, R. P., Gschwend, P. M., & Imboden, D. M. (1993). Environmental organic chemistry pp. 90–108. New York, USA: Wiley.
Sciera, K. L., Isley, J. J., Tomasso, J. R., & Klaine, S. J. (2004). Influence of multiple water-quality characteristics on copper toxicity to fathead minnows (Pimephales promelas). Environmental Toxicology and Chemistry, 23, 2900–2905. doi:10.1897/03–574.1.
South Florida Water Management District. (2001–2006). Reports, submitted to. South Florida Water Management District for Phase I/II Environmental Site Assessments. South Florida Water Management District, West Palm Beach, FL.
Steenbergen, N. T. T. M., Iaccino, F., Winkel, M. D., Reijnders, L., & Peijnenburg, W. J. G. M. (2005). Development of a biotic ligand model and a regression model predicting acute copper toxicity to the earthworm, Aporrectodea caliginosa. Environmental Science & Technology, 39, 5694–5702. doi:10.1021/es0501971.
Stemmer, B. L., Burton Jr, G. A., & Leibfritz-Frederick, S. (1990). Effect of sediment test variables on selenium toxicity to Daphnia Magna. Environmental Toxicology and Chemistry, 9, 381–389. doi:10.1897/1552-8618(1990)9[381:EOSTVO]2.0.CO;2.
Thurman, E. M. (1986a). Organic geochemistry of natural waters. Dordrecht: Martinus Nijhoff, pp 87–102.
Thurman, E. M. (1986b). Organic geochemistry of natural waters. Dordrecht: Martinus Nijhoff, pp 365–423.
US Department of Agriculture. (2005). Agricultural chemical usage summary, 2005. Fruit Summary, National Agricultural Statistical Service, Washington, DC, 2006.
US EPA. (2002). Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms: EPA-821-R-02-012; Office of Water, Washington, DC.
Zhang, M. K., He, Z. L., Calvert, D. V., Stoffella, P. J., & Yang, X. E. (2003). Surface runoff losses of copper and zinc in sandy soils. Journal of Environmental Quality, 32, 909–915.
Acknowledgements
We thank Kathy Moore of Clemson University and Sayed Hassan of the University of Georgia for their help on chemical analyses and Tim Bargar from US Fish and Wildlife Service for his help collecting soils. This study is SERC contribution no. 404.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoang, T.C., Schuler, L.J., Rogevich, E.C. et al. Copper Release, Speciation, and Toxicity Following Multiple Floodings of Copper Enriched Agriculture Soils: Implications in Everglades Restoration. Water Air Soil Pollut 199, 79–93 (2009). https://doi.org/10.1007/s11270-008-9861-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9861-2