Abstract
Purpose
Floodwaters contain a mixture of organic contaminants which affect both the water and soil quality within flooded regions. This study is the first to describe the chemical behavior of organic contaminants mobilized during the 2010 flooding event of the Rio Grande. The study compares two sites: one directly impacted by outfall from municipal sources and one primarily impacted by rural runoff.
Materials and methods
This study characterized the in situ cycling of organic contaminants (triclosan, ciprofloxacin, and atrazine), their depositional mechanisms, and the post-flood dynamics within riparian soils over 6 months. Water quality (pH, salinity, alkalinity, turbidity, N, and P) of the Rio Grande at four sites within Webb County, TX, was monitored for 3 months pre-flooding and 6 months post-flooding in order to determine contaminant levels along the Webb County border. Soil physicochemical properties (pH, CEC, and EC) were assessed for 6 months at two sites post-flooding: within the municipal area of both Laredo, TX, USA, and Nuevo Laredo, Mexico (LCC site), and upstream of the site near the edge of Laredo, TX (MR site).
Results and discussion
Low levels of contaminants were detected in the watershed year-round with levels increasing during the flood. Floodwaters had a lower pH than nonflooding conditions, resulting in greater partitioning of both triclosan and ciprofloxacin to particles within the water column with no change detected in atrazine behavior. Soil samples (0–2-cm depth) showed an increase in clay-sized particles (up to 6–14 %) and contaminant concentrations similar to that of water column particles. Organic contaminants were uniform in concentration throughout the soil profile (2–30 cm), demonstrating the impact of infiltration. Both triclosan and atrazine decayed away at rates of 23 ± 2 and 16 ± 1 days, respectively, with no decay of ciprofloxacin detected. Vertical mobility of contaminants within soils was not detected.
Conclusions
This study was able to distinguish the influx of contamination contributed by particle deposition from that due to infiltration of the contaminated floodwaters. Further, our data indicates that outfall/effluent contaminants, such as ciprofloxacin, may persist in riparian soils post-flooding. Agricultural management should consider potential exposure and accumulation of floodwater contaminants in crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Flooding of agricultural, industrial, mining, shipping, and municipal areas allows for the mobilization of a suite of organic contaminants in mobilized sediments and runoff. As floodwaters recede, contaminants are deposited in soils adjacent to streams and rivers. The behavior of these compounds in floodwaters and soils, to which they are transported, still needs to be better defined. Both water and soils are dynamic media requiring that sampling be performed immediately in order to properly model contaminant dynamics and retention within the system during periods of flooding.
Between the years of 1999 and 2002, pharmaceutical sales increased by over 25 % worldwide with 51 % of the total sales occurring in the USA by 2002 (Xia et al. 2005). These compounds are continuously released into the environment due to human consumption and pose a potentially significant threat to the integrity of aquatic ecosystems (Barnes et al. 2008; Focazio et al. 2008). Triclosan, a chemical of emerging concern, is one of the most widely used broad spectrum antimicrobials found in detergents, personal hygiene products, plastics, and textiles (Daughton and Ternes 1999; USEPA 2009), and it is one of the most prevalent PPCPs found in freshwater systems (Kolpin et al. 2002). Both triclosan and ciprofloxacin have the potential to be transported to soils through floodwaters and/or sewage sludge applications possibly affecting nontarget species and increasing bacterial resistance (Halling-Sørensen et al. 1998, 2003; Wilson et al. 2003; Hinther et al. 2011; Svenningsen et al. 2011; Drury et al. 2013). There has also been a tenfold increase in pesticide use in the USA from 1945 to 2000 despite concerns of environmental damage they may cause (Pimentel 2009). Atrazine application rates range between 70 and 80 million pounds per year based on surveys conducted from 1992 to 2007 (Thelin and Stone 2010). The pressure for continued development of new compounds for both PPCPs and agricultural use poses difficulties for management in regard to the options available to alleviate concerns regarding these compounds entering aquatic ecosystems. Once present in the watershed, contaminants are mobilized and transported in floodwaters potentially impacting floodplains hundreds of miles from the initial contamination point.
It is commonly accepted that the pH of a water body is the primary factor in determining partitioning behavior of an organic compound, thereby determining if the compound will remain aqueous or adsorbed to particles (Hari et al. 2005; Robberson et al. 2006). Additional alterations of organic contaminants in the water column may also be due to hydrolysis and photolytic processes (Wilson et al. 2009; Kummerer 2009).
Modeling of contaminant depositional patterns within riverine systems is primarily based on hydraulic and geospatial techniques, with the efficiency of these models dependent on post-depositional field data (Stewart et al. 1998; Schultz et al. 2009). During flood events, sediments and riparian soils serve as a repository for a number of particulate bound contaminants in riverine and estuarine systems, and therefore serve as a major source of contaminant mobilization (Pizzuto 1987; Cooper et al. 2003; Forstner et al. 2004; Schwartz et al. 2006; Hilscherova et al. 2007). Continued monitoring of historically contaminated areas has shown that the distribution of organic contaminants within recovering systems is a complicated fluctuation between storage and redistribution of materials based on flow regime (Pizzuto 1987; Cantwell et al. 2010; Micić et al. 2013). One of the primary concerns in modeling is the quantification of particle deposition based on hydrological flow models used to identify areas receiving disproportionate quantities of contaminated particulate matter (Stewart et al. 1998). Sediment modeling demonstrates that changes in water column parameters and boundary conditions are significant factors in contaminant dynamics (Schultz et al. 2009).
