Abstract
Leakage of jet fuel from an oil skimmer has resulted in soil and groundwater contamination at the fire fighting training site of the Oslo international airport. The site is located on an unconfined sandy aquifer formed by the Gardermoen ice contact delta. A strong relationship between hydrogeological settings and spatial distribution of the hydrocarbons has been observed. Leaching hydrocarbons constitute a source of dissolved BTEX and naphthalene. Concentration of the dissolved hydrocarbons, electron acceptors and metabolic by-products has revealed concomitant changes in space and in time, in response to groundwater fluctuations. Correlation between high concentrations of hydrocarbons, elevated alkalinity and depleted electron acceptors supports the hypothesis that intrinsic bioremediation has been taking place at the site. Naphthalene, however, has been found persistent under anoxic conditions. Calculations of biodegradation potential indicates that Fe(III) reduction, methanogenesis, nitrate and sulphate reduction are the main factors controlling biodegradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
One of the side effects of widespread use of petroleum hydrocarbons is an uncontrolled release of these chemicals into the natural environment. Due to relatively high aqueous solubilities and toxicity, aromatic hydrocarbons, like: benzene, toluene, ethylbenzene and xylenes (BTEX) as well as naphthalene are known to be relatively mobile and potentially dangerous for the groundwater resources (Bennett et al. 1993; Chapelle 2001; Eganhouse et al. 1993; Ward et al. 1997).
Occurrence of hydrocarbons in groundwater, including drinking water, has raised a strong interest in research concerning their transport and degradation. Several naturally occurring processes, like dispersion, dilution, volatilisation, sorption, biotic and abiotic transformations, termed natural attenuation, result in reduction of the contaminant concentration (Wiedemeier et al. 1999). Not only concentration reduction, but also reduction of mass, toxicity, mobility, or volume is considered as natural attenuation (USEPA 1997). The component of the natural attenuation processes taking place due to biological degradation mechanisms is termed intrinsic bioremediation (Wiedemeier et al. 1999).
Dissolved hydrocarbons can be utilized by the in situ heterotrophic microorganisms as electron donors and a source of energy crucial to bacterial growth, protein synthesis and reproduction (Schlegel 1992). Thus such microorganisms are responsible for degradation of the hydrocarbons in both pristine and contaminated aquifers (Thomas et al. 1997). Terminal electron accepting processes (TEAPs), involving reduced inorganic species as electron acceptors, are necessary in order to accomplish these reactions (Chapelle 2001). The hydrocarbon degradation reactions are microbially mediated and follow diverse – aerobic and anaerobic, microbial respiration pathways in a sequence dependent on the potential energy yield, namely: aerobic respiration, denitrification, Mn(IV) reduction, Fe(III) reduction, sulphate reduction and methanogenesis (Grbic-Galic 1991; Löser et al. 1998; Lovley et al. 1994). In effect, hydrocarbon biodegradation results in zonation of redox processes and distinct changes of aquatic chemistry (Chapelle et al. 1995; Christensen et al. 2000a, b). Observation of space and time changes of concentrations of the dissolved organics, electron acceptors and metabolic by-products of biodegradation can be used for evaluation of natural attenuation and intrinsic biodegradation (McAllister and Chiang 1994; Salanitro 1993; Schirmer et al. 1999; Vroblesky and Chapelle 1994). Despite the fact that capabilities of microorganisms to degrade dissolved aromatic compounds have been clearly demonstrated, the biodegradation potential and rate of the processes may vary from site to site due to a number of limiting factors. Among others, presence of the appropriate heterotrophic microorganisms (Chapelle 2000; Haack and Bekins 2000), concentration of the potential electron acceptors as well as heterogeneities of aquifer material and hydrogeological conditions should be considered (Davis et al. 1999; Lee et al. 2001; Phelps and Young 1999; Röling and Verseveld van 2002).
The concept of natural attenuation offers, to some extent, an alternative approach to the conventional remediation actions for cleanup of hydrocarbon contaminated aquifers (Christensen et al. 2000b). Successful implementation of natural attenuation as a remedial approach requires usually a long term monitoring of spatial and time distribution of the plume (Bekins et al. 2001; Cozzarelli et al. 2001) and is referred to as monitored natural attenuation (USEPA 1997). In order to demonstrate that the natural attenuation and intrinsic bioremediation processes are actually taking place different lines of evidence can be used (Council 1994; Wiedemeier et al. 1999). Those include historical contaminant data showing stabilization of the plume and/or loss of contaminant mass over time, distinct changes of groundwater chemistry caused by microbial activity as well as microbiological data showing the presence of organisms able to degrade the contaminants. Occurrence of natural attenuation and intrinsic bioremediation have been reported to be responsible for successful hydrocarbon removal at a number of contaminated sites connected to storage and distribution facilities, industrial activities and leakage from waste disposal sites (Barker et al. 1987; Baun et al. 2003; Borden et al. 1997; Chiang et al. 1989; Klecka et al. 1990; Schirmer and Barker 1998).
The site studied in this paper is located at the fire fighting training facility of the Oslo international airport, Gardermoen. Site investigations completed in 1998 by the Norwegian Geotechnical Institute revealed contamination of the soil and groundwater in the vicinity of a leaking oil skimmer connected with the water drainage system (Rudolph-Lund and Sparrevik 1999a). The contamination source has been removed and simultaneously 17.5 l of a hydrocarbons free phase as well as about 6,000 l of the contaminated groundwater have been retrieved and treated (Aagaard et al. 2001). Based on the observations of groundwater chemistry, a remediation procedure using natural degradation of the groundwater contaminants has been suggested (Rudolph-Lund and Sparrevik 1999b). This has been approved by the Norwegian State Pollution Control Agency as a pilot study on natural attenuation. In order to monitor the natural attenuation processes within the plume a monitoring network, including multilevel samplers (MLS) has been established (Kłonowski et al. 2002).
The main goals of this paper are: firstly – to describe the conditions under which the hydrocarbon plume has developed, secondly – to analyse the spatial and temporal distribution of hydrocarbons as well as electron acceptors and metabolic by-products, resulting from the natural attenuation processes, and thirdly – to evaluate the natural potential of the aquifer for biodegradation of dissolved organic compounds.
2 Materials and Methods
2.1 Site Description
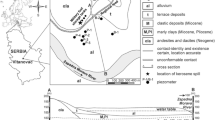
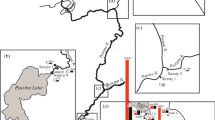
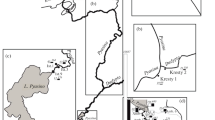
The studied site is situated at the fire fighting training facility of the Gardermoen airport, about 50 km northeast of Oslo, in southeastern Norway (Fig. 1). The major hazardous substance handled at this site is jet fuel. The studied plume of jet fuel derived hydrocarbons has developed due to leakage from an oil skimmer. The skimmer was dismantled and replaced by the observation well BR1, further on referred to as a contamination source zone or shortly source zone (Kłonowski et al. 2005).
The airport is situated on a top of an unconfined aquifer formed by the sediments of an ice contact, glacio-marine fan delta deposited during the early Holocene (Soerensen 1979). The aquifer is mainly recharged, up to 60%, by snow melting, which results in significant seasonal fluctuation of the groundwater table (Joergensen and Oestmo 1992). The contaminated part of the aquifer is situated within the topset and foreset of the delta drained by the Sogna river catchment (Oestmo 1976). The topset and foreset consist of, respectively: cobbly gravel and coarse sands as well as interbeded layers of the gravely, coarse sands and fine, silty sands, dipping southwest (Tuttle et al. 1997).
