Abstract
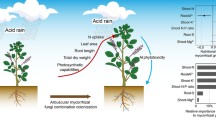
Aluminum in acidic conditions is toxic to plants. Aluminum tolerance in some plant species has been ascribed to arbuscular mycorrhizal fungal symbiosis. In this study, the application of aluminum was found to inhibit mycelia development of saprobe fungi Fusarium concolor and Trichoderma koningii and the hyphal length of the arbuscular mycorrhizal fungi Glomus mosseae and Glomus deserticola in vitro. Several levels of aluminum were applied to Eucalyptus globulus plants and inoculated with arbuscular mycorrhizal fungi alone or together with both saprobe fungi. The application of 1,500 mg kg−1 decreased the shoot and root dry weight, chlorophyll content and total P, Mg, and Ca concentrations in the shoot of E. globulus. However, both mycorrhizal fungi G. mosseae and G. deserticola inoculated alone increased the shoot dry weight of Eucalyptus, compared with a non- arbuscular mycorrhizal inoculated control treated with 1,500 mg kg−1 of aluminum. When 1,500 mg kg−1 of aluminum was applied, T. koningii increased the effect of G. deserticola on the shoot weight of eucalyptus, whereas with 3,000 mg kg−1, shoot weight and arbuscular mycorrhizal colonization decreased in all treatments. With 1,500 mg kg−1, the highest accumulation of aluminum in the shoot was obtained when G. deserticola was inoculated together with T. koningii. The possibility of manipulating an arbuscular mycorrhizal inoculation together with a saprobe fungus confers a high aluminum resistance in E. globulus. The effect of such combined inoculation is particularly important in some Chilean volcanic acid soils, mainly those which have been intensively cropped and are without lime addition, which facilitates the increase of phytotoxic aluminum species and limits their agricultural use. Therefore, such dual inoculation in field conditions deserves further investigation. Overall, the arbuscular mycorrhizal and saprobe fungi contribute to the increase in resistance of E. globulus to aluminium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aluminun (Al) is the most abundant metal and the third most common element, after oxygen and silicon, found in the earth’s crust (Fuente-Martinez & Herrera-Estrella, 1999). The bulk of aluminum in mineral soils is locked into oxides and alumino-silicates and these aluminum forms are usually harmless to plants. In soils with pH 5.5 or lower, however, which comprise about 40% of the world’s arable land, aluminum phytotoxicity is one of the major factors limiting crop production (Kochian, 1995; Von Uexküll & Mutert, 1995). Aluminum is found in the soil solution as different chemical species, each one with different phytotoxicity degrees according to the pH. Moreover, it is widely recognized that Al+3 is the species which presents the highest toxicity (Kinraide, 1991). The most important symptom of aluminum toxicity in plants is root elongation inhibition, which can usually be detected within minutes even at micromolar concentrations (Barcelo & Poschenrieder, 2002; Jones & Kochian, 1995). The subsequent effects on root growth inhibition leads to a reduction in essential nutrient acquisition (P, Ca, K, Mg and Fe) and water uptake. The final consequence is a severe reduction in growth and plant productivity (Baligar, Campbell, & Wright, 1993; Fuente-Martinez & Herrera-Estrella, 1999).
Arbuscular mycorrhizal fungi (AMF) are large components of the soil microbial biomass. Their symbiosis benefits plant growth, particularly through enhanced phosphorus, water and mineral nutrient uptake (Li, Marschner, & George, 1991; Pearson & Jakobsen, 1993) even under stressful environmental conditions (Smith & Read, 1997). It is also recognized that AMF protect plants against the toxic effects of excessive concentrations of toxic compounds including heavy metals (Arriagada, Herrera, & Ocampo, 2005; Haselwandter & Berreck, 1994; Heggo, Angle, & Chaney, 1990; Rivera-Becerril et al., 2002).
In soils containing elevated concentrations of phytotoxic metals, both arbuscular and ecto-mycorrhizal fungal symbioses can increase the metal resistance of the respective host plants (Gildon & Tinker, 1983; Tonin, Vandenkoornhuyse, Joner, Straczek, & Leyval, 2001; Weissenhorn, Leyval, Belgy, & Berthelin, 1995). The mechanisms involved in conferring resistance on plants growing in such habitats are unclear but surely differ among the toxic metals, fungal species and ecotypes involved and may be related to an increase in P uptake (Khan, Kuek, Chaudhry, Khoo, & Hayes, 2000). Furthermore, it has been reported that metal uptake and translocation to plant shoots may be reduced by metal chelation in the mycorrhyzosphere, metal binding to hyphal cell walls (Gonzalez-Chavez, Carrillo-Gonzalez, Wright, & Nichols, 2004) or by intracellular sequestration (Jentschke & Godbold, 2000; Tonin et al., 2001). In addition, AMF are widely established in acidic or moderately acidic soils (Borie & Rubio, 1999; Clark, 1997; Cuenca, De Andrade, & Meneses, 2001; Mendoza & Borie, 1998), often improving seedling survival and enhancing plant growth. It has been suggested that the symbiosis in those habitats may be playing a crucial role in protecting the plant against aluminum toxicity through mechanisms which are not yet well understood. A limited number of experiments have been reported that demonstrated that arbuscular mycorrhizal fungal colonization improves aluminum toxicity in crop plants (Borie & Rubio, 1999; Medeiros, Clark, & Ellis, 1994; Ning & Cumming, 2001) and tree species (Lux & Cumming, 2001). Aluminum resistance in Andropogon virginicus (broomsedge) has recently been associated with AMF, which influence organic acid exudation by plant roots, suggesting that this is potentially one of the mechanisms involved (Cumming & Ning, 2003). For ectomycorrhizal tree species, Barros and Novais (1996) found mycorrhizal Eucalyptus cloeziana to be more tolerant to high aluminum species concentration than non-mycorrhizal plants, and the same behaviour has been reported in Pinus rigida inoculated with Pisolithus tinctorius (Cumming & Weinstein, 1990b) fungus, which is recognized as being able to produce oxalic acid (Cumming, Swiger, Kurnik, & Panaccione, 2001), a powerful chelating agent for aluminum.