Evidence of contaminant mobilization and deposition associated with flooding has also been shown in post-flood studies of floodplain soils and associated organisms. Sampling of sediments redeposited in areas with low turbulence, typical of floodplains and stream banks, has shown that elevated levels of contamination in flooded areas are due to a redistribution of contaminants within the watershed (Hamers et al. 2006; Cobb et al. 2006; Hilscherova et al. 2007). Flooding of agricultural lands mobilizes herbicides within floodwaters, increasing herbicide concentrations within floodplain soils downstream of the application site due to the deposition of particle-adsorbed herbicides (Chong et al. 1998). A change in plant growth has also been associated with an influx of contaminated particles due to flooding (Kooistra et al. 2004). Lastly, soil invertebrates have demonstrated bioaccumulation of contaminants in post-Hurricane Katrina flooded soils (Harmon and Wyatt 2008).
In terrestrial systems, it has been determined that the absorption of a number of compounds in soils is correlated to the combined effect of the cation exchange capacity (CEC), electrical conductivity (EC), and pH of a system. Other studies have indicated that triclosan readily decays in soils with neutral to alkaline pH and high organic content (Al-Rajab et al. 2009; Xu et al. 2009). Ciprofloxacin’s mobility and bioavailability is primarily dependent on CEC, and it appears to be persistent in soils (Golet et al. 2003; Vasudevan et al. 2009). Atrazine has also been documented as a persistent soil contaminant with low levels of percolation; the primary driving force in the removal of atrazine is biodegradation (Lupi et al. 1988). Few studies have demonstrated that there is an interaction between the anionic exchange in aqueous systems as well (Robberson et al. 2006).
This research was performed during the flooding of the Rio Grande which began on July 5, 2010, due to a combination of tropical storms and water releases from Amistad Reservoir. The flood peaked on July 8, 2010, at 42 ft above the river base level. This was the second largest flood in 60 years; the largest flood occurred in 1954, peaking at 62 ft. Since then, the reservoir system has been developed for water storage and flood control of the region. However, excessive rainfall caused by Hurricane Alex overflowed the storage capacity of Amistad Reservoir, resulting in mass releases to protect the dam and to limit damage downstream. Controlled releases of water (>4000 m3/s) from Amistad Reservoir resulted in flow levels exceeding flood controls for the lands between the Amistad and Falcon Reservoirs, approximately 250 mi. of river (Montero 2010).
The Rio Grande outside of Laredo, TX, has been regularly monitored for contaminant levels prior to the flood event. The monitoring program allowed us to assess the differences in chemical concentrations and behavior within the Rio Grande during both flooding and typical flow conditions. Flooding can alter a number of parameters that may influence the distribution of contaminants within the watershed, most importantly particulate mobilization/redistribution and shifts in the pH of the water column, potentially altering the partitioning of compounds. Secondly, having the water column data from the flood itself allowed us to better assess the mechanisms distributing the contaminants within the floodplain soils. Lastly, this project assesses, within the root zone, the impact and retention of organic contaminants potentially available to plants over a period of 6 months. This data can be used in flood-prone regions to assist management organizations and property owners in identifying areas in which contamination may be most severe.
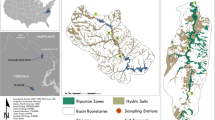
2 Materials and methods
2.1 Water sampling
Water sampling sites were monitored monthly for general water quality and contaminant levels beginning 3 months prior to the flood through the 6 month period of post-flood analysis. Due to the availability of bridges and structural access to the river, monitoring was also conducted during peak flood conditions. Samples were collected from four sites within the Laredo, TX, city limits: (1) at Rio Bravo-Laredo city limits; (2) at the International Bridge II; (3) the Jefferson Water Treatment Plant (at the intake for the drinking water plant); and (4) McNaboe Park. Two of these sites, the International Bridge II and Del Rio Park, are adjacent to the field sampling sites used for this research (see below). The Rio Bravo-Laredo city limits were selected as a representative site to determine the cumulative contamination released into the river from both Laredo, TX, and Nuevo Laredo, Mexico. Del Rio Park was selected as the point furthest upstream in the city to determine the contamination levels flowing into the city limits. These sites were originally selected due to accessibility to the river and because they are areas of concern for contamination even during low-flow conditions.
In situ data were collected using a YSI 6600 V2 multiple probe sondes (with interchangeable ROX sensors for dissolved oxygen, pH, salinity, alkalinity, turbidity, fluorescence, and temperature) and recorded with a YSI 650MDS data logger. Flow rates were collected from the in situ gauges monitored by the International Boundary Water Commission (IBWC) and the Texas Commission on Environmental Quality Control (TCEQ) via flow gauges at Columbia Bridge, International Bridge II and Del Rio. Water samples were placed into HDPE amber bottles, filtered, acidified with H2SO4 and stored at 4 °C for further analysis. Each 1 l sample was filtered through 0.2 μm filters to collect the suspended matter for contaminant extractions and to determine the total suspended matter of the water column. The total suspended matter was further used to determine the concentration of contaminants adsorbed to the suspended particulates within the water column and to determine partitioning coefficients for the contaminants.
2.2 Soil sampling and physicochemical analysis
For the purposes of this study, two sites were selected for sampling based on proximity to the river with similar plant diversity/type and protection from industrial/agricultural processes. The two sites were as follows: (1) the Lamar Bruni Vergara Environmental Science Center Nature Walk maintained by the Laredo Community College (LCC Site) near downtown Laredo, TX, and (2) undeveloped lands near Mines Road used by US Border Patrol for monitoring the river (MR site) at the northern (upstream) edge of the city. Both sites were dominated by buffelgrass, Pennisetum ciliare (L.), and sparsely populated with common native woody species, making them ideal for sampling and retaining transects with visible flags. The MR sampling site contained a cryptobiotic crust present in both flooded and nonflooded areas which was not present at the LCC sampling site. Thus, the microbial community may differ between the two sampling areas.