2.2 Sediment Sampling and Analyses
The multilevel samplers MLS 8 to MLS 19 were installed using rotary drilling which allows sampling of the sediment cuttings. The samples were obtained from nine boreholes with depth resolution of 1 m. The sampling profiles corresponding to the multilevel samplers MLS 8, 9, 10, 11, 12, 13 and MLS 15 are 5 m deep, while those corresponding to the samplers MLS 19 and MLS 16 6 and 12 m deep, respectively. Totally 53 samples of the Quaternary sediments were collected. The sediment samples are referred to in the following manner: SP, number of the sampling profile and sampling depth, for example SP 10/3.
The analyses of grain size distribution were carried out for all the samples (Tucker 1988) in order to estimate hydraulic conductivity by the Hazen (Fetter 2001) and Gustafson (Andersson et al. 1984) methods.
X-ray diffraction (XRD) was used to determine mineralogical composition of the bulk sediment samples as well as for the individual grain size fractions of the samples obtained from the profile SP 16. Powder XRD analyses were carried out on a Philips X’Pert X-ray diffractometer (Philips, Eindhoven, the Netherlands) equipped with θ–θ goniometer and Cu–Kα radiation. In order to complete the semi-quantitative analysis of the main minerals relative intensity was multiplied by a weight factor and eventually, weight percentage for individual minerals was calculated (Ramm 1991; Tucker 1988).
The content of organic contaminants was determined for all the sediment samples by hexane extraction using sonication. Hexane extracts were dried with sodium sulphate and. analyzed using a Chrompack CP9001 gas chromatograph equipped with a split/splitless injector, CP-Sil 8 CB column (25 m × 0.25 mm i.d. × 1.2 μm) and a flame ionization detector. Total hydrocarbon levels were quantified by integrating the area between n-C8 and n-C40 using a 5-point external calibration curve.
Total carbon content (TC) and total organic carbon content (TOC), after removal of inorganic carbon using hydrochloric acid, were determined for all the bulk sediment samples as well as for the grain size fraction <0.063 mm of the samples SP 16/1–16/4 and SP 16/9–16/12. The determination of carbon content was accomplished using a CR-412 Carbon Analyser (Leco Corporation, St. Joseph, USA) measuring CO2 release after thermal oxidation. The uncertainty of the method was calculated to be 0.03% C in both TC and TOC determinations. Total inorganic carbon (TIC) was calculated as the difference between TC and TOC.
2.3 Groundwater Monitoring
The groundwater monitoring system consists of nine piezometers: P1–P6 and P8–P10, two observation wells: Br1 and Ano11, and sixteen multilevel samplers: MLS 1–MLS 13, MLS 15–MLS 16 and MLS 19. Please note that P7 as well as MLS 14, MLS 17 and MLS 18 do not exist. Location of the individual points of the monitoring network is illustrated in Fig. 2. The piezometers and the observation wells were used for monitoring of groundwater table elevation, while the multilevel samplers were used for monitoring of groundwater quality.
Construction of the multilevel samplers – each sampling tube terminates at a specific depth with a separate filter, further on referred to as sampling port, enables groundwater sampling from different depths. Vertical spacing of the sampling ports for all the multilevel samplers is 0.5 m, while the depths of the sampling ports vary between 2 and 11 m below the surface. The samplers were assembled with Teflon tubing (inner diameter 1/8 in) and a stainless steel mesh, in order to minimise the sorption effects.
All the multilevel samplers consist of several sampling ports. Numbering of the sampling ports starts at the bottom. Further on, the multilevel samplers are referred to as MLS, its number and depth of the sampling port, for example: MLS 10/3. The multilevel samplers MLS 1–MLS 7 were installed in October 2000, the samplers MLS 8–MLS 15 in August 2001 and MLS 16 and MLS 19 in August 2002. Installation of the multilevel samplers and piezometers was completed using the diverse drilling methods. The samplers MLS 1 to MLS 7 as well as all the piezometers, were installed with a direct push-vibration method, whereas the remaining samplers were installed by rotary drilling, using casing. The multilevel samplers MLS 1 and MLS 8 were installed in the background of the plume, MLS 2, 16 and 10 in the contamination source zone, MLS 11, 3, 9, 15, 4, 19 and 5 in the various zones of the plume, while MLS 6, 7, 12 and 13 aside the plume. Prior to installation the direction of the plume extent had been estimated based on a groundwater contour map.
Four groundwater sampling events, covering different seasons of the year and different groundwater table elevations, were completed between September 2001 and September 2003, namely: in September and October 2001, in April and May 2002, in March and April 2003 and in September 2003. The groundwater was sampled using a peristaltic pump Ismatec (Ismatec SA, Zurich, Switzerland) equipped with the tygon tubing. The pump was directly coupled to a set of tightly capped glass bottles. This procedure, called in-line sampling, ensures minimum contact of the groundwater with atmospheric oxygen. All the groundwater samples were stored in a dark place at ambient temperature and transported to the laboratory on the sampling day.
2.4 Field Measurements and Laboratory Procedures
Determination of the following groundwater parameters: pH, electroconductivity, temperature and dissolved oxygen content, were accomplished in the field. An Orion instrument, model 250A (Orion Research Inc., Boston, USA) was used for determination of pH. Electroconductivity and temperature were determined using a WTW Conductivity meter, model 330i (WTW, Weilheim, Germany), while oxygen was measured by an Oxi instrument, model 330 (WTW, Weilheim, Germany). The measurements were taken in a flow-through cell and the groundwater sampling started when stable readings of the measured parameters were obtained. This usually took about 5 to 10 min pumping at the average rate of 60 ml/min, purging approximately 0.5 l of groundwater.
Alkalinity was determined in the field – in a laboratory caravan, immediately after sampling, using a direct Gran titration method (Appelo and Postma 1996; Stumm and Morgan 1996). A 30 ml groundwater sample was titrated by 0.01-normal hydrochloric acid up to pH about 4.2. Titration was carried out using a digital burette BRAND (Rudolf Brand GMBH+Co, Wertheim, Germany), while pH measurements were taken using the Orion pH meter.
The samples for determination of dissolved hydrocarbon concentrations were collected in the 120 ml glass bottles. In order to minimise volatilisation the bottles were tightly capped with Teflon lined silicon septa and stored upside down. Extractions were conducted on the sampling day by adding 2 ml of pentane and 1-Cl-4F benzene as internal standard. The bottles were shaken vigorously and the pentane phase was removed. The pentane extracts were analysed on a Chrompack CP9001 gas chromatograph (Chrompack, Middelburg, the Netherlands) equipped with a split/splitless injector, CP-Sil 8 CB column (25 m × 0.25 mm i.d. × 1.2 μm) and a flame ionization detector. Levels of individual hydrocarbons were determined using a 5-point external standard calibration. Based on the used volumes of the groundwater sample and pentane, detection limits were calculated to be approximately 0.1 μg·l−1 for each hydrocarbon and the accuracy was within 20%.
The groundwater samples for determination of major ions were filtered through a 0.45 micrometers filter tightly fitted into the in-line sampling system. The samples were collected in 27 ml glass bottles. An ion chromatograph Dionex QIC (Dionex, Sunnyvale, USA) was used for determination of major anion concentrations: F−, Cl−, \( {\text{NO}}_{3} ^{ - } \) and \( {\text{SO}}_{4} ^{{2 - }} \). The groundwater samples for determination of major cations: Na+, K+, Ca2+, Mg2+, Fe2+ and Mn2+, were conserved in the field with 3-normal nitric acid. The samples were stored in a cooling room and analysed after the completion of the sampling event. Determination was accomplished by atomic adsorption spectroscopy using a Varian SpectrAA-300 instrument (Varian Techtron, Springvale, USA).