Although research on aluminum resistance has focused on agricultural crops, it is acknowledged that some forest species are aluminum-tolerant and such tolerance is usually greater compared with annual crops (Schaedle, Thornton, Raynal, & Tepper, 1989). Eucalypt species are known to be aluminum tolerant, growing without limitation in acidic soils and showing scant or no response to liming (Vale, Furtini-Neto, Reno, Fernandez, & Resende, 1996). The possible mechanism involved in such aluminum tolerance of eucalypt species has been related to an internal aluminum detoxification mechanism produced through the formation of stable aluminum complexes with short-chain organic acids such as oxalic acid (Silva et al., 2004).
Other soil micro-organisms affect arbuscular mycorrhizal symbiosis that function both antagonistically or synergistically. Saprobe fungi are important and common components of rhizosphere soil, where they obtain enhanced nutritional benefit from organic and inorganic compounds released from living roots, together with sloughed cells (Alexander, 1977; Dix & Webster, 1995). Their importance lies in the large microbial biomass they supply to soil and in their capacity to degrade toxic substances (Madrid, De La Rubia, & Martinez, 1996; Wainwright, 1992). Some experimental results confirm the existence of synergistic effects of the saprobe fungi Fusarium concolor and Trichoderma koningii on plant roots colonized by AMF (Arriagada et al., 2005; García-Romera et al., 1998).
The aim of this work was to study the response of Eucalyptus globulus plants to aluminum and the influence of AMF and saprobe fungi inoculation on the growth and nutrition of plants exposed to different aluminum levels.
2 Materials and Methods
We tested the effect of aluminum on the saprobe fungi Fusarium concolor Schlecht. BAFC Cult. No. 2183 (Booth, 1977) and Trichoderma koningii Rifai (BAFC Cult. no. F8844; Rifai, 1969). These fungi were isolated from the rhizosphere soil and roots of maize cultivated in the province of Buenos Aires, Argentina by the particle washing method, using a multi-chamber washing apparatus (Widden & Bisset, 1972). Strains are currently kept in the fungal culture collection of the Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina. Both saprobe fungi were transferred to tubes with potato dextrose agar medium (PDA, DIFCO) and 2% malt extract at 4°C as stock culture. An aqueous suspension in sterile distilled water containing approximately 106 spores ml−1 of each saprobe fungus was prepared from cultures grown in potato dextrose agar (PDA, DIFCO) for 1 week at 27°C. Two milliliters of this suspension were transferred to 250 ml flasks containing 125 ml of sterile AG liquid medium (Galvagno, 1976) in a shaker at 28°C. The AG medium consisted of 1 g glucose, 0.4 g asparagine, 0.05 g MgSO4, 0.05 KH2PO4 and 100 ml distilled water. Al Cl3 was added to AG medium at a final concentration of 0, 500 and 1,000 mg l−1; aluminum and pH was adjusted daily to a constant 4.5. After 2 weeks, the number of spores per ml of culture medium was evaluated using a Neubauer chamber (McAllister, 1992). The culture medium was filtered through a disk of filter paper, dried at 80°C for 72 h, and the dry mycelium of each saprobe fungi was weighed (McAllister, 1992). In aluminum treatment, the concentration of aluminum was analysed in the AG medium after 1 and 2 weeks culture of F. concolor and T. koningii (Mingorance, 2002). AG medium with 500 and 1,000 mg L−1 of aluminum without fungal culture was used as a control. Ten replicates were used in these experiments.
The effect of aluminum on hyphal length of Glomus mosseae (Nicol. and Gerd.) (Gerd. and Trappe) (BEG no. 12) and Glomus deserticola (Trappe, Bloss and Menge) from Rothamsted Experimental Station was tested in Petri dishes 9 cm in diameter. Sporocarps of G. mosseae and spores of G. deserticola were isolated from the soil by wet sieving (Gerdemann, 1955) from the soil of alfalfa plant pot cultures and stored in water at 4°C. The spores of G. mosseae, obtained by dissecting the sporocarps, were surface sterilized as described by Mosse (1962). Ten surface-sterilized spores per plate were placed 1 cm from the edge of a Petri dish with 10 mL of 10 mM 2-(N-morpholin) ethane sulphonic acid (MES) plus 0.04 g of Gel-Gro (ICN Biochemicals, Aurora, OH, USA). Aluminum chloride was added to the Petri dishes to a final concentration of 0, 25, 50, 100, 200 and 500 mg l−1 of aluminum. Ten replicates for each AMF were used. The plates were incubated at 25°C in the dark for 21 days, and were sealed to reduce dehydration and contamination. The hyphal lengths of the germinated G. mosseae and G. deserticola spores from five replicates were quantified at the end of the experiment using the gridline intersect method under a binocular microscope at 40× magnification (Brundrett, Bougher, Grove, & Malajczuk, 1996; Marsh, 1971). All fungal mycelia were measured. In order to visualise whether the aluminum toxicity effect on arbuscular mycorrhizal hyphal length was of a fungistatic nature, arbuscular mycorrhizal spores from five replicates were transferred to new plates of Gel-Gro without aluminum and the fungal mycelia were measured after 10 days of incubation. Both saprobe fungi as well as mycorrhizal inocula have been used recently with success in an experiment testing heavy metal tolerance conferred to plants via the synergistic effect of free and symbiotic fungi (Arriagada, Herrera, García-Romera, & Ocampo, 2004).