Immediately after floodwaters had receded, transects were delineated at the two sites and initial samples were collected on July 16, 2010. Samples were collected immediately in order to document initial conditions prior to any environmental and biological alterations of the soils (such as rain events or animal disturbance) and to minimize the effects of microbial activity within the rhizosphere. Additional samples were collected from these transects at 6, 14, and 24 weeks following the flood. Transects were laid out perpendicular to the topography (to the maximum slope) at each site, geo-referenced and marked for ongoing sampling. Each transect was positioned to span an equal distance into both the flooded and nonflooded areas with the flood line denoted by the fine layer of silt on both the plants and soils. Three transects were delineated at each of the sites with alternate 1-m2 quadrats sampled at every point: a 50-m transect with sampling points at every 10 m and two 25-m transects with sampling points at every 5 m (Fig. 1). Soil within each quadrat were sampled at five equidistant points and consolidated for each quadrat. The dominant plant in both sampling areas was buffelgrass that had a shallow rhizosphere of <10 cm in all locations. Our initial intent was to sample at the surface (0–2 cm) and at a depth of at least twice that of the root zone (10 cm, 2–20 cm) to assess the chemical availability to plants, and eventually determine if the organics were retained within this zone. After the initial sampling, we extended this depth down to 30 cm (collected at 20–30 cm) to further assess the penetration and mobility of these compounds within the flood plain, post-deposition. At the initial sampling (0 weeks), 36 samples were collected from each site (18 quadrats at two depths per sampling site); 54 samples were then collected at every subsequent sampling time (18 quadrats with three depths each per sampling site) at 6, 14, and 24 weeks post-flooding. Number of samples increased due to the addition of a third depth (20–30 cm) to the sampling regime. The initial samples were recoded so that each position and depth had the appropriate sample number for statistical comparisons.
Samples were collected using a 1″ diameter nickel-plated soil probe to a depth of 20 cm in July 2010 and down to 30 cm for the remaining sampling periods. Samples were then segmented by horizon: the upper (0–2 cm, surface/particle deposition), root zone (2–20 cm), and deep soils (20–30 cm, below the root zone). All samples were collected into airtight zip-top bags and stored on ice for transport. From these samples, 25 g were partitioned and immediately dried at 50 °C (to a constant mass) for organic contaminant analysis. Soil moisture was determined from these samples. The remaining soil samples were then air-dried in paper bags for 7 days, then sieved with a 2-mm mesh screen prior to organic carbon/nitrogen, pH, EC, CEC, available phosphate, and soil texture analysis.
Soil pH and EC were determined using a 2:1 ratio of deionized water to air-dried, sieved soils (v/w) that were shaken and centrifuged. The resulting supernatant was then analyzed using a VWR Symphony SB80PC pH/conductivity meter. CEC was analyzed using the sodium acetate method as described by Chapman (1965). Available phosphorous was extracted using the Olsen method (Pierzynski 2000). Soil texture was determined using the hydrometer method by Gee and Bauder (1982). Total organic carbon and total nitrogen were evaluated using combustion methods modified (with increased acidification) from Wallace et al. (2004).
Changes in soil pH, EC, and contaminant concentrations over time were further examined by a repeated measures ANOVA to determine the effect of time, flooding, and depth along with the interactions between factors. Since the 20–30-cm depth was missing from the first sampling time (week 0), a PROC MIXED was used followed by a Tukey’s post hoc test to determine differences between samples. For soil pH and EC, only transects 1 (LCC site) and 4 (MR site) were used in this analysis since these were the longest transects (50 m). For each depth, the overall main effects of flooding and time were examined further using a repeated measures ANOVA along with a MANOVA to determine the effects of these factors over time. Where flooding was significant, this analysis was followed by a one-way ANOVA and Tukey’s HSD post hoc test to determine differences between quadrats based on location relative to the river for each sampling time (see the Tables in the Electronic Supplementary Material for complete data). For the contaminant analysis, all six transects were evaluated to determine differences between quadrats based on relative distances from the river. Statistics were performed using SAS version 9.3 software (SAS Inc., Cary, NC) for the repeated measures ANOVA analysis.
2.3 Contaminant analysis
Procedures for extracting triclosan from both water and soil/suspended particulate samples were originally described by Wilson et al. (2009). Water column samples (1 l) were filtered through 15-cm Whatman GF/F filters via N2 pressure filtration (<15 psi). Samples were spiked with 10 ng (per liter water) of 13C-labeled triclosan (Cambridge Isotopes, MA) as a recovery standard. Water was extracted using dichloromethane (DCM) separations with a sep-funnel (DCM; 10 ml/250 ml of sample four times, then repeated until a full liter was extracted). Samples were then concentrated and passed through sodium sulfate and solid phase extraction disks (Oasis silica disks), eluted with ethyl acetate, and then concentrated to 1 ml prior to derivatization. Suspended particulate and soil samples were dried at 50 °C (to a constant mass), hand ground, and spiked with 25 ng of 13C-labeled triclosan (Cambridge Isotopes, MA) as a recovery standard. For extractions, 50–250 mg of particulate was used, depending on the amount available, or 2 g of dry soil was utilized. Solid-phase extractions were performed using sonication in a 1:1 mixture by volume of acetone and methanol at 25 °C (15 min, three times). Samples were concentrated and passed through sodium sulfate and solid-phase extraction disks (Oasis silica disks), eluted with methanol, and then concentrated to 1 ml prior to derivatization. Aliquots (200 μl) from all extracts were dried under N2 gas and then derivatized with MTBSTFA followed by resuspension in 200 μl of ethyl acetate. Samples were then analyzed using gas chromatography–mass spectrometry (GC-MS) operating in electron impact select ion monitoring (ELI-Sim) mode.