Concentration of methane in groundwater was determined for selected locations sampled in April 2003. The ground water samples were collected in 120 ml glass bottles – without any head space. The bottles were tightly capped with Teflon lined silicon septa and stored upside down at ambient temperature. Methane concentrations were completed on the sampling day by injection of 2 ml of analytical purity nitrogen gas, while equal amounts of water were allowed to drain from the bottles. The samples have been shaken for about 30 min and the methane concentration was analysed in the nitrogen head space using the above mentioned gas chromatograph and a PoraPlot column. Aqueous concentrations were calculated using air–water partitioning coefficients.
Dissolved organic carbon (DOC) content was measured for selected locations in April 2003. The groundwater samples were collected into 120 ml bottles, and tightly capped with Teflon lined silicon septa. The samples were conserved in the field with 1-normal hydrochloric acid, stored in a cooling room and analysed using a total organic carbon analyser model TOC-5000A (Shimadzu Corporation, Japan).
2.5 Soil Gas Sampling
Soil gas was sampled through the sampling ports of the multilevel samplers located within the unsaturated zone. The concentrations of carbon dioxide, oxygen and methane were determined as volume percentage directly in the field using a direct reading field instrument Multiwarn II (Dräger, Lübeck, Germany), tightly connected to the multilevel samplers via a water trap. Two sampling sessions at different groundwater table elevations and growing seasons were carried out, namely in April 2003 and September 2003.
2.6 Data Processing
Hydraulic conductivity was estimated based on the grain size distribution according to the methods by Hazen (Fetter 2001) and Gustafson (Andersson et al. 1984). The average linear groundwater flow velocity – V x was calculated according to the following equation (Appelo and Postma 1996; Fetter 2001):
Where K is hydraulic conductivity, θ is effective porosity and dh/dl is hydraulic gradient. The hydraulic gradient was inferred from the groundwater table elevation data. Determination of porosity and bulk density was carried out by Knudsen (2003) for the undisturbed sediment cores of the Gardermoen aquifer sampled at the Moreppen field research station (Fig. 1). Like the studied site, the station is located within the distal part of the delta, about 1,000 m north of the studied area. Determination yielded porosity equal to 0.367 (dimensionless, given as a fraction) and bulk density equal to 1.68 (g·cm−3; Knudsen 2003), which were used for further calculations in this paper.
The plots illustrating piezometric surface of the studied aquifer as well as two dimensional (2D) distribution of the selected electron acceptors and metabolic by-products were prepared in Surfer software, version 8.0, with use of a kriging interpolation method (Golden Software 2002). Anisotropy ratio was assumed to be 1:10, based on the previous studies at the Moreppen research station (Alfnes et al. 2003a,b; Knudsen 2003).
Calculations of biodegradation potential of the dissolved hydrocarbons were carried out for the April 2003 sampling event, since methane concentrations are available only for this sampling event. Major geochemical changes took part within the core of the hydrocarbon plume, therefore calculations focussed on this part of the plume. The term core of the plume is described more precisely in the next chapter. Calculations were conducted using degradation of o-xylene as a model organic substrate – electron donor, since contribution of the individual hydrocarbons could not be determined. The individual half-cell reactions for oxygen consumption, nitrate reduction, Mn(IV) reduction, Fe(III) reduction, sulphate reduction and methanogenesis are given in Table 3. Based upon the concentration changes of the redox sensitive species the potential electron acceptor capacity could be calculated in mol electrons. This could be balanced with mol of electrons donated by o-xylene degradation. The potential biodegradation capacity (in o-xylene equivalents) could be calculated in this way. Consumption of the monitored electron acceptors and production of metabolic by-products were calculated between the subsequent samplers, namely: MLS 8, MLS 10, MLS 11, MLS 9, MLS 15 and MLS 4. This estimation method could be hindered by the presence of degradable natural organic matter in the groundwater. However, at this site very low DOC levels were observed in the background wells. In addition electron acceptor levels in the background well confirmed the absence of electron acceptor consuming processes out site the plume area.
3 Results
3.1 Hydrogeological Setting
3.1.1 Sedimentary Structure and Hydrogeological Properties
Texture of the sediments samples (total number = 53) was examined by macroscopic inspection and analysis of grain size distribution. A wide range varying from fine, silty sands through medium to coarse sands with pebbles was found. This diversity and spatial distribution of sediment texture influences heterogeneity of (hydro)geological settings of the studied site. It was found that the delta topset unit, up to 2.5 m thick, consists of poorly stratified, medium and fine sands, while underlying foreset is strongly heterogeneous and consists of dipping, interbeded layers of fine silty sands to coarse sands. The sediments sampled at SP 16 show the tendency to become finer with depth.
Based on grain size distribution analysis hydraulic conductivity (K) was determined, by the Hazen and Gustafson methods. Due to limitations of Hazen formula eight samples (MLS 4/1 and 4/2, MLS 15/1–15/3 and MLS 16/10–16/12) were excluded from further analysis. Estimates of hydraulic conductivity vary between 1.17·10−4 and 1.37·10−2 (m·s−1), according to the Hazen method, while Gustafson formula gave higher values. Vertical distribution of estimated hydraulic conductivity for the sampling profile SP 16 is illustrated in Fig. 3.
3.1.2 Groundwater Table Fluctuations
The groundwater table position was regularly monitored between October 2000 and December 2003. In this period the measured change in groundwater table elevation exceeded 1.5 m. In October 2000, the groundwater table was about 4 m below the ground level. It reached a maximum in May 2001, in response to very high precipitation in autumn 2000 and winter 2000/2001. Since then the groundwater level was gradually decreasing, showing some seasonal trends. The results of monitoring of the groundwater table elevation for the selected piezometers are illustrated in Fig. 4. The groundwater contour maps prepared for each sampling event show that the groundwater flow direction was stable during this study – towards southwest (Fig. 1).
The horizontal hydraulic gradient within the studied area calculated for the piezometers P2 and P10, for the individual sampling events in September 2001, April 2002, April 2003 and September 2003 yielded: 0.004598, 0.005208, 0.004705 and 0.004384 (m.m−1), respectively. Therefore, calculated average groundwater velocities varied between 10−7 and 10−5 (m·s−1). The lowest values are applicable for the sediments showing low hydraulic conductivity.
3.2 Mineralogical and Chemical Composition of Sediments
Determination of mineralogical composition by the X-ray diffraction (XRD) method revealed that the sampled sediments were mainly composed of quartz (20–42 wt%), plagioclase (17–38 wt%) and K-feldspar (9–26 wt%). Content of clay minerals varied along the profile and increased with depth. Pyrite and calcite were not detected in the upper 5 m of the sampling profiles, however they occurred in the deeper part of the aquifer. Weight percentage of pyrite and calcite were slightly lower for the bulk samples than for the clay fraction samples. Weight percentage of pyrite and calcite versus altitude (metres above sea level) for the SP 16 are shown in Fig. 5. The altitude of the ground surface is nearly 202 m above sea level. Pyrite reached a maximum of about 0.5%, while calcite was at 2.5% of the total sample weight.
Content of total organic carbon (TOC) and total inorganic carbon (TIC) was determined for all the sediment samples. Obtained values varied between 0.08 and 0.41% for TOC and between 0.00 and 0.59% for TIC. For the bulk samples of the sediment profile SP 16 an abrupt increase of TIC was found below 197 m above sea level. This is between 6 and 7 m of depth – the altitude of the ground surface is nearly 202 m above sea level. The determined TIC values for the top part are below 0.1%, while those for the bottom part are by one order of magnitude higher. Values of TOC do not show any correlation with hydrocarbon contamination and are rather stable along the profile. Differences in TIC between top and bottom of the profile were found also for the grain size fraction <0.063 mm. For this fraction TOC values decrease gradually with depth, from about 0.8 to about 0.3% and do not show any correlation with hydrocarbon contamination. The results of TOC and TIC determination for the sediment profile SP 16 are illustrated in Fig. 6a and b.