Seeds of Eucalyptus globulus Labill., previously surface sterilised (HgCl2 for 10 min), and thoroughly rinsed with sterilised water, were sown in moistened sand. After germination, uniform seedlings were planted in 0.3 l pots filled with a mixture of sterilized sand:vermiculite:sepiolite (Named substrate pot) at a volume proportion of 1:1:1. Plants were grown in a greenhouse with supplementary light provided by Sylvania incandescent and cool-white lamps, 400 E m−2 s−1, 400–700 nm, with a 16/8 h day/night cycle at 25/19°C and 50% relative humidity. Plants were watered from below and fed every week with 10 ml of a nutrient solution plus 50 mg l−1 of P (Hewitt, 1952).
The arbuscular mycorrhizal fungal inoculum was a root and soil mixture consisting of rhizosphere soil containing spores and colonized root fragments of Medicago sativa L. in amounts of 8 g per pot, which were predetermined to achieve high levels of root colonization. A water filtrate (Whatman no. 1 paper) of the inoculum was applied to the uninoculated pots containing common soil microflora but free of arbuscular mycorrhizal fungal propagules.
An aqueous suspension in sterile distilled water containing approximately 108 spores ml−1 of F. concolor and T. koningii was prepared from cultures grown in potato dextrose agar (PDA, DIFCO) for 1 week at 27°C, and 2.5 ml of this suspension was inoculated into each pot.
Treatments used were: (1) Uninoculated controls (2) Substrate pot inoculated with F. concolor or T. koningii (3) Substrate pot inoculated with G. mosseae or G. deserticola, and (4) Substrate pot inoculated with F. concolor or T. koningii plus either G. mosseae or G. deserticola. Plants were inoculated at transplantation (after 4 weeks of growth). The saprobe fungi were inoculated at the same time as G. mosseae or G. deserticola. Five replicates per pot were used.
Aluminum was applied to Eucalyptus pots at a concentration of 0, 150, 600, 1,500 and 3,000 mg Al kg−1 per substrate pot. These concentrations were selected to show the significant toxic effect on agricultural and forest plant development (Barros & Novais, 1996; Jansen, Broadley, Robbrecht, & Smets, 2002). The pH substrate pot was adjusted to 4.2 every 15 days.
Plants were harvested after 16 weeks and dry mass was determined. After the harvest, two samples of fresh weight were randomly taken from the entire root system. One of the samples was cleared and stained (Phillips & Hayman, 1970) and the percentage of root length colonization was measured (Giovannetti & Mosse, 1980). In a second sample, succinate dehydrogenase (EC 1.3.99.1) (SDH) activity was measured in fungal mycelia through the reduction of tetrazolium salts at the expense of added succinate (MacDonald & Lewis, 1978); the percentage of arbuscular mycorrhizal fungal mycelia with SDH activity was determined under a compound microscope (Ocampo & Barea, 1985). Phosphorous (P), magnesium (Mg), calcium (Ca) and aluminum contents in the Eucalyptus plant shoots were determined as described by Mingorance (2002). To determine total chlorophyll, chlorophyll a and chlorophyll b, the leaves were extracted with 80% (V:V) acetone at the same developmental stage (after 16 weeks transplanting) and measured according Lichtenthaler (1987).
The values were arcsine transformed before statistical analysis. The data were analysed by factorial analysis of variance with AMF treatment (Control, G.mosseae and G.deserticola), Saprobe fungi treatment (Control, F.concolor and T.koningii), aluminum in soil treatment (0, 150, 600, 1,500 and 3,000 mg kg−1) and their interaction as sources of variation.
3 Results
Mycelium dry weight and spore number of saprobe fungi F. concolor decreased significantly in the presence of 500 and 1,000 mg l−1 (Table 1), whereas T. koningii tolerated 500 mg l−1, but not the highest level of aluminum (1,000 mg l−1). These results are in accordance with fungal aluminium absorption, since F. concolor absorbed 15.3 and 11.6% of the aluminum at 2 weeks’ growth when this element was supplied at a rate of 1,000 and 500 mg l−1, respectively. On the other hand, T. koningii absorbed higher amounts of aluminum with around 26% at 1,000 mg l−1 at 2 weeks and 77.2% at 500 mg l−1 (data not shown), reinforcing the results shown in Table 1, where T. koningii appears to be more aluminum resistant than F. concolor.
Aluminum concentration differently affected the mycelium length of both mycorrhizal fungi (Fig. 1). Therefore, G. mosseae mycelium was strongly affected by aluminum presence even at the lowest concentration (25 mg l−1); hyphae were scarcely observed at medium aluminum concentrations and no mycelium was observed at 200 and 500 mg l−1. Comparatively, G. deserticola tolerated the presence of aluminum much better and mycelium decreased according to the aluminum concentration, but was not totally inhibited by the higher level of aluminum (500 mg l−1). A significant decrease in G. mosseae hyphal length at all aluminum levels was observed (Fig. 1). When spores of G. mosseae were transferred from media with 0, 25, 50, 100, 200 and 500 mg l−1 of aluminum to new Gel-Gro media without aluminum, the hyphal length was 17 ± 0.31; 6.1 ± 0.34, 2 ± 0.29, 0 and 0 mm after 10 days of incubation, respectively (data not shown). In the dose assays, G. deserticola hyphal length always showed development.
The results of factorial ANOVA can be seen in Table 2. The saprobe fungi F. concolor and T. koningii were not significant on all analyzed variables. The shoot dry weight average for each factor and their interaction in Fig. 2 illustrates that neither saprobe fungi gave any additional aluminum tolerance to E. globulus. However, both mycorrhizal fungi G. mosseae and G. deserticola inoculated alone increased the shoot dry weight of E. globulus, with the latter being significantly higher, even at the application rate of 1,500 mg kg−1. No differences at 3,000 mg kg−1 between the inoculation of either G. mosseae or G. deserticola were observed. The inoculation of saprobe fungi T. koningii only produced an increase in shoot weight at 1,500 mg kg−1; when inoculated together, G. deserticola increased. The application of 3,000 mg kg−1 decreased the shoot dry weight of plants in all treatments tested. (Fig. 2, Table 2).