Procedures for extracting ciprofloxacin from water were modified from Golet et al. (2001) and Lee et al. (2007). Water column samples (1 l) were spiked with 10 ng of 13C-labeled ciprofloxacin (Cambridge Isotopes, MA) as a recovery standard. Samples of the acidified and filtered water samples were extracted using 30-μm Oasis WCX-SPE cartridges (mixed-phase cation-exchange) pretreated with methanol. Disks were washed with acidified water (pH ≤3) then eluted with a mixture (v/v) of 20:75:5 methanol/acetonitrile/formic acid. Suspended particulate and soil extraction procedures for ciprofloxacin were modified from Golet et al. (2001) and Pico and Andreu (2007). Suspended particulate and soil samples were dried at 50 °C (to a constant mass), hand ground, and spiked with 25 ng of 13C-labeled ciprofloxacin, as a recovery standard. For extractions, 50–250 mg of particulate was used, depending on the amount available, and 2 g of dry soil was utilized. Solid-phase extractions were performed using sonication in a 1:1 mixture by volume of aqueous phosphoric acid (pH = 2) and acetonitrile at 100 °C. The extract was then filtered using 30-μm Oasis WCX SPE cartridges pretreated with methanol; disks were washed with acidified water (pH ≤3) and then eluted with a mixture (v/v) of 20:75:5 methanol/acetonitrile/formic acid. Aliquots (200 μl) from all extracts were dried under N2 gas then derivatized with MTBSTFA, followed by resuspension in 200 μl of ethyl acetate and analyzed using GC-MS operating in ELI-Sim mode.
The procedure for extracting atrazine from water samples was originally described by Eisenreich et al. (1994). Water column samples (1 l) were filtered through 15-cm Whatman GF/F filters via N2 pressure filtration (<15 psi). Samples were then spiked with 10 ng (per liter water) of 13C-labeled atrazine (Cambridge Isotopes, MA) as a recovery standard. Water samples were extracted using solid-phase extraction disks (Oasis silica disks) with 7 ml of 85 % dichloromethane (DCM) and 15 % methanol (MeOH) solution, by volume. Suspended particulate and soil samples were dried at 50 °C (to a constant mass), hand ground, and spiked with 25 ng of 13C-labeled atrazine (Cambridge Isotopes, MA), as a recovery standard. For extractions, 50–250 mg of particulate was used, depending on the amount available, or 2 g of dry soil was utilized. Solid-phase extractions were performed using sonication in a 1:1 mixture, by volume, of acetone and methanol at 25 °C (15 min, three times). Samples were concentrated and passed through sodium sulfate and solid-phase extraction disks (Oasis silica disks), eluted with acetone. The final extract was then concentrated to 1 ml prior to analysis. Final quantification for all samples of atrazine was determined using a GCMS operating in ELI-Sim mode.
The particle-to-water partitioning coefficient is defined as the concentration of contaminant per gram of sediment to the concentration in 1 g of water. The empirical partitioning coefficient was derived from the in situ concentration of organic contaminants on water column particles divided by the aqueous concentrations. Previous research has also allowed for the optimization of both water column and soil extractions. Based on 13C-internal standards and spiked reference materials, recoveries were at 90–93 % with a detection limit of 1 ng/g.
A one-way ANOVA was employed to determine significant (p < 0.05) differences in water factors during flooded and nonflooded periods. When significant differences were detected, the ANOVA was followed by a Tukey’s HSD post hoc test. Statistical analysis was performed using the SPSS for Windows version 16 Statistical Software (SPSS Inc., Chicago, IL).
3 Results
3.1 Water column concentrations and partitioning behavior
Monitoring of the Rio Grande began in the spring of 2010 in order to monitor the overall water quality of this region (dissolved oxygen (DO), nutrient levels, pH, flow, and total suspended matter (TSM)). These samples were then utilized for analysis of the contaminants of interest (triclosan, atrazine, ciprofloxacin). Assessment of pre- and post-flood concentrations within the river system was possible due to an ongoing monitoring program for the Rio Grande implemented by TAMIU in the fall of 2009.
The floodwaters were significantly different than waters typically found in this region (Table 1). There was a significant decrease (p < 0.01) in the pH and DO (p < 0.001) during the flood. There was also a significant increase (p < 0.05) in both nutrients and TSM. Both nitrogen and phosphorous more than doubled and the TSM in the system was over five times as high compared to pre-flood conditions. Typical flow rates for the Rio Grande within the Laredo, TX, city limits range from 50 to 100 m3/s. The flow of the river during the flood exceeded 4000 m3/s with an average of almost 1300 m3/s for the month of July.
The Rio Grande contained trace amounts of triclosan, atrazine, and ciprofloxacin during all flow conditions (Table 2). During the flooding of the Rio Grande, there was a significant increase (p < 0.01) in the concentration of all three organic contaminants in both the water column and on suspended particulate matter in the system. In addition, we were able to determine that the partitioning coefficient significantly (p < 0.05) increased for both triclosan and ciprofloxacin in floodwaters with a lower pH (Table 2). There was not a significant difference (p > 0.05) in the partitioning of atrazine associated with changes in the water chemistry. Partitioning was determined as the concentration on 1 g of particulate to 1 g of water.
3.2 Contaminant distribution and subsequent behavior within the floodplain soils
In general, the highest concentrations of triclosan, atrazine, and ciprofloxacin were in the 0–2-cm surface depth of flooded quadrats (Figs. 2, 3, and 4). Of these flooded quadrats, those located nearest to the river contained the highest concentrations and the highest amount of particle deposition as indicated by visual inspection and particle size analysis. Contaminant concentrations decreased with depth. Concentrations of these compounds were generally higher at the LCC site, which is near an outfall for the sewage treatment plant in Nuevo Laredo, Mexico. The MR site received sediments from upstream of the city where agricultural lands using atrazine are commonly found. Therefore, the MR site was expected to contain higher concentrations of atrazine than the LCC site located nearer to the sewage outfall; this however was not what was observed. Low levels of particulate matter were found at the MR site, indicating a lower level of particle deposition and inundation overall. This also indicates that the overall particle loading may be the primary factor in determining contaminant concentrations during flood events, rather than proximity to contaminant sources. Organic contaminants were not detected in nonflooded soils. Therefore, the presence and behavior of these compounds are attributed to the flooding experienced in the affected quadrats.