3.3 Hydrocarbon Contamination
3.3.1 Hydrocarbons in Sediments
The gas chromatography analysis of the organic compounds content revealed that 25 out of 53 sediment samples were contaminated by hydrocarbons in the range of n-C10 to n-C16. The concentration varied from 27 to 3,900 (mg of hydrocarbons per kilogram dry weight sediment). The hydrocarbon concentrations is given in Table 1. No detectable hydrocarbon concentrations were found for the sediment samples in the background of the plume-SP 8, located 20.2 m upgradient of the source zone. The highest concentration was found for SP 16, located 1.17 m upgradient from the source zone, with a maximum concentration detected at the depth of four m-sample SP 16/4. The hydrocarbon concentrations for the remaining samples at this profile were smaller by one–two orders of magnitude. Vertical distribution of the hydrocarbons was similar for SP 10 and SP 12, located respectively 1.1 and 1.5 m downgradient from the source zone. For these sampling profiles hydrocarbons were present in the top 5–5 m, which might, to some extent, be a result from the disturbance caused by the excavation of the leaking oil skimmer and installation of the observation well Br1. Hydrocarbon contamination for the profiles SP 13, SP 9 and SP 15, located respectively 1.5, 7.5 and 17.0 m downgradient of the source zone, was restricted to the depth of 4–5 m. In the case of SP 11, located 3.2 m downgradient of the source zone, contamination was detected at a depth of 3 to 5 m. A very steep vertical concentration gradient at the profiles SP 12, 13, 9 and 15 illustrates well the plume division into a mobile and immobile zones (Fetter 1992; Fitts 2002). Formation of an immobile zone might, to some extent, be an effect of vertical smearing of the free phase product due to groundwater table fluctuation. Except from SP 10 and 11, the maximum concentration was detected at the depth of 4 m. This coincides with the zone of higher permeability as well as minimal measured elevation of the groundwater table. At the profile SP 4, located 37.5 m downgradient from the source zone, no measurable hydrocarbon concentrations were detected in the sediment samples.
3.3.2 Hydrocarbons in Groundwater
The groundwater has been sampled via the multilevel samplers, therefore, the groundwater samples have been marked and are referred to as MLS, number of the sampler and number of the sampling port, for example MLS 10/3. Analysis of the groundwater samples revealed presence of the following hydrocarbons: toluene, ethylbenzene, o-xylene, m/p-xylene, 1,3,5-trimethylbenzene (1,3,5-TMB), 1,2,4-trimethylbenzene (1,2,4-TMB) and naphthalene. For each sampling event, the maximum concentration of the dissolved hydrocarbons was detected at exactly the same sampling ports, namely: MLS 10/2, MLS 12/3, MLS 13/3, MLS 11/3, MLS 9/2, MLS 15/6 and MLS 4/14. This strongly coincides with the detected maximum hydrocarbon concentrations in the sediment samples – the contamination core. The data obtained on hydrocarbon concentrations were used to study contribution of the various aromatic hydrocarbons to the total dissolved hydrocarbon concentrations and to analyse natural attenuation processes. Determined concentrations of hydrocarbons correlated well with determined DOC data.
Spatial analysis of the dissolved hydrocarbons for the September 2001 sampling event revealed very low toluene concentrations – below 5 (μg·l−1), constrained to the vicinity of the contamination source zone. Concentration of o-xylene was also rather low–maximum 61 (μg·l−1) and was found at MLS 10/2–1.1 m downgradient the source zone. Naphthalene, in contrast, was the dominant compound in the dissolved fraction showing high concentration within a distance of 17 m downgradient from the source zone, with a maximum of 706 (μg·l−1) detected for MLS 9/2. Further downgradient, a decrease to 6 (μg·l−1) for MLS 4/14, located 37.2 m downgradient from the source zone was observed. Concentration of 1,3,5-TMB revealed similar patterns, however the absolute values were much lower – maximally 126 (μg·l−1), detected in MLS 9/2, 7.5 m downgradient from the source zone. Ethylbenzene, m/p-xylene and 1,2,4-TMB showed some intermediate concentration patterns. Concentrations of the individual dissolved organic compounds determined for the plume core in September 2001 are illustrated in Fig. 7.
Concentration of o-xylene and naphthalene, determined for the plume core for each sampling event are presented in Fig. 8. Naphthalene showed stable concentrations within 7.5 m distance from the source zone. Some fluctuation was found further downgradient. Concentration of o-xylene decreased rapidly within a very short distance from the source zone and showed much higher variations than naphthalene. For the September 2001 and April 2002 sampling events the maximum concentrations were detected at the sampler MLS 9, located 7.5 m downgradient, while for the sampling events in April and September 2003 at the sampler MLS 10, located 1.1 m downgradient of the source zone. Changes in maximum concentration of the total dissolved hydrocarbons, naphthalene and o-xylene detected within the plume compared to the groundwater table elevation are illustrated in Fig. 3. No clear trend for the whole plume was found.
The concentrations of naphthalene and o-xylene decreased by time in the samples collected at MLS 9/2. At this location the following concentrations were found: for naphthalene – 706, 665, 484 and 535 (μg·l−1), and for o-xylene – 17, 5, 6 and 3 (μg·l−1), in September 2001, April 2002, April 2003 and September 2003, respectively. Similar fluctuation patterns in time were observed for the remaining compounds concentration.
The spatial dimensions of the hydrocarbons plume were found to decrease since September 2001 until April 2003 and increase again in September 2003. These changes can be correlated to the groundwater table position. The minimum thickness, observed in April 2003, occurred at a very low groundwater table position. Then, the groundwater table dropped below the core of the plume at MLS 11/3, resulting in reduced dissolution of hydrocarbons into the groundwater.
3.4 Natural Attenuation Processes
3.4.1 Electron Acceptors and Metabolic By-products
Analysis of the spatial distribution revealed concomitant depletion of the electron acceptors and increase of metabolic by-product concentrations. Such distribution patterns can be correlated with spatial distribution of the dissolved hydrocarbons. Spatial distribution of oxygen, nitrate, Mn(II), Fe(II), sulphate and bicarbonate observed in the September 2001 sampling event for the cross-section MLS 8–MLS 4, is illustrated in Fig. 9.
Spatial distribution of: a total dissolved hydrocarbons, b dissolved O2, c NO3 -, d Fe2+, e Mn2+, f SO4 2-, g HCO3 -, along the cross-section SW–NE (MLS 4 MLS 8), determined for September 2001 sampling event. Vertical scale: altitude (metres above sea level), horizontal scale: distance from source zone in metres, black bold dotted line isoconcentration line 100 (g TDH·l )
In September 2001 depleted concentrations of oxygen, about one (mg·l−1) or lower, were found within a distance of around 7 m downgradient from the source zone. In contrast, oxygen concentration upgradient from the contamination source zone was very close to saturation. The lowest concentration was detected within the plume core. Nitrate was completely depleted within most of the hydrocarbon plume. High concentrations – up to about 15 (mg·l−1) were found in the background and underneath the plume. One surprisingly high value of almost 40 (mg·l−1) was found about 5 m under the source zone. Nitrate was not detected at all below 7–8 m of depth. Concentrations of Mn(II), increased up to about 5 (mg·l−1) within 3.2 m distance from the source zone, were limited to the zone immediately below the groundwater surface. In contrast, increased Fe(II) concentrations were detected within the whole hydrocarbons plume, up to a distance of 17 m downgradient from the source zone, with the highest concentrations coinciding with the plume core. The maximum concentrations – up to 70 (mg·l−1), were detected 1.1 m downgradient the source zone. Concentrations of both Mn(II) and Fe(II) in the background and underneath the plume were smaller by two orders of magnitude. Sulphate concentrations revealed a complete depletion in the plume core, up to about 7 m downgradient from the source zone. Concentrations in the background were slightly above 15 (mg·l−1). Sulphate concentrations increased with depth, up to about 30 (mg·l−1), as a result of pyrite oxidation. Pyrite oxidation in the deeper sediments also causes depletion of dissolved oxygen and nitrate.