Shoot and root dry weight of Eucalyptus globulus inoculated or not with AMF or saprobe fungi in soil with different aluminum concentrations. C: Control; Fc: F. concolor; Tk: T. koningii; Gm: Glomus. mosseae; Gm+Fc: G.mosseae+F.concolor; Gm+Tk: G.mosseae+T.koningii; Gd: G. deserticola; Gd+Fc: G.deserticola+F.concolor; Gd+Tk: G.deserticola+T.koningii
The root dry weight mean for each factor and their interaction, illustrated in Fig. 2, shows that G. mosseae and G. deserticola did not increase the root dry weight of E. globulus. The application of 600 mg kg−1 decreased the shoot dry weight of E. globulus; when G. deserticola and T. koningii were inoculated together, however, the root and shoot dry weight (Fig. 2) were significantly increased, even at 1,500 mg kg−1. Weight increase corresponding to increasing aluminum could be due to a habitual response of root tips and lateral roots, which become thicker and brown when the roots are exposed to aluminum. Nevertheless, the shoot:root ratio increased with both strains of mycorrhizal inoculum, with G. deserticola being higher; this suggests a greater beneficial effect on plant growth produced by such a mycorrhizal strain.
The AMF caused the highest beneficial effect on chlorophyll content (Fig. 3, Table 2). G. mosseae did not increase the chlorophyll content of E. globulus, but this parameter was significantly improved by G. deserticola inoculated alone or together with the saprobe fungi. Doses of 1,500 and 3,000 mg kg−1 decreased this content in all E. globulus plants.
Chlorophyll content of Eucalyptus globulus inoculated or not with AMF or saprobe fungi in soil with different aluminum concentrations. C: Control; Fc: F. concolor; Tk: T. koningii; Gm: Glomus. mosseae; Gm+Fc: G.mosseae+F.concolor; Gm+Tk: G.mosseae+T.koningii; Gd: G. deserticola; Gd+Fc: G.deserticola+F.concolor; Gd+Tk: G.deserticola+T.koningii
The effect of aluminum treatment in mycorrhizal root colonization and SDH activity of E. globulus decreased in the presence of 1,500 mg kg−1. Plants inoculated with F. concolor did not see an affect on the arbuscular mycorrhizal root colonization and SDH activity of Eucalyptus in any treatment. However, dual inoculation with G. deserticola and T. koningii increased the percentage of root colonization and SDH activity at 0 and 150 mg kg−1. By contrast, the application of 1,500 mg kg−1 dramatically decreased the percentage of root length colonization and SDH activity of Eucalyptus in all treatments (Fig. 4).
Effect of AMF and Saprobe fungi on root length colonization and percentage of AMF mycelium with SDH activity of Eucalyptus globulus in soil with different Aluminum concentrations. Gm: Glomus. mosseae; Gm+Fc: G.mosseae+F.concolor; Gm+Tk: G.mosseae+T.koningii; Gd: G. deserticola; Gd+Fc: G.deserticola+F.concolor; Gd+Tk: G.deserticola+T.koningii
G. deserticola was the only arbuscular mycorrhizal fungus that increased total P (F = 10.03; p < 0.01), Mg (F = 8.76; p < 0.01) and Ca concentrations (F = 9.27; p < 0.01) of Eucalyptus shoots at 600 mg kg−1. Dual inoculation with T. koningii increased the beneficial effect of G. deserticola at low aluminum levels in the media. The application of 1,500 mg kg−1 decreased the total P, Mg and Ca concentrations of Eucalyptus in all treatments and all interaction factors (Fig. 5).
Mineral nutrition (Phosphorous, Magnesium and: Calcium) in shoot of Eucalyptus globulus inoculated or not with AMF or saprobe fungi in soil with different Aluminum concentrations. C: Control; Fc: F. concolor; Tk: T. koningii; Gm: Glomus. mosseae; Gm+Fc: G.mosseae+F.concolor; Gm+Tk: G.mosseae+T.koningii; Gd: G. deserticola; Gd+Fc: G.deserticola+F.concolor; Gd+Tk: G.deserticola+T.koningii
Aluminum concentration in shoots of E. globulus plants did not show any differences either at lowest (150 mg kg−1) or highest (3,000 mg kg −1) concentrations, when plants growing at such concentrations of aluminum were not affected either by AMF or saprobe fungi inoculation (Fig. 6, Table 2). The same occurred with aluminum content. However, at 600 mg kg−1, both Glomus strains produced a significant increase in shoot aluminum concentration, which was not reinforced by the two inoculated saprobe fungi. At 1,500 mg kg−1, shoot aluminum concentration was dramatically increased by arbuscular mycorrhizal inoculation, particularly with G. deserticola. In addition, at the same aluminum level in the growth media, the effect of T. koningii was synergistic with what was presented by G. deserticola inoculation and the highest aluminum concentration was obtained (approximately 27 mg kg−1). Aluminum shoot content in this last treatment increased approximately sixfold in comparison to those obtained in control plants.