Over the subsequent 24 weeks, both triclosan and atrazine were significantly degraded (repeated measures ANOVA p < 0.001) and were not detected by week 24. The first quadrats at each site had the highest concentrations of triclosan and atrazine (Figs. 2 and 3); however, atrazine was only found in the first quadrat at the surface of the MR site. Triclosan at the surface layer of the LCC site was significantly higher than all other depths and time points (p < 0.05), while at the MR site, triclosan concentrations at both depths examined initially were similar. Atrazine concentrations were also similar at the surface layer and root zone immediately following the flood. The half-life for triclosan in this system was 23 ± 3 days, and for atrazine, it was 16 ± 1 days. Half-lives were calculated individually for all layers in which the compounds were present and then averaged. Triclosan did not decay between 0 and 6 weeks at the Mines Road site in all but one quadrat, then was not detected at 14 weeks; the half-life at this site was not calculable.
Overall, ciprofloxacin did not decay over the course of this study and remained present in these soils (Fig. 4) at concentrations similar to those determined for the soils immediately following the flood (±3 ng). Concentrations did not increase with depth following rain events occurring over the study period (Figs. 2, 3, and 4).
3.3 Physiochemical behavior over 6-month monitoring period post-flood
This study examined the in situ impacts of a flooding event on contaminant deposition and fate in a riparian ecosystem. Overall, soils at the LCC site showed a greater change in soil composition due to floodwaters when compared to the MR site. Riparian soils were chemically altered by the inundation of standing floodwaters with a significant decrease (p = 0.0022) in soil pH that was reduced to a mean of 7.8 in flooded soils immediately following the flood, as opposed to 8.2 in nonflooded soils. Soil pH at the MR site was not affected by flooding (see Tables 1Sa and 2Sa in the Electronic Supplementary Material). There was also a significant increase (p < 0.0001) in EC immediately following the flood at both sites (see Tables 1Sb and 2Sb in the Electronic Supplementary Material). However, flooding effects were more drastic at the LCC site with an EC mean of 0.98 dS/m in flooded soils and 0.23 dS/m in nonflooded soils; flooded soils were moderately saline and nonflooded soils were nonsaline as defined by Scianna et al. (2007). At the upper soil layers, mean soil EC at the LCC site decreased over time, while EC increased at the 20–30-cm deep soil layer, suggesting that salts eventually leached from the upper surface layers over the sampling period. The flooded surface layer became nonsaline by week 24. Salt deposition at the MR site was minimal as flooded soils were considered to be slightly saline (0.37 dS/m) and nonflooded soils were very slightly saline (0.28 dS/m). As observed at the LCC site, the mean soil EC in the quadrats closest to the river at the 0–2-cm surface layer were significantly higher in mean soil EC (p < 0.01) than all other quadrats immediately following flooding (week 0) and 6 weeks later. Soils of the first two quadrats nearest the Rio Grande at the LCC and MR sites demonstrated a greater deposition of clay-sized (<2 μm) particles at the surface layer (0–2 cm), while a higher proportion of clay-sized particles were found in the root zone (2–20 cm) of the LCC site compared to the MR site (Table 3). Based on the particle size analysis, all soils would be classified as silt except at the root zone of quadrat 6 (silt loam), and the surface layer of the first two quadrats at the MR site (silt loam). The CEC was highest at the surface of the first quadrat of the LCC site where higher concentrations of organics were also detected. Overall, there was not a significant change in mean CEC over time (p < 0.05) except in the first quadrats of each transect (data not shown).
All other soil parameters (organic carbon, nitrogen, phosphate, and soil moisture) did not differ significantly between flooded and nonflooded soils. At 14 weeks, differences between flooded and nonflooded soils were not significant for pH, EC, or CEC as observed initially. This demonstrated recovery of the system to baseline values, assumed to be equivalent to the nonflooded soils.
4 Discussion
4.1 Sediment contamination and impacts on contaminant levels
Contaminants can be brought into floodwaters by various sources. In addition to floodwaters released from Amistad Reservoir, upstream point sources likely included runoff from industrial and agricultural lands adjacent to the Rio Grande that were impacted by the flood. Triclosan and ciprofloxacin are likely sourced from municipal and agricultural lands, while atrazine is most commonly found in agricultural farmlands. Sewage overflows occurred in both Laredo, TX, and Nuevo Laredo, Mexico, with more than 800,000 gals/day of raw sewage released from July 8 to 16, 2010 (LDH 2010). These municipal sources were likely the primary source of both triclosan and ciprofloxacin which resulted in higher concentrations in the water column found at sampling sites downstream of the lift stations. There are four primary fates for these contaminants within the flood waters: (1) they will remain aqueous within the water column; (2) they will be adsorbed and deposited within the river sediments; (3) they will be adsorbed then deposited with sediments on floodplain soils; and/or (4) they will infiltrate floodplain soils with the flood waters, then remain in the soils, Fig. 5.
The partitioning coefficients (discussed above) show that the majority of the observed contaminants were adsorbed onto particles within the water column. Water column concentration increased as expected during the flood, with an increase in the partitioning coefficient of triclosan and ciprofloxacin. As pH increases these compounds will deprotonate, allowing them to associate with other cations within the water column and particle surfaces (Wilson et al. 2009). There was not an increase in the partitioning of atrazine. Partitioning of atrazine is more closely associated with the organic content of particles rather than the pH of the system (Basta et al. 1997); therefore, without a significant change in the organic content of particles in the Rio Grande floodwaters, partitioning did not increase.