Elevated concentrations of bicarbonate were found within the whole plume, especially in the contamination core. Maximum concentrations – above 160 (mg·l−), were found within 1.1 m distance from the source zone. Concentrations in the background were about 130 (mg·l−). As a result of calcite dissolution an increase of alkalinity can be observed in the deeper part of the aquifer.
Analysis of temporal changes revealed some variations of concentrations of electron acceptors and metabolic by-products. The major changes always occurred at the core of the plume. The concentrations of sulphate, Fe(II), bicarbonate and total dissolved hydrocarbons (TDH), determined at the plume core for each sampling event are illustrated in Fig. 10. Elevated bicarbonate concentration – a product of hydrocarbon degradation, was found to coincide with the hydrocarbons plume for each sampling event. Maximum values were observed in April 2003 and slightly lower in September 2003, while much lower concentrations were found for the April 2002 and September 2001 sampling events. Exactly the same patterns were found for Fe(II) concentration. Sulphate concentration was completely depleted within the distance of 17 m downgradient from the source zone in September 2001 and April 2002, however, detectable sulphate concentration within the plume core occurred in April and September 2003. For correlation reasons, concentration of total dissolve hydrocarbons have been shown as well. Those showed very high values, up to about 1400 (μg·l−1), within the distance of about 7 m downgradient of the source zone. Largest fluctuation in time was observed for the sampler MLS 9, with a maximum of 1472 (μg·l−1) in September 2001 and the following concentrations: 1284, 815 and 477 (μg·l−1), for April 2002, April 2003 and September 2003, respectively.
Methane concentration in the groundwater was detected in the plume core, for the following samples: MLS 10/2, MLS 12/3, MLS 11/2, MLS 9/2 and MLS 15/6. The results are given in Table 2. The remaining samples did not show any detectable methane concentration. The maximum methane concentration – 4.38 (mg·l−1) was detected for MLS 10/2, located 1.1 m downgradient the source zone.
Significant changes in the concentration of some hydrocarbons, electron acceptors and metabolic by-products in time were observed at the sampling profile along the samplers MLS 9 and MLS 3 – in vertical dimension. Figure 11 illustrates observed concentration of sulphate, Fe(II), bicarbonate and total dissolved hydrocarbons (TDH) for each sampling event versus altitude (the altitude of the ground surface is nearly 202 m above sea level). Major changes in sulphate concentration were observed in the top part of the aquifer corresponding to the hydrocarbons plume. Relatively high concentrations were observed immediately below the groundwater table. Within the plume sulphate concentration was strongly depleted in September 2001 and April 2002 – completely, while in April and September 2003 slightly higher concentrations were detected. In the deeper part of the aquifer the concentration gradually increased with depth – up to about 192 m above sea level and then slightly decreased. Main changes of Fe(II) concentration occurred in the top part of the aquifer. Very high concentration, around 100 (mg·l−), were observed in April 2002 and September 2003, while much lower, around 50 (mg·l−), in April 2002 and September 2001. In the deeper part of the aquifer the concentration was lower by one to two orders of magnitude. Significant changes of bicarbonate concentration were observed within the plume, with maximum values at its core. Very high concentrations, of about 300 (mg·l−), were detected in April and September 2003, while much smaller, of about 100 – 160 (mg·l−), in September 2001 and April 2002. Below the plume bicarbonate showed an abrupt increase, up to about 130 (mg·l−), for each sampling event, and remained stable over the depth. For correlation reasons concentration of the total dissolved hydrocarbons were illustrated in Fig. 11 as well. The bottom of the plume was observed at about 198.5 m above sea level, for each sampling event.
An effect of the groundwater table elevation on concentrations of hydrocarbons, electron acceptors and metabolic by-products was observed. The highest observed elevation of the groundwater table was observed in September 2001. Then, high concentrations of total dissolved hydrocarbons, whereas relatively low levels of Fe(II), sulphate and bicarbonate were observed. In contrast, at the lowest elevation of the groundwater table – April 2003, relatively low hydrocarbon concentrations as well as very high concentration of Fe(II), sulphate and bicarbonate were observed.
3.4.2 Soil Gas Content
Concentration of soil gases, measured in April and September 2003, revealed that the maximum concentration of carbon dioxide, about 14% by volume, was detected at the source zone and in its close vicinity – up to 1.1 m downgradient. The concentration decreased downgradient from the source zone and towards the ground surface. The minimum zone of oxygen concentration corresponds to the zone of maximum concentration of carbon dioxide. A minimum of 3.20% oxygen was measured 3.2 m downgradient from the source zone. Oxygen content increased downgradient from the source zone and towards the ground surface. No methane was detected in the soil atmosphere, despite its presence in the groundwater. The measured volume % concentrations of carbon dioxide and oxygen, for the sampling ports located directly above the groundwater table, for the cross-section MLS 8–MLS 19, are presented in Fig. 12.
3.4.3 Biodegradation Potential
In order to evaluate the contribution of biodegradation to the overall process of natural attenuation of the dissolved hydrocarbons, biodegradation potential was determined for the April 2003 sampling event. Calculation of the electron donor, electron acceptor balance in various zones of the plume revealed that major geochemical processes leading to degradation of the hydrocarbons take part within the source zone and its close vicinity – up to MLS 10, located 1.1 m downgradient. Table 3 shows the results of calculations for this part of the plume. It was observed that within the studied plume the biodegradation is mainly related to Fe(III) reduction, showing biodegradation potential up to 0.0507 (mmol o-xylene equivalents·l−1). Other important degradation processes are methanogenesis, nitrate reduction and sulphate reduction, showing degradation potential of 0.0438, 0.0388 and 0.0334 (mmol o-xylene equivalents·l−1), respectively. Oxygen consumption and Mn(IV) reduction showed a much lower biodegradation potential of 0.0093 and 0.0016 (mmol o-xylene equivalents·l−1) respectively. For more details on calculation of the biodegradation potential the reader is asked to refer to earlier publication by the authors (Kłonowski et al. 2005) regarding natural gradient experiment conducted on the same plume.
4 Discussion
4.1 Hydrogeological and Geochemical Settings
The hydrogeological settings of the studied site are very complex. This is a result of a strong heterogeneity of the sediments, especially of the delta foreset unit which forms a major part of the aquifer. Within this unit highly variable values of hydraulic conductivities and groundwater flow velocities were observed. Such heterogeneities and their significant influence on the groundwater flow patterns were found for other locations at the Gardermoen aquifer, for both saturated and unsaturated zones (Alfnes et al. 2003a, b; French 1999; Knudsen 2003; Sabir 2001; Soevik and Aagaard 2003; Soevik et al. 2002).
Monitoring of the groundwater table elevation revealed strong fluctuations – between September 2000 and September 2003 the groundwater table dropped by almost 2 m. The position of the groundwater table influenced the groundwater flow velocity – low values were found for low elevations and vice versa. Nevertheless, the direction of the groundwater flow was stable during the monitoring period.