Aluminum concentration and Aluminum-content in shoot of Eucalyptus globulus inoculated or not with AMF or saprobe fungi in soil with different Aluminum concentrations. C: Control; Fc: F. concolor; Tk: T. koningii; Gm: Glomus. mosseae; Gm+Fc: G.mosseae+F.concolor; Gm+Tk: G.mosseae+T.koningii; Gd: G. deserticola; Gd+Fc: G.deserticola+F.concolor; Gd+Tk: G.deserticola+T.koningii
4 Discussion
Aluminum inhibited the hyphal length of G. mosseae and G. deserticola spores. However, this inhibition seems to be of a fungistatic nature, because when these spores were transferred from media with aluminum to media without aluminum, they were able to develop their hyphae, albeit smaller than those of the spores grown in medium without aluminum. These results suggest that soils with high aluminum concentration could decrease the development of AMF in soil, but these fungi can recover their functionality when the concentrations of metal inhibitors decrease (Hepper, 1979). Kelly, Morton, and Cumming (2005) suggested that the functional diversity of aluminum resistance displayed by AMF reflects a variation in the acclimation mechanisms operating in the mycorrhizal symbiosis under environmental stress. The presence of aluminum decreased the mycelial weight and the spore number of F. concolor and T. koningii. However, these saprobe fungi were able to absorb aluminum from the culture media, indicating the ability of these fungi to take aluminum up from the media. In fact, some microorganisms are able to absorb and to store heavy metals in their fungal structures (Alexander, 1999; Arriagada et al., 2005; J. W. Huang, C. P. Huang, & Morehart, 1990).
High amounts of aluminum in soil decrease plant growth and nutrient uptake (Cumming & Weinstein, 1990a; Fabig, 1982; Hentschel, Godbold, Marschner, Schlegel, & Jentschke, 1993). It has also been described that aluminum disables the biosynthesis of chlorophyll (Ridolfi & Garrec, 2000), which will produce an alteration in plant photosynthesis (Ouzounidou, 1995; Sinha, Srivastava, & Tripathi, 1993). Higher plant dry weight and P, Ca and Mg uptakes by mycorrhizal plants compared with non-mycorrhizal ones in the presence of aluminum in soil have also been observed (Anderson, 1988; Borie, Redel, Rubio, Rouanet, & Barea, 2002; Cumming & Weinstein, 1990b; Hentschel et al., 1993). However, plant protection from aluminum toxicity by AMF has been reported to be dependent on the type of micro-organism and aluminum concentration (Heggo et al., 1990; Lux & Cumming, 2001). In this study, only G. deserticola increased the E. globulus shoot dry weight and total P concentration at 1500 mg kg−1 of aluminum. However, at 3,000 mg kg−1 shoot dry weight, the total P decreased, which indicates toxic effects of aluminum on the plant growth at a higher aluminum concentrations. Studies have revealed that aluminum interferes with Ca and Mg uptake and translocation in plants (Lux and Cumming, 2001; Rengel, Pineros, & Tester, 1995; Tan, Keltjens, & Findenegg, 1993). Cumming and Ning (2003) found that aluminum significantly reduced Ca and Mg tissue concentrations in both mycorrhizal and non-mycorrhizal plants. These effects result in nutrient imbalances in plants, producing plant growth reduction. In metal-contaminated soils, mycorrhizal plants have shown increased P uptake compared with non-mycorrhizal plants (Chen, Li, Tao, Christie, & Wong, 2003). The improved P nutrition might be a mechanism involved in the alleviation of aluminum toxicity as a result of mycorrhizal colonization (Borie et al., 2002). These increases in P acquisition protect root tips from Al3+ toxicity in acidic soils (Hocking, 2001). The increase in Mg and Ca acquisition in plants inoculated with G. deserticola alone or together with T. koningii could have contributed to an increase in total chlorophyll synthesis (Cordeiro, Alcántara, & Barranco, 1995). Therefore, the major production of total chlorophyll by E. globulus colonized with AMF indicates that they were more efficient at light absorption, affecting plant photosynthetic efficiency (Gil, 1995). These effects induced by saprobe and AMF should be taken into consideration when studying the effect of heavy metals on Ca and Mg deficiencies. The decrease in the pH of the rhizosphere can increase the concentration of aluminum and decrease those of Ca and Mg (Göttlein, Heim, & Matzner, 1999).
The synergic effect of saprobe fungi belonging to Fusarium concolor and Trichoderma koningii on the arbuscular mycorrhizal colonization of root has already been observed (Arriagada et al., 2005; García-Romera et al., 1998). In this study, the fact that saprobe fungi could absorb aluminum and increase root arbuscular mycorrhizal colonization may explain why combined inoculation of G. deserticola and T. koningii increased the tolerance of E. globulus to the application of 1,500 mg kg−1. Nevertheless, when 3,000 mg kg−1 was applied, the protective effect disappeared, probably due to reduced arbuscular mycorrhizal root length colonization and metabolic activity of G. deserticola. These results indicate that the presence of high aluminum concentration in soil also decreases the development of the AMF inside the root and decreases its contribution to aluminum accumulation in the plant. On the other hand, the saprobe fungi inoculated alone did not decrease the toxic action of aluminum on E. globulus. This indicates that the beneficial effect of T. koningii was attributable to its synergistic effect on root colonization by G. deserticola more than on aluminum uptake.
Accumulation and exclusion are two basic strategies by which plants respond to elevated concentrations of heavy metals. Concentrations of available aluminum between 1–500 mg kg−1 are considered toxic for most plants (Anderson, 1988). However, some Eucalyptus species have been reported to tolerate a concentration of 500 mg kg−1 (Barros & Novais, 1996). Arbuscular mycorrhizal fungi may play a role in the protection of roots from aluminum toxicity by mediating interactions between aluminum, phosphorous and plant roots (Kruger & Sucoff, 1989; Marschner, 1995). In the present study, higher aluminum concentrations and content were observed in E. globulus shoots when colonized with G. mosseae and G. deserticola at 1,500 mg kg−1. Arriagada et al. (2004, 2005) suggest that the main metal concentration is found in the stem of E. globulus, where the harmful effects on plant development have a smaller incidence. In our research, this could explain why G. deserticola increased the resistance of E. globulus to aluminum toxicity in spite of a high aluminum concentration in the plant shoot. Dual inoculation between G. deserticola and T. koningii benefited and conferred the highest tolerance and translocation of available aluminum.
