Abstract
A comparison was made of the ability of three different methods to describe the deposition and distribution of chloride from deicing salt in the roadside environment along a highway: direct sampling of airborne deposition (including snow ploughing) in containers; soil sampling and analysis of chloride content in the topsoil; and direct current resistivity measurements. Each method showed a distribution with significant decreasing values with increasing distance from the road. Two transport mechanisms, splash and spray, were identified when describing the airborne deposition. A mathematical model that includes these two transport mechanisms was adopted, and the total amount of airborne deposition on the ground 0–100 m from the road was estimated to approximately 45% of the salt applied on the road. The main part of the chloride spread by air and ploughing ended up within 10 m from the road. The soil sampling and resistivity measurements also showed the highest impact within this distance. The variation in chloride content in the soils reflected a poorer drainage ability of fine-grained soils compared to more coarse-grained soils. The resistivity measurements represented an integrated value of the differences in geology, water content and salinity. The increase in resistivity with distance from road in the topsoil was interpreted to reflect the distribution of chloride from deicing salt.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Winter maintenance of roads often includes the use of deicing salt, which is applied to keep roads clear from snow and ice and to improve traffic safety throughout winter. In Sweden, the most frequently used deicing salt is sodium chloride (NaCl). The Swedish Road Administration (SRA) uses about 200,000–250,000 tonnes of NaCl annually for deicing purposes on national roads, with an application rate of ca. 5–15 tonnes/km road. After being applied to the road, the salt eventually spreads to the surrounding area, following different pathways and having different fates. The deicing salt can be transported off the road with runoff or by airborne spread and by snow ploughing. Poor application methods may also throw salt directly into the roadside. The salt that is deposited on the ground adjacent to the road may infiltrate the soil with snowmelt or precipitation. The roadside vegetation that is exposed to this salt can consequently be damaged (Bäckman & Folkesson, 1995; Bernhardt-Römermann, Kirchner, Kudernatsch, Jakobi, & Fischer, 2006; Blomqvist, 1999, 2001; Kayama et al., 2003; Pedersen & Fostad, 1996; Pedersen, Randrup, & Ingerslev, 2000; Viskari & Kärenlampi, 2000). Plants may be damage both by salt spray deposited directly on the aboveground parts and by salt in the soil. Fostad and Pedersen (2000) showed that different tree species have different tolerance to soil salinity and the damage to plants varied with soil type.
The salt that infiltrates the soil is temporarily retained in the vadose zone between the soil surface and the groundwater, which, except being potentially hazardous to plants, also poses a future risk for groundwater contamination. This salt storage depends on where and when the salt infiltrates the soil, i.e. the deposition pattern. Naturally, different soils also have different abilities to store the salt in the vadose zone. Sodium takes part in chemical processes in the soil and is to a great extent retained in the upper part of the soil profile. Chloride, on the other hand, usually follows the water as a conservative element as it does not participate in chemical reactions. The chloride is therefore transported through the vadose zone down to the groundwater, from where it may then be further transported to various surface waters. Constructed drainage systems or roadside ditches can also intercept the deicer and carry it away to some recipient surface waters or to other areas where it may infiltrate the soil. Increasing salt concentration due to deicing salt application has been observed in groundwater (Foos, 2003; Howard, 1998; Nystén, 1998; Thunqvist, 2003) and surface water (Godwin, Hafner, & Buff, 2003; Koryak, Stafford, Reilly, & Magnuson, 2001; Mason, Norton, Fernandez, & Katz, 1999; Thunqvist, 2004). Salt application thus has environmental impacts when the salt spreads from the road to the surroundings, as it influences the vegetation, soil, groundwater and surface water. To predict the extent of this impact, it is important to quantify the different pathways and fate of the deicing salt.
Different transport mechanisms are able to carry the salt to various distances from the road. Runoff infiltrates in close proximity to the asphalt surface and generally ends up in ditches or drainage systems, or may be directly transported down to groundwater. Airborne spread, including splash and spray, may transport the salt to different distances before the salt is deposited on the ground or vegetation. While splash generally is deposited in proximity to the road, salt spray may be transported longer distances by wind. The transport distance is influenced by various factors such as: road and traffic characteristics, type and amount of precipitation, and wind conditions (Blomqvist, 1999). Finally, snow ploughing moves the snow from the road surface to the side of the road, where consequently more water may infiltrate during snowmelt. The snowpack may be considered as a reservoir retaining and storing pollutants during winter (Bartosová & Novotny, 1999). If the infiltration rate of the soil is exceeded during snowmelt, overland flow of salt meltwater may occur, which may transport the salt further away from the road. Horizontal transport of salt in shallow groundwater above impermeable soil layers may lead to the occurrence of high chloride concentrations in soils at large distances from the road (Pedersen & Fostad, 1996). These different transport mechanisms contribute to a deposition and distribution pattern that varies both in space and with time.
Different approaches can be used to study the deposition and distribution of deicing salt. Several studies have concentrated on airborne deposition of deicing salt (Blomqvist & Johansson, 1999; Gustafsson & Blomqvist, 2004; Pedersen et al., 2000), road salt accumulation in roadside snow (Buttle & Labadia, 1999; Hautala, Rekilä, Tarhanen, & Ruuskanen, 1995; Viskari et al., 1997) and in soil (Labadia & Buttle, 1996; Pedersen & Fostad, 1996; Pedersen et al., 2000). Both Blomqvist (1999) and Pedersen et al. (2000) showed that the largest airborne deposition occurred in close proximity to the road and seemed to decline exponentially with distance from road.
A non-invasive technique that has been used in a range of applications is direct current (DC) resistivity measurements. This method of measurement has for example been used in studies on pollutant leaching from landfills (Aaltonen & Olofsson, 2002; Ahmed & Sulaiman, 2001), saltwater intrusion (Nowroozi, Horrocks, & Henderson, 1999) or more recently on transport of deicing salt in groundwater (Leroux & Dahlin, 2006). The resistivity in soils exhibits large variations, from 1 ohmm in saline soil to several thousand ohmm in dry soils (Tabbagh, Dabas, Hesse, & Panissod, 2000). Different factors control the magnitude of the resistivity (or its inverse, conductivity) in soils. Of these the soil water content and salinity, the clay and fine-particle content, and the ground temperature are the most significant. The resistivity may also be affected by anthropogenic sources that increase the salinity in soil and groundwater. The resistivity method could thus be useful for monitoring the distribution of deicing salt in roadside soils.
The objective of this study was to examine the deposition pattern and distribution of chloride from deicing salt in soils along a section of a major highway in Sweden, representing different soil and vegetation types. Both components of deicing salt, chloride and sodium, may cause impacts on the roadside environment. This study has focused on the most mobile element chloride, because of its simplicity, as it is essentially non-reactive and easily soluble and transported throughout the soil profile. The different drainage and water storage ability of soils may hence be assessed. Three different methods for capturing the chloride deposition and distribution with distance from the road were tested: airborne deposition sampling; soil sampling; and DC resistivity measurements. The chloride deposition and distribution was assumed to differ with respect to type of soil and vegetation.
2 Materials and Methods
2.1 Site Description