Neither calcite nor pyrite was found in the top 7–8 m of the sediment profile. Dissolution of calcite and pyrite oxidation occurred in the deeper part of the aquifer, far below the hydrocarbon plume. This is reflected by the groundwater chemistry – elevated concentration of alkalinity and sulphate in the deeper parts of the aquifer as well as depletion of oxygen and nitrate. Presence of geochemical fronts of calcite dissolution and pyrite oxidation at similar depths were reported for other locations of the Gardermoen aquifer (Basberg et al. 1998; Dagestad 1998; Knudsen 2003).
4.2 Spatial Distribution of Hydrocarbons
The plume of jet fuel derived hydrocarbons spilled from the leaking oil skimmer, was present within the delta foreset unit, in both saturated and unsaturated zones. The maximum hydrocarbon concentration – about 3,900 (mg·kg−1) of dry weight within the sediments was found in the close vicinity of the source zone – 1.17 m upgradient. There, and within the distance of 1.5 m downgradient from the source zone most of the sampling profile was contaminated, which to some extent, can be an effect of the excavation works. Further downgradient, up to about 17 m, contamination within the sediments was limited to the depth of about 4–5 m. Effects of multiphase flow and vertical smearing due to the groundwater table fluctuations were found, dividing the plume into mobile and immobile parts,. Hydrogeological conditions were another factor controlling development of the hydrocarbons plume. The zone of elevated hydrocarbon concentration within the sediments can be correlated with the zone of increased permeability and the lowest observed groundwater table elevation. A strong influence of groundwater table fluctuation on distribution and degradation of hydrocarbons has also been found for other contaminated sites (Cheol-Hyo et al. 2001).
The hydrocarbons present within the sediments constitute the source of the hydrocarbons dissolved in groundwater, namely toluene, o-xylene, m/p-xylene, ethylbenzene, 1,3,5-TMB, 1,2,4-TMB and naphthalene. Maximum concentration of dissolved hydrocarbons was observed at the zone of elevated hydrocarbon concentration in the sediments, within a distance of 17 m downgradient from the source zone. Dissolved hydrocarbons were found further downgradient, though concentration was much lower. Distribution of the individual organic compounds differed along the plume. Toluene and o-xylene were rather limited to the vicinity of the source zone, while naphthalene and 1,2,4-TMB could be found as far as 37.2 m downgradient.
4.3 Temporal Distribution of Hydrocarbons
A significant change in spatial distribution of the hydrocarbons occurred during the monitoring period. In September 2001 the thickness of the plume reached a maximum and was decreasing gradually with time until April 2003. This process was accompanied by groundwater table lowering. A slight increase of the plume’s dimensions and elevation of the groundwater table were noticed in September 2003.
Maximum concentrations of dissolved organic compounds were detected 7.5 m downgradient from the source zone in September 2003 and April 2002, while in April and September 2003 the maximum was observed 1.1 m downgradient from the source zone. In the distal part of the plume, about 7.5 m from the source zone and further downgradient, concentrations of naphthalene and o-xylene were higher in September 2001 and April 2004 than for the sampling events in 2003. For the vertical profile at 7.5 m downgradient from the source zone – MLS 9 and MLS 3, distinct changes in concentration of hydrocarbons with time were found. At this location, in April 2002, the groundwater table dropped below some part of the contaminated sediments and lower concentrations of dissolved hydrocarbons were detected as a result of reduced dissolution rates.
4.4 Attenuation and Degradation of Dissolved Hydrocarbons
The observed differences in spatial distribution of the individual dissolved aromatic hydrocarbons resulted from the physiochemical properties of the contaminants, natural attenuation processes and hydrogeological conditions. Naphthalene was detected at the highest concentrations and formed the most extensive plume. Similar distribution patterns were found for 1,2,4-TMB. These compounds were rather stable over a distance of 7.5 m downgradient from the source zone, and then decreased noticeable with increasing distance. Contrary, toluene and o-xylene were attenuated rapidly and could be found only close to the contamination source zone. Similar patterns of biodegradation of the dissolved aromatic compounds were observed in the laboratory batch studies using sediment samples from the site studied in this paper and a similar mixture of the dissolved organics (Zheng et al. 2001). Preferred biodegradation of toluene and o-xylene and recalcitrance of naphthalene and 1,2,4-TMB were detected. This can be explained by differences in microbial transformation under anaerobic conditions as well as differences in aqueous solubility. Recalcitrance of naphthalene under anaerobic conditions in the polluted aquifer was also found for other contaminated sites (Bjerg et al. 1999).
The main process controlling reduction of mass and concentration of hydrocarbons is intrinsic biodegradation. Its effects can be observed as changes in concentration, spatial and temporal distribution of the terminal electron acceptors and metabolic by-products of the degradation reactions. Distinct depletion of the electron acceptors – oxygen, nitrate and sulphate as well as elevation of the metabolic by-products – Fe(II), Mn(II), bicarbonate and methane, can be correlated to the presence of the dissolved hydrocarbons, within a distance of up to about 17 m downgradient from the source zone.
Hydrogeochemical data indicating active biodegradation processes were confirmed by the measurement of soil gas concentrations. Elevated concentrations of carbon dioxide were found in the contamination source zone. Oxygen concentrations were depleted in the same region. Since no methane was detected in the soil atmosphere, immediate mineralization to carbon dioxide in the capillary fringe is suspected.
Groundwater table fluctuation was also found to affect biodegradation processes, with higher degradation activity at low groundwater levels compared to higher hydrocarbon levels at higher groundwater table elevations. Due to the groundwater table lowering – from September 2001 to April 2003, the zone of contaminated sediments was gradually becoming exposed to aerobic conditions. Between April and September 2003 the groundwater table raised again.
Variation of hydrocarbon concentrations as well as some electron acceptors and degradation by-products suggested that the individual hydrocarbons were degraded under different conditions, though contribution of the individual terminal electron accepting processes (TEAP) could not be identified. This might be a result of several TEAP’s acting simultaneously as well as small scale variations within the aquifer (Cozzarelli et al. 2001).
Based upon calculations of the consumed electron acceptors and produced metabolic by-products the biodegradation potential was calculated. It was found that Fe(III) reduction is the main degradation process. Nearly as efficient are methanogenesis, denitrification and sulphate reduction, while oxygen consumption and Mn(IV) reduction show rather a small contribution to the overall biodegradation potential. No significant changes, like shifts between the individual degradation processes, were observed which reflex stability of the system during the monitoring period.
5 Summary and Conclusions
These studies deal with spatial and temporal changes of jet fuel derived hydrocarbons. A leaking oil skimmer released hydrocarbons into the strongly heterogeneous hydrogeological system of the Gardermoen fan delta. Concentrations of dissolved hydrocarbons, major anions and cations as well as soil gases were monitored via multilevel samplers. The content of hydrocarbons was determined for the sediment samples.
Spatial distribution of hydrocarbons within the sediments and groundwater was found to be highly influenced by the hydrogeological conditions – permeability of the sediments and fluctuation of the groundwater table. The zone of maximal hydrocarbon concentrationss – the core of the plume, coincided with the zone of increased permeability and the lowest recorded position of the groundwater table – at the depth of about 4 m. As an effect of the groundwater table fluctuation the hydrocarbons were smeared in vertical direction, forming an immobile part of the plume.
The dissolved hydrocarbons are constantly leaching out from the contamination source zone. The hydrocarbons were detected within the sediments up to 17 m downgradient from the source zone, while the dissolved hydrocarbons were present as far as about 37 m downgradient from the source zone. The individual hydrocarbons showed different concentration along the flow paths. The longest and the most extensive plumes were formed by naphthalene and 1,2,4-TMB, while those of toluene and o-xylene were detected only close to the source zone. The other dissolved aromatics – ethylbenzene, m/p-xylene, and 1,3,5-TMB, showed some intermediate behaviour.