In conclusion, we have clearly identified that inoculation with specific microbial (AMF and saprobe fungi) combinations can improve plant establishment. The possibility of manipulating an arbuscular mycorrhizal inoculation together with a saprobe fungus conferring high aluminum tolerance and accumulation in the shoot by E. globulus could be a good alternative for stimulating plant growth under adverse conditions, such as in soils where acidic conditions and low levels of P, Ca and Mg may contribute to aluminum toxicity. This is particularly important in some volcanic soils in southern Chile, where the acidic soils, mainly those intensively cropped and without lime additions, facilitate the increase of toxic aluminum species, thus limiting the opportunities for these soils to be used for agricultural purposes. Therefore, such dual inoculation in field conditions deserves further investigation.
References
Alexander, M. (1977). Introduction to soil microbiology. New York: Wiley (467 pp).
Alexander, M. (1999). Biodegradation and bioremediation. San Diego, CA: Academic (302 pp).
Anderson, M. (1988). Toxicity and tolerance of aluminum in vascular plants. Water, Air and Soil Pollution, 39, 439–462.
Arriagada, C. A., Herrera, M. A., García-Romera, I., & Ocampo, J. A. (2004). Tolerance to Cd of soybean (Glycine max) and eucalyptus (Eucalyptus globulus) inoculated with arbuscular mycorrhizal and saprobe fungi. Symbiosis, 36, 285–299.
Arriagada, C. A., Herrera, M. A., & Ocampo, J. A. (2005). Contribution of arbuscular mycorrhizal and saprobe fungi to the tolerance of Eucalyptus globulus to Pb. Water, Air and Soil Pollution, 166, 31–47.
Baligar, V. C., Campbell, T. A., & Wright, R. J.(1993).Differential responses of alfalfa clones to aluminum-toxic acid soil. Journal of Plant Nutrition, 16, 219–233.
Barcelo, J., & Poschenrieder, C. (2002). Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environmental and Experimental Botany, 48, 75–92.
Barros, N. F., & Novais, R. F. (1996). Eucalypt nutrition and fertilizer regimes in Brazil. In P. M. Attiwill & M. A. Adams (Eds.), Nutrition of eucalyptus (pp. 335–355). Australia: CSIRO Publishing.
Booth, C. (1977). Fusarium. Laboratory guide to the identification of the major species (58 pp). Ferry Lane, Kew, Surrey, England: Commonwealth Mycological Institute.
Borie, F., Redel, Y., Rubio, R., Rouanet, J. L., & Barea, J. M. (2002). Interactions between crop residues application and mycorrhizal developments and some soil–root interface properties and mineral acquisition by plants in an acidic soil. Biology and Fertility of Soils, 36, 151–160.
Borie, F., & Rubio, R. (1999). Effects of arbuscular mycorrhizae and liming on growth and mineral acquisition of aluminum-tolerant and aluminum-sensitive barley cultivars. Journal of Plant Nutrition, 22, 121–137.
Brundrett, M., Bougher, N., Grove, T., & Malajczuk, N. (1996). Working with mycorrhizas in forestry and agriculture (374 pp). Canberra: ACIAR.
Clark, R. B. (1997). Arbuscular mycorrhizal adaptation, spore germination, root colonization, and host plant growth and mineral acquisition at low pH. Plant and Soil, 192, 15–22.
Cordeiro, A. M., Alcántara, E., & Barranco, D. (1995). Differences in tolerances to iron deficiency among olive (Olea europaea L.) cultivars. In J. Abadía (Ed.), Iron nutrition in soils and plants (pp. 197–200). The Netherlands: Kluwer.
Cuenca, G., De Andrade, Z., & Meneses, E. (2001). The presence of aluminum in arbuscular mycorrhizas of Clusia multiflora exposed to increased acidity. Plant and Soil, 231, 233–241.
Cumming, J. R., & Ning, J. (2003). Arbuscular mycorrhizal fungi enhance aluminium resistance of broomsedge (Andropogon virginicus L.). Journal of Experimental Botany, 54, 1447–1459.
Cumming, J. R., Swiger, T. D., Kurnik, B. S., & Panaccione, D. G. (2001). Organic acid exudation by Laccaria bicolor and Pisolithus tinctorius exposed to aluminum in vitro. Canadian Journal of Forest Research – Revue Canadienne de Recherche Forestiere, 31, 703–710.
Cumming, J. R., & Weinstein, L. H. (1990a). Aluminum-mycorrhizal interactions in the physiology of pitch pine-seedlings. Plant and Soil, 125, 7–18.
Cumming, J. R., & Weinstein, L. H. (1990b). Utilization of A1Po4 as a phosphorus source by ectomycorrhizal pinus-rigida mill seedlings. New Phytologist, 116, 99–106.
Chen, B. D., Li, X. L., Tao, H. Q., Christie, P., & Wong, M. H. (2003). The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere, 50, 839–846.
Dix, N. J., & Webster, J. (1995). Fungal ecology (594 pp). London, UK: Chapman & Hall.
Fabig, B. (1982). Einfluß von Al und den Schwermetallen Fe, Mn, Zn, Cu, Pb und Cd auf die efficienz der VA-Mykorrhiza bei tropischen und subtropischen Pflanzen. PhD Thesis, University of Göttingen, Germany.
Fuente-Martinez, J. M., & Herrera-Estrella, L. (1999). Advances in the understanding of aluminum toxicity and the development of aluminum-tolerant transgenic plants. Advances in Agronomy, 66, 103–120.