Contaminated particles were then deposited onto the floodplain soils sampled in this project. Surface samples contained high levels of litter, usually indicative of soils with a high organic carbon content. A fine layer of newly deposited sediment covered both the topsoil and plants in the area. Within areas of dense plant cover, the sediment layer was up to 2 cm in depth in quadrats closest to the river, with scoured areas having as little as 1–2 mm of deposition. This increase in sediment was also apparent in the soil texture analysis, with an increase in clay-sized particles in flooded quadrats when compared to the nonflooded quadrats. Therefore, contaminant levels were highest at the surface (Figs. 2 and 3), yet the presence of contaminants throughout the soil profile indicates that floodwaters inundated soil pore spaces followed by direct adsorption to soil particles at lower depths.
Overall, greater differences in organic contamination and impacted soil factors occurred in the first quadrat of each transect, which was closest to the river and under water for the longest period of time, increasing contaminant exposure. Further, these differences were more notable at the LCC site where contamination from sewage outfalls along with municipal runoff likely occurred. The LCC site is located at a bend in the Rio Grande which may have contributed to the additional flooding and particulate deposition at this site. Given that clay-sized particles appeared greater within the first two quadrats of both the LCC and MR site, these quadrats were likely those that had been flooded longer than the third quadrat examined at each transect. However, a higher infiltration of floodwaters occurred at the first quadrats of the LCC site when compared to the MR site, which coincides with contaminant levels.
4.2 Organic contaminant behavior within soils
Triclosan and atrazine were both found to degrade within the soils over a 6-month period with a half-life of 23 ± 3 and 16 ± 1, respectively. These results are similar to other studies indicating that the soil type and the method of contaminant deposition can affect the degradation rates for these compounds. In an agricultural field, the half-life of triclosan was 107 days after a single biosolid application (Lozano et al. 2010), while the half-life of triclosan when applied as a solution was similar to that observed at the LCC site. Soils with a high pH (>7.4) have a short degradation half-life of 2 to 3 weeks due to the high bioavailability of triclosan and subsequent biodegradation in aerobic conditions (Ying et al. 2007; Xu et al. 2009; Carr et al. 2011). Ciprofloxacin likely sorbed to clay particles as observed with fluoroquinolones at moderately alkaline conditions during which ciprofloxacin becomes protonated (Nowara et al. 1997; Golet et al. 2003). Benchtop studies indicate that ciprofloxacin is generally persistent in sludge unless it is aerated or subjected to photolysis in aquatic systems (Burhenne et al. 1997; Halling-Sørensen et al. 2000, 2003; Pereira et al. 2007). Our data suggests that ciprofloxacin in soils after a flood will remain for at least 6 months. Long-term field experiments are needed to understand the persistence of ciprofloxacin and if disturbance of soils would affect its degradation. Further, the absence of changes in concentrations of all contaminants with depth over time, despite rain events, indicates that organic contaminants were not mobile in the system.
Previous studies have shown that the mobility of these compounds in soils is correlated to the combined effect of the CEC, EC, and pH of a system. In areas with a high pH and EC, triclosan has limited mobility in soils, as was shown in this study. Additionally, triclosan decayed within a month in this study as similarly found in other studies when triclosan-contaminated biosolids were applied when wet (Al-Rajab et al. 2009; Xu et al. 2009). Ciprofloxacin’s behavior and bioavailability is also closely related to the CEC of a system as it is likely able to bind strongly to cations, thereby reducing the ability of a system to remove this contaminant (Golet et al. 2003).
5 Conclusions and managerial considerations
The completion of this project has provided a better understanding of the behavior of contaminants mobilized by flooding events and deposited onto soils. Our continued study of contaminant dynamics will greatly impact our understanding of agricultural lands as managed ecosystems. Here, we have shown that substantial quantities of organic contaminants, specifically herbicides from agricultural lands and antimicrobials from wastewater treatment plants/sewage lines, can be distributed to floodplains via particle adsorption and deposition. Additionally, this study of a natural flooding event has demonstrated that contaminants found at lower depths in floodplains are due to the initial infiltration of flood waters rather than leaching from post-flood particulate deposition. Our data indicates that triclosan and atrazine are undetectable between 6 and 14 weeks post-flood; however, ciprofloxacin remains in soils for at least 24 weeks with no vertical mobility. Most agricultural lands are within the floodplain of major rivers in the USA (FEMA 2014). Periodic flooding may potentially allow for contaminants, such as those examined, to become available to commercial crops. In turn, this would constitute an indirect exposure to the people relying on these food sources. Further, the deposition of antimicrobials and persistence of these compounds may increase populations of antimicrobial resistant microbes. Floodwaters likely deposited microbes with some antimicrobial resistance, given that sources included sewage outfalls and wastewater effluent and may have influenced changes in the microbial community (not addressed here). We are now beginning to address behavior of organic contaminants and impact on microbial communities in agricultural soils due to the use of recycled water from municipal/industrial sources used for irrigation in nonfloodplain regions.