As an effect of biologically mediated degradation of hydrocarbons, consumption of the electron acceptors and production of metabolic by-products were detected. These processes formed a distinct zonation of O2, \({\text{NO}}_{\text{3}} ^ - \), Mn2+, Fe2+, \({\text{SO}}_{\text{4}} ^{2 - } \) and CH4 concentrations in groundwater, within and around the hydrocarbon plume. Calculations of biodegradation potential indicated that Fe(III) reduction, methanogenesis, nitrate and sulphate reductions are the major contributing processes.
The observed temporal changes of hydrocarbons distribution and resulting distribution of the electron acceptors and metabolic by-products were, to a great extend, an effect of groundwater table fluctuation. Higher TDH concentrations were detected for lower groundwater table elevations – in April and September 2003, then for the higher ones – in September 2001 and April 2002. Such patterns could be an effect of lower dissolution rates caused by dropping of the groundwater table below the zone of maximum hydrocarbon content in the sediments.
In order to distinguish between the major processes of natural attenuation of hydrocarbons, like sorption and intrinsic biodegradation, further research involving examination of solute transport and degradation processes in the field is needed. A field experiment involving injection of conservative and reactive tracers would be the most informative method.
References
Aagaard, P., Breedveld, G., Rudolph-Lund, K., Zheng, Z. & Kłonowski, M. R. (2001). Natural attenuation of jet fuels contaminated sediments and groundwater at the fire fighting training place of the international airport Gardermoen (in Norwegian). Paper presented at the 10th Seminar on Hydrogeology and Environmental Geochemistry, Trondheim.
Alfnes, E., Breedveld, G. D., Kinzelbach, W. & Aagaard, P. (2003a). Investigation of hydrogeologic processes in a dipping layer structure. 2. Transport and biodegradation of organics. Journal of Contaminant Hydrology, DOI 10.1016/j.jconhyd.2003.08.006
Alfnes, E., Kinzelbach, W. & Aagaard, P. (2003b). Investigation of hydrogeologic processes in a dipping layer structure: 1. The flow barrier effect. Journal of Contaminant Hydrology, DOI 10.1016/j.jconhyd.2003.08.003
Andersson, A. C., Andersson, O. & Gustafson, G. (1984). Wells, Exploration-Dimensioning-Drilling-Use. Report R42 (in Swedish). Stokholm: Byggforskningsradet.
Appelo, C. A. J. & Postma, D. (1996). Geochemistry, groundwater and pollution. Rotterdam: A.A.Balkema.
Barker, J. F., Patric, G. C. & Major, D. (1987). Natural attenuation of aromatic hydrocarbons in a shallow sand aquifer. Groundwater Monitoring Review, 64–71, Winter.
Basberg, L., Dagestad, A. & Engesgaard, P. (1998). Geochemical modelling of natural geo-/hydrochemical stratification dominated by pyrite oxidation and calcite dissolution in a glaciofluvial Quaternary deposit, Gardermoen, Norway. NGU bulettine, 434, 10.
Baun, A., Reitzel, L. A., Ledin, A., Christensen, T. H. & Bjerg, P. L. (2003). Natural attenuation of xenobiotic organic compounds in a landfill leachate plume (Vejen, Denmark). Journal of Contaminant Hydrology, 65, 269–291.
Bekins, B. A., Cozzarelli, I. M., Godsy, E. M., Ean, W., Essaid, H. I. & Tucillo, M. E. (2001). Progression of natural attenuation processes at a crude oil spill site: II. Controls on spatial distribution of microbial populations. Journal of Contaminant Hydrology, 53, 387–406.
Bennett, P. C., Siegel, D. E., Baedecker, M. J. & Hult, M. F. (1993). Crude oil in a shallow sand and gravel aquifer – I. Hydrogeology and inorganic geochemistry. Applied Geochemistry, 8, 529–549.
Bjerg, P. L., Rugge, K., Cortsen, J., Nielsen, P. H. & Christensen, T. H. (1999). Degradation of aromatic and chlorinated aliphatic hydrocarbons in the anaerobic part of the Grindsted Landfill leachate plume: in situ microcosm and laboratory batch experiments. Ground Water, 37, 113–121.
Borden, R. C., Daniel, R. A., LeBrun IV, L. & Davis, C. W. (1997). Intrinsic biodegradation of MTBE and BTEX in a gasoline-contaminated aquifer. Water Resources Research, 33, 1105–1115.
Chapelle, F. H. (2000). The significance of microbial processes in hydrogeology and geochemistry. Hydrogeology Journal, 8, 41–46.
Chapelle, F. H. (2001). Ground-Water Microbiology and Geochemistry. New York, USA: Wiley.
Chapelle, F. H., McMahon, P. B., Dubrovsky, N. M., Fujii, R., Oaksford, E. T. & Vroblesky, D. A. (1995). Deducing the distribution of terminal electron-accepting processes in hydrologically diverse groundwater systems. Water Resources Research, 31, 359–371.
Cheol-Hyo, L., Jin-Yong, L., Jeong-Yong, C. & Kang-Kun, L. (2001). Attenuation of petroleum hydrocarbons in smear zones: a case study. Journal of Environmental Engineering, 639–647, July.
Chiang, C. Y., Salanitro, J. P., Chai, E. Y., Colthart, J. D. & Klein, C. L. (1989). Aerobic biodegradation of benzene, toluene, and xylene in a sandy aquifer-data analysis and computer modeling. Ground Water, 27, 823–834.
Christensen, T. H., Bjerg, P. L., Banwart, S. A., Jakobsen, R., Heron, G. & Albrechtsen, H.-J. (2000a). Characterization of redox conditions in groundwater contaminant plumes. Journal of Contaminant Hydrology, 45, 165–241.
Christensen, T. H., Bjerg, P. L. & Kjeldsen, P. (2000b). Natural attenuation: a feasible approach to remediation of groundwater pollution at landfills? Groundwater Monitoring and Remediation, 69–77, Winter.
Council, N. R. (1994). Alternatives for groundwater cleanup. Washington: National Academy Press.
Cozzarelli, I. M., Bekins, B. A., Baedecker, M. J., Aiken, G. R., Eganhouse, R. P. & Tucillo, M. E. (2001). Progression of natural attenuation processes at a crude-oil spill site: I. Geochemical evolution of the plume. Journal of Contaminant Hydrology, 53, 369–385.
Dagestad, A. (1998). In situ airsparging as a remedial action at the Gardermoen aquifer, Southeast Norway (in Norwegian). Dissertation, Norwegian University of Science and Technology.
Davis, G. B., Barber, C., Power, T. R., Thierrin, J., Petterson, B. M., Rayner, J. L. & Wu, Q. (1999). The variability and intrinsic remediation of a BTEX plume in anaerobic sulphate-rich groundwater. Journal of Contaminant Hydrology, 36, 265–290.
Eganhouse, R. P., Baedecker, M. J., Cozzarelli, I. M., Aiken, G. R., Thorn, K. A. & Dorsey, T. F. (1993). Crude oil in a shallow sand and gravel aquifer-II. Organic geochemistry. Applied Geochemistry, 8, 551–567
Fetter, C. W. (1992). Contaminant Hydrogeology. London: Prentice Hall.
Fetter, C. W. (2001). Applied Hydrogeology. London: Prentice Hall.
Fitts, C. R. (2002). Groundwater Science. Amsterdam: Academic.
French, H. K. (1999). Transport and degradation of deicing chemicals in a heterogenous unsaturated soil. Dissertation, Agricultural University of Norway.
Golden Software, I. (2002). Surfer 8. User’s Guide. Contouring and 3D Surface Mapping for Scientists and Engineers. Golden: Golden Software.
Grbic-Galic, D. (Eds.) (1991). Anaerobic microbial degradation of aromatic hydrocarbons. Amsterdam: Elsevier.