Galvagno, M. A. (1976). Ensayos de nutrición en Ascobolus crenulatus P. Karst (Fungi, Ascomycete). Boletin de la Sociedad Argentina de Botanica, 17, 95–118.
García-Romera, I., García-Garrido, J. M., Martín, J., Fracchia, S., Mujica, M. T., Godeas, A., et al. (1998). Interactions between saprotrophic Fusarium strains and arbuscular mycorrhizas of soybean plants. Symbiosis, 24, 235–246.
Gerdemann, J. W. (1955). Relation of a large soil-borne spore to phycomycetous mycorrhizal infections. Mycologia, 47, 619–632.
Gil, M. F. (1995). Elementos de fisiología vegetal (1047 pp). Madrid: Mundi-Prensa.
Gildon, A., & Tinker, P. B. (1983). Interactions of vesicular-arbuscular mycorrhizal infection and heavy metals in plants. I. The effects of heavy metals on the development of vesicular-arbuscular mycorrhizas. New Phytologist, 95, 247–261.
Giovannetti, M., & Mosse, B. (1980). An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytologist, 84, 489–500.
Gonzalez-Chavez, M. C., Carrillo-Gonzalez, R., Wright, S. F., & Nichols, K. A. (2004). The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environmental Pollution, 130, 317–323.
Göttlein, A., Heim, A., & Matzner, E. (1999). Mobilization of aluminium in the rhizosphere soil solution of growing tree roots in an acidic soil. Plant and Soil, 211, 41–49.
Haselwandter, K., & Berreck, M. (1994). Accumulation of radionuclides in fungi. In G. Winkelmann & D. R. Winge (Eds.), Metal ions in fungi (pp. 259–277). New York: Marcel Dekker.
Heggo, A., Angle, J. S., & Chaney, R. L. (1990). Effects of vesicular-arbuscular mycorrhizal fungi on heavy metal uptake by soybeans. Soil Biology and Biochemistry, 22, 865–869.
Hentschel, E., Godbold, D. L., Marschner, P., Schlegel, H., & Jentschke, G. (1993). The effect of Paxillus-involutus Fr on aluminum sensitivity of Norway spruce seedlings. Tree Physiology, 12, 379–390.
Hepper, C. M. (1979). Germination and growth of Glomus caledonius spores: The effects of inhibitors and nutrients. Soil Biology and Biochemistry, 11, 269–277.
Hewitt, E. J. (1952). Sand water culture methods used in the study of plant nutrition. Commonwealth Agricultural Bureau, Technical Communication No. 22.
Hocking, P. J. (2001). Organic acids exuded from roots in phosphorus uptake and aluminum tolerance of plants in acid soils. Advances in Agronomy, 74, 63–97.
Huang, J. W., Huang, C. P., & Morehart, A. L. (1990). The removal of Cu(II) from dilute aqueous solutions by Saccharomyces cerevisiae. Water Research, 24, 433–439.
Jansen, S., Broadley, M. R., Robbrecht, E., & Smets, E. (2002). Aluminum hyperaccumulation in angiosperms: A review of its phylogenetic significance. Botanical Review, 68, 235–269.
Jentschke, G., & Goldbold, D. L. (2000). Metal toxicity and ectomycorrhizas. Physiologia Plantarum, 109, 107–116.
Jones, D. L., & Kochian, L. V. (1995). Aluminum inhibition of the inositol 1,4,5-trisphosphate signal-transduction pathway in wheat roots – A role in aluminum toxicity. Plant Cell, 7, 1913–1922.
Khan, G., Kuek, C., Chaudhry, T. M., Khoo, C. S., & Hayes, W. J. (2000). Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere, 41, 197–207.
Kelly, C. N., Morton, J. B., & Cumming, J. R. (2005). Variation in aluminum resistance among arbuscular mycorrhizal fungi. Mycorrhiza, 15, 193–201.
Kinraide, T. B. (1991). Identity of the rhizotoxic aluminum species. Plant and Soil, 134, 167–178.
Kochian, L. V. (1995). Cellular mechanisms of aluminum toxicity and resistance in plants. Annual Review of Plant Physiology and Plant Molecular Biology, 46, 237–260.
Kruger, E., & Sucoff, E. (1989). Growth and nutrient status of Quercus-Rubra l in response to Al and Ca. Journal of Experimental Botany, 40, 653–658.
Li, X. L., Marschner, H., & George, E. (1991) Acquisition of phosphorus and copper by VA-mycorrhizal hyphae and root-to-shoot transport in white clover. Plant and Soil, 136, 49–57.
Lichtenthaler, H. K. (1987). Chlorophylls and carotinoids: Pigments of photosynthetic biomembranes. Methods in Enzymology, 148, 350–382.
Lux, H. B., & Cumming, J. R. (2001). Mycorrhizae confer aluminum resistance to tulip-poplar seedlings. Canadian Journal of Forest Research – Revue Canadienne de Recherche Forestiere, 31, 694–702.
MacDonald, R. M., & Lewis, M. (1978). The occurrence of some acid-phosphatases and dehydrogenases in the vesicular-arbuscular mycorrhizal fungus G. mosseae. New Phytologist, 80, 135–141.
Madrid, F., De La Rubia, T., & Martinez, J. (1996). Effect of Phanerochaete flavido-alba on aromatic acids in olive oil mill waste waters. Technological Environmental Chemistry, 51, 161–168.
Marschner, H. (1995). Mineral nutrition of higher plants (889 pp). London: Academic.
Marsh, B. A. B. (1971). Measurement of length in random arrangements of lines. Journal of Applied Ecology, 8, 265–270.
McAllister, C. B. (1992). Interacción entre hongos saprofitos y hongos formadores de micorrizas vesículo-arbusculares. PhD Thesis, University of Buenos Aires, Argentina (203 pp).
Medeiros, C. A. B., Clark, R. B., & Ellis, J. R. (1994). Effects of excess aluminum on mineral uptake in mycorrhizal sorghum. Journal of Plant Nutrition, 17, 1399–1416.
Mendoza, J., & Borie, F. (1998). Effect of Glomus etunicatum inoculation on aluminum, phosphorus, calcium, and magnesium uptake of two barley genotypes with different aluminum tolerance. Communications in Soil Science and Plant Analysis, 29, 681–695.
Mingorance, M. D. (2002). Focused microwave-assisted digestion of vegetal materials for the determination of essential mineral nutrients. Analitical Bioanalitical Chemistry, 373, 153–158.
Mosse, B. (1962). The establishment of vesicular arbuscular mycorrhiza under aseptic conditions. Journal of General Microbiology, 27, 509–520.
Ning, J. C., & Cumming, J. R. (2001). Arbuscular mycorrhizal fungi alter phosphorus relations of broomsedge (Andropogon virginicus L.) plants. Journal of Experimental Botany, 52, 1883–1891.
Ocampo, J. A., & Barea, J. M. (1985). Effect of carbamate herbicides on VA mycorrhizal infection and plant growth. Plant and Soil, 85, 375–383.
Ouzounidou, G. (1995). Cu-ions mediated changes in growth, chlorophyll and other ion contents in a Cu-tolerant Koeleria splendens. Biologia Plantarum, 37, 71–79.
Pearson, J. N., & Jakobsen, I. (1993). The relative contribution of hyphae and roots to phosphorus uptake by arbuscular mycorrhizal plants, measured by dual labeling with 32P and 33P. The New Phytologist, 124, 489–494.
Phillips, J. M., & Hayman, D. S. (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transaction British Mycological Society, 55, 158–161.
Rengel, Z., Pineros, M., & Tester, M. (1995). Transmembrane calcium fluxes during Al stress. Plant and Soil, 171, 125–130.
Ridolfi, M., & Garrec, J. P. (2000). Consequences of an excess Al and a deficiency in Ca and Mg for stomatal functioning and net carbon assimilation of beech leaves. Annals of Forest Science, 57, 209–218.
Rifai, M. A. (1969). A revision of the genus Trichoderma. Mycological Papers, 116, 1–56.
Rivera-Becerril, F., Calantzis, C., Turnau, K., Caussanel, J. P., Belimov, A. A., Gianinazzi, S., et al. (2002). Cadmium accumulation and buffering of cadmium-induced stress by arbuscular mycorrhiza in three Pisum sativum L. genotypes. Journal of Experimental Botany, 53, 1177–1185.
Schaedle, M., Thornton, F. C., Raynal, D. J., & Tepper, H. B. (1989). Response of tree seedlings to aluminum. Tree Physiology, 5, 337–356.
Silva, I. R., Novais, R. F., Jham, G. N., Barros, N. F., Gebrim, F. O., Nunes, F. N., et al. (2004). Responses of eucalypt species to aluminum: The possible involvement of low molecular weight organic acids in the Al tolerance mechanism. Tree Physiology, 24, 1267–1277.
Sinha, S. K., Srivastava, H. S., & Tripathi, R. D. (1993). Influence of some growth regulators and cations on the inhibition of chlorophyll biosynthesis by lead in maize. Bulletin of Environmental Contamination Toxicology, 51, 241–246.
Smith, S. E., & Read, D. J. (1997). Mycorrhizal symbiosis (2nd ed.). San Diego, CA: Academic.
Tan, K. Z., Keltjens, W. G., & Findenegg, G. R. (1993). Aluminum toxicity in sorghum genotypes as influenced by solution acidity. Soil Science and Plant Nutrition, 39, 291–298.
Tonin, C., Vandenkoornhuyse, P., Joner, E. J., Straczek, J., & Leyval, C. (2001). Assessment of arbuscular mycorrhizal fungi diversity in the rhizosphere of Viola calaminaria and effect of these fungi on heavy metal uptake by clover. Mycorrhiza, 10, 161–168.
Vale, F. R., Furtini-Neto, A. F., Reno, N. B., Fernandez, L. A., & Resende, A. V. (1996). Root growth of forest species in acid soil. Pesquisa Agropecuária Brasileira, 31, 609–616.
Von Uexküll, H. R., & Mutert, E. (1995). Global extent, development and economic-impact of acid soils. Plant and Soil, 171, 1–15.
Wainwright, M. (1992). The impact of fungi on environmental biogeochemistry. In G. C. Carrol & D. T. Wicklow (Eds.), The fungal community (pp. 601–618). New York: Marcel Dekker.
Weissenhorn, I., Leyval, C., Belgy, G., & Berthelin, J. (1995). Arbuscular mycorrhizal contribution to heavy-metal uptake by maize (Zea-mays L) in pot culture with contaminated soil. Mycorrhiza, 5, 245–251.
Widden, P., & Bisset, J. (1972). An automatic multichamber soil washing apparatus for removing fungal spores from soil. Canadian Journal of Microbiology, 18, 1399–1404.
Acknowledgements
Financial support of this study was provided by the Comision Interministerial de Ciencia y Tecnologia, Spain. The senior author is thankful to the Secretaría de Estado de Educación y Universidades, Spain by providing financial support. Written in memory of Gabriel Victoriano Gutierrez Sandaña (1950–2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arriagada, C.A., Herrera, M.A., Borie, F. et al. Contribution of Arbuscular Mycorrhizal and Saprobe Fungi to the Aluminum Resistance of Eucalyptus globulus . Water Air Soil Pollut 182, 383–394 (2007). https://doi.org/10.1007/s11270-007-9349-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9349-5