References
Al-Rajab AJ, Sabourin L, Scott A, Lapen DR, Topp E (2009) Impact of biosolids on the persistence and dissipation pathways of triclosan and triclocarbon in an agricultural soil. Sci Total Environ 407:5978–5985
Barnes KK, Kolpin DW, Furlong ET, Zaugg SD, Meyer MT, Barber LB (2008) A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States – I) Groundwater. Sci Total Environ 402:192–200
Basta NT, Huhnke RL, Stiegler JH (1997) Atrazine runoff from conservation tillage systems: a simulated rainfall study. J Soil Water 52:44–48
Burhenne J, Ludwig M, Nikoloudis P, Spiteller M (1997) Photolytic degradation of fluoroquinolone carboxylic acids in aqueous solution. Primary photoproducts and half-lives. Environ Sci Pollut R 4:10–15
Cantwell M, Wilson B, Zhu J, King J, Wallace G, Smith J, Olsen C, Burgess R (2010) Temporal trends of triclosan contamination in dated sediment cores from four urbanized estuaries: evidence of preservation and accumulation. Chemosphere 78:347–352
Carr DL, Morse AN, GZak JC, Anderson TA (2011) Microbially mediated degradation of common pharmaceuticals and personal care products in soil under aerobic and reduced oxygen conditions. Water Air Soil Pollut 216:633–642
Chapman HD (1965) Cation exchange capacity. In: Black CA (ed) Methods of soil analysis. Part 2: Chemical and microbiological properties. 1965. ASA, CCSA, and SSSA, Madison, pp 891–900
Chong SK, Klubek BP, Weber JT (1998) Herbicide contamination by the 1993 great flood along the Mississippi river. J Amer Water Resour Assoc 34:687–693
Cobb GP, Abel MT, Rainwater TR, Austin GP, Cox SB, Kendall RJ, Marsland EJ, Anderson TA, Leftwich BD, Zak JC, Presley SM (2006) Metal distributions in New Orleans following hurricanes Katrina and Rita: a continuation study. Environ Sci Technol 40:4571–4577
Cooper C, Smith S, Moore M (2003) Surface water, ground water and sediment quality in three oxbow lake watersheds in the Mississippi delta agriculture region: pesticides. Int J Ecol Environ Sci 29:171–184
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change. Environ Health Perspect 107:907–936
Drury B, Scott J, Rosi-Marshall EJ, Kelly JJ (2013) Triclosan exposure increases triclosan resistance and influences taxonomic composition of benthic bacterial communities. Environ Sci Technol 47:8923–8930
Eisenreich S, Schottler S, Hines N (1994) Standard operating procedure for isolation, extraction and analysis of atrazine, DEA and DIA. Rutgers University P.O. Box 231 New Brunswick, NJ 08903
FEMA (2014) Flood map service center. http://msc.fema.gov/portal, accessed June 2014
Focazio MJ, Kolpin DW, Barnes KK, Furlong ET, Meyer MT, Zaugg SD, Meyer MT, Barber LB (2008) A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States—II) Untreated drinking water sources. Sci Total Environ 402:201–216
Forstner U, Heise S, Schwartz R, Westrich B, Ahlf W (2004) Historical contaminated sediments and soils at the river basin scale. J Soils Sediments 4:247–260
Gee, GW, Bauder, JW (1982) Cation-Exchange Capacity. In Klut A (ed) Methods of soil analysis. Part1, physical and mineralogical methods. Am Soc Agron, Inc., Madison, WI, pp 383–411
Golet E, Alder A, Hartmann A, Ternes T, and Giger W (2001) Trace determination of fluoroquinolone antibacterial agents in urban wastewater by solid-phase extraction and liquid chromatography with fluorescence detection. Anal Chem 73:3632–3638
Golet EM, Xifra I, Siegrist H, Alder AC, Giger W (2003) Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environ Sci Technol 37:3243–3249
Halling-Sørensen B, Nielsen SN, Lanzky PF, Holten Lützhøft H-C, Jorgensen SE (1998) Occurrence, fate and effects of pharmaceutical substances in the environment. Chemosphere 36:357–393
Halling-Sørensen B, Holten Lützhøft H-C, Andersen HR, Ingerslev F (2000) Environmental risk assessment of antibiotics: comparison of mecillinam, trimethoprim and ciprofloxacin. J Antimicrob Chemother 46:53–58
Halling-Sørensen B, Sengeløv G, Ingerslev F, Jensen LB (2003) Reduced antimicrobial potencies of oxytetracycline, tylosin, sulfadiazine, streptomycin, ciprofloxacin, and olaquindox due to environmental process. Arch Environ Contam Toxicol 44:7–16
Hamers T, van den Berg JHJ, van Gestel CAM, van Schooten FJ, Murk AJ (2006) Risk assessment of metals and organic pollutants for herbivorous and carnivorous small mammal food chains in a polluted floodplain (Biesbosch, The Netherlands). Environ Pollut 144:581–595
Hari A, Paruchuri R, Sabatini D, Kibby T (2005) Effects of pH and cationic and nonionic surfactants on the adsorption of pharmaceuticals to a natural aquifer material. Environ Sci Technol 39:2592–2598
Harmon SM, Wyatt DE (2008) Evaluation of post-Katrina flooded soils for contaminants and toxicity to the soil invertebrates Eisenia fetida and Caenorhabditis elegans. Chemosphere 70:1857–1864
Hilscherova K, Dusek L, Kubik V, Cupr P, Hofman J, Klanova J, Holoubek I (2007) Redistribution of organic pollutants in river sediments and alluvial soils related to major floods. J Soils Sediments 7:167–177
Hinther A, Broomba CM, Wulff JE, Helbing CC (2011) Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ Sci Technol 45:5395–5402
Kolpin DW, Furlon ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, a national reconnaissance. Environ Sci Technol 36:1202–1211
Kooistra L, Salas EAL, Clevers JGPW, Wehren R, Leuven RSEW, Nienhuis PH, Buydens LMC (2004) Exploring field vegetation reflectance as an indicator of soil contamination in river floodplains. Environ Pollut 127:281–290
Kummerer K (2009) Antibiotics in the aquatic environment—a review—Part I. Chemosphere 75:417–434