Haack, S. K. & Bekins, B. A. (2000). Microbial populations in contaminant plumes. Hydrogeology Journal, 8, 63–76
Joergensen, P. & Oestmo, S. R. (1992). Research Programme. The Environment of the Subsurface. Part I: The Gardermoen Project, 1992–1994. Introductory Report. Literature Review and Project catalogue. Hydrogeology at Romerike.
Klecka, G. M., Davis, J. W., Gray, D. R. & Madsen, S. S. (1990). Natural bioremediation of organic contaminants in ground water: Cliffs-Dow Superfund site. Ground Water, 28, 534–543.
Kłonowski, M. R., Zheng, Z., Aagaard, P., Breedveld, G. D. & Rudolph-Lund, K. (2002). Persistence of Naphthalenein a Jet Fuel contaminated Aquifer. Paper presented at European Conference on Natural Attenuation, Heidelberg.
Kłonowski, M. R., Bredveld, G. D. & Aagaard, P. (2005). Natural gradient experiment on transport of jet fuel derived hydrocarbons in an unconfined sandy aquifer. Environmental Geology, 48, 1040–1057. DOI 10.1007/s00254-005-0042-y
Knudsen, J. B. S. (2003). Reactive transport of dissolved aromatic compounds under oxygen limiting conditions in sandy aquifer sediments. Dissertation, University of Oslo.
Lee, J.-Y., Cheon, J.-Y., Lee, K.-K., Lee, S.-Y. & Lee, M.-H. (2001). Factors affecting the distribution of hydrocarbon contaminants and hydrogeochemical parameters in a shallow sand aquifer. Journal of Contaminant Hydrology, 50, 139–158.
Löser, C., Seidel, H., Zehnsdorf, A. & Stottmeister, U. (1998). Microbial degradation of hydrocarbons in soil during aerobic/anaerobic changes and under purely aerobic conditions. Applied Microbiology and Biotechnology, 49, 631–636.
Lovley, D. R., Chapelle, F. H. & Woodward, J. C. (1994). Use of dissolved H2 concentrations to determine distribution of microbially catalyzed redox reactions in anoxic groundwater. Environmental Science & Technology, 28, 1205–1210.
McAllister, P. M. & Chiang, C. Y. (1994). A practical approach to evaluating natural attenuation of contaminants in ground water. Ground Water Monitoring Review, 161–173, Spring.
Oestmo, S. R. (1976). Hydrogeological Map of Ovre Romerike; groundwater in loose sediments between Jessheim and Hurdalsjone-scale. 1:20 000: Oslo, Norwegian Geological Survey.
Phelps, C. D. & Young, L. Y. (1999). Anaerobic biodegradation of BTEX and gasoline in various aquatic sediments. Biodegradation, 10, 15–25.
Ramm, M. (1991). On quantitative mineral analysis of sandstones using XRD. University of Oslo Department of Geology.
Röling, W. F. M. & Verseveld van, H. W. (2002). Natural attenuation: What does the subsurface have in store? Biodegradation, 13, 53–64.
Rudolph-Lund, K. & Sparrevik, M. (1999a). Mapping of contamination spreading at the fire fighting training place of the Gardermoen international airport (in Norwegian). Norwegian Geotechnical Institute.
Rudolph-Lund, K. & Sparrevik, M. (1999b). A strategy of natural attenuation at the fire fighting training place (in Norwegian). Norwegian Geotechnical Institute.
Sabir, I. H. (2001). Transport of a de-icing chemical and synthetic DNA tracers in groundwater at Oslo Airport, Gardermoen. Field experiments and numerical modelling. Dissertation, Agricultural University of Norway.
Salanitro, J. P. (1993). The role of bioattenuation in the management of aromatic hydrocarbon plumes in aquifers. Groundwater Monitoring and Remediation, 150–161, Fall.
Schirmer, M. & Barker, J. F. (1998). A study of long-term MTBE attenuation in the Borden aquifer, Ontario, Canda. Groundwater Monitoring and Remediation, 113–122, 1998.
Schirmer, M., Butler, B. J., Barker, J. F., Church, C. D. & Schirmer, K. (1999). Evaluation of biodegradation and dispersion as natural attenuation processes of MTBE and benzene at the Borden Field. Physical, Chemical & Earth Sciences, 24, 557–560.
Schlegel, H. G. (1992). Allgemeine Mikrobiologie (in German). Stuttgart, Germany: Georg Thieme Verlag.
Soerensen, R. (1979). Late Weichselian deglaciation in the Oslofjord area, south Norway. Boreas, 8, 241–246.
Soevik, A. K. & Aagaard, P. (2003). Spatial variability of a solid porous framework with regard to chemical and physical properties. Geoderma, 113, 47–76.
Soevik, A. K., Alfnes, E., Breedveld, G. D., French, H., Pedersen, T. S. & Aagaard, P. (2002). Transport and Degradation of toluene and o-xylene in an unsaturated soil with dipping sedimentary layers. Journal of Environmental Quality, 31, 1809–1823.
Stumm, W. & Morgan, J. J. (1996). Aquatic Chemistry. An Introduction Emphasizing Equilibria in Natural Waters. New York: Wiley.
Thomas, J. M., Wilson, J. T. & Ward, C. H. (Eds.) (1997). Microbial processes in the subsurface. Chelsea: Ann Arbor.
Tucker, M. (1988). Techniques in Sedimentology. Oxford: Blackwell Scientific.
Tuttle, K. J., Ostmo, S. R. & Andersen, B. G. (1997). Quantitative study of the distributory braidplain of the Preboreal ice-contact Gardermoen delta complex, southeastern Norway. Boreas, 26, 141–156.
USEPA (1997). Use of Monitored Natural Attenuation at Superfund, RCRA Corrective Action, and Underground Storage Tank Sites. Directive 9200.4–17. US Environmental Protection Agency.
Vroblesky, D. A. & Chapelle, F. H. (1994). Temporal and spatial changes of terminal electron-accepting processes in a petroleum hydrocarbon-contaminated aquifer and the significance for contaminant biodegradation. Water Resources Research, 30, 1561–1570
Ward, C. H., Cherry, J. A. & Scalf, M. R. (Eds.) (1997). Subsurface Restoration. Chelsea: Ann Arbor.
Wiedemeier, T. H., Rifai, H. S., Newell, C. J. & Wilson, J. T. (1999). Natural Attenuation of Fuels and Chlorinated Solvents in the Subsurface. New York: Wiley.
Zheng, Z., Bredveld, G. D. & Aagaard, P. (2001). Biodegradation of soluble aromatic compounds of jet fuel under anearobic conditions: laboratory batch experiments. Applied Microbiology and Biotechnology, 57, 572–578.
Acknowledgements
The first author is deeply grateful to the management and colleagues of the Polish Geological Institute for granting a long study leave in order to complete this research. We all would like to thank Alf Nielsen, Jana Mahačkova, Mufak Naoroz, Kim Rudolf-Lund, Rebecca Worsley and Zouping Zheng for their great help in conducting the field work. Also contribution of Berit Løken Berg, Mufak Naoroz, Turid Winje and Øyvind Kvalvåg to completion of the laboratory analyses is acknowledged. Assistance of the employees of the Gardermoen Airport – Jarl Øvstedal, Stig Moen and Knut Ringheim is acknowledged. The first author would like to thank especially the Donald Kuennen Foundation (the Netherlands) which provided the grants for the scientific books and software.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kłonowski, M.R., Breedveld, G.D. & Aagaard, P. Spatial and Temporal Changes of Jet Fuel Contamination in an Unconfined Sandy Aquifer. Water Air Soil Pollut 188, 9–30 (2008). https://doi.org/10.1007/s11270-007-9492-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9492-z