Lee HB, Peart TE, Svoboda ML (2007) Determination of ofloxacin, norfloxacin, and ciprofloxacin in sewage by selective solid-phase extraction, liquid chromatography with fluorescence detection and liquid chromatography – tandem mass spectrometry. J Chromatrography 1139:45–52
Lozano N, Rice CP, Ramirez M, Torrents A (2010) Fate of triclosan in agricultural soils after biosolid applications. Chemosphere 78:760–766
Lupi C, Bucchi AR, Piccioni A, Zapponi GA (1988) The environmental behavior of chemicals in soil: atrazine as an example. Ecotox Environ Safe 16:133–142
Micić VI, Kruge MA, Hofmann T (2013) Variations of common riverine contaminants in reservoir sediments. Sci Total Environ 458–460:90–100
Montero R (2010) Hurricane Alex flood operations, lower Rio Grande flood control project, 2010. International Boundary and Water Commission, Control Lower Rio Grande Citizens Forum, El Paso, TX, USA
Nowara A, Buhrenne J, Spiteller M (1997) Binding of fluoroquinolone carboxylic acid derivatives to clay minerals. J Agric Food Chem 45:1459–1463
Pereira VJ, Linden KG, Weinberg HS (2007) Evaluation of UV for photolytic and oxidative degradation of pharmaceutical compounds in water. Water Res 41:4413–4423
Pico Y, Andreu V (2007) Fluoroquinolones in soil—risks and challenges. Anal Bioanal Chem 387:1287–1299
Pierzynski GM (ed) (2000) Methods of phosphorous analysis for soils, sediments, residuals, and waters. Southern Cooperative Series Bulletin 396
Pimentel D (2009) Environmental and economic costs of the application of pesticides primarily in the United States. Environ Dev Sustain 7:229–252
Pizzuto JE (1987) Sediment diffusion during overbank flows. Sedimentology 34:301–331
Robberson K, Waghe A, Sabatini D, Butler E (2006) Adsorption of the quinolone antibiotic nalidixic acid onto anion-exchange and neutral polymers. Chemosphere 63:934–941
Schultz M, Buttner O, Barorowski M, Bohme M, Matthies M, von Tumpling W (2009) A dynamic model to simulate arsenic, lead and mercury contamination in the terrestrial environment during extreme floods of rivers. Clean - Soil Air Water 37:209–217
Schwartz R, Gerth J, Neumann-Hensel H, Forstner U (2006) Assessment of highly polluted fluvisol in the Spittelwasser floodplain based on national guideline values and MNA-criteria. J Soils Sediments 6:145–155
Scianna J, Logar R, Pick T (2007) Testing and interpreting salt-affected soil for tree and shrub planting, Plant Materials Technical Note No. MT-60. United States Department of Agriculture, Natural Resources Conservation Service, Plant Materials Center, Bridger, MT, USA
Stewart MD, Bates PD, Price DA, Burt TP (1998) Modelling the spatial variability in floodplain soil contamination during flood events to improve chemical mass balance estimates. Hydro Proc 12:1233–1255
Svenningsen H, Henriksen T, Prieme A, Johnsen AR (2011) Triclosan affects the microbial community in simulated sewagedrain-field soil and slows down xenobiotic degradation. Environ Pollut 159:1599–1605
Thelin GP, Stone WW (2010) Method for Estimating Annual Atrazine Use for Counties in the Conterminous United States, 1992–2007: Scientific Investigations Report 2010. US Geological Survey, Washington, DC, USA
USEPA (2009) Occurrence of contaminants of emerging concern in wastewater from nine publicly owned treatment works, EPA-821-R-09-009. Environmental Protection Agency, Office of Water (4303T), Washington, DC, USA
Vasudevan D, Bruland GL, Torrance BS, Upchurch VG, MacKay AA (2009) pH-dependent ciprofloxacin sorption to soils: interaction mechanisms and soil factors influencing sorption. Geoderma 151:68–76
Wallace G, Oktay S, Pala F, Ferraro M, Gnatek M, Luce D (2004) Determination of recent inputs of mercury to lakes/ponds in the Merrimack Valley using sediment cores: a feasibility study. Final report for Massachusetts Department of Environmental Protection. University of Massachusetts, Boston, MA, USA
Wilson B, Smith V, deNoyelles J, Larive C (2003) The effects of three pharmaceutical and personal care products on natural freshwater algal assemblages. Environ Sci Technol 37:1713–1719
Wilson BA, Chen R, Olsen C, Cantwell M, Gontz A, Zhu J (2009) The partitioning of triclosan between aqueous and particulate bound phases in the Hudson River Estuary. Mar Pollut Bull 59:207–212
Xia K, Bhandari A, Das K, Pillar G (2005) Occurrence and fate of pharmaceuticals and personal care products (PPCPs) in biosolids. J Environ Qual 34:91–104
Xu J, Wu L, Chang AC (2009) Degradation and adsorption of selected pharmaceuticals and personal care products (PPCPs) in agricultural soils. Chemosphere 77:1299–1305
Ying GG, Yu XY, Kookana RS (2007) Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with environmental fate modeling. Environ Pollut 150:300–305
Acknowledgments
We would like to thank our undergraduate research students for their work throughout this project. A sampling of this magnitude is not possible without a strong group of contributing individuals. This includes Cynthia de le Miyar, Anna Garza, Keith Almeida, Adrian Villarreal, Paulina Gonzalez, Maria Gallegos, Melissa Espino, Erik Perez, Raul Castro, Ediza Martinez, Edwin Rodriguez, and Melissa Rodela. We would also like to thank the National Science Foundation for supporting this research (#EAR 1054466).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jianming Xu
Rights and permissions
About this article
Cite this article
Wilson, B.A., Addo-Mensah, A.K. & Mendez, M.O. In situ impacts of a flooding event on contaminant deposition and fate in a riparian ecosystem. J Soils Sediments 15, 2244–2256 (2015). https://doi.org/10.1007/s11368-015-1145-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1145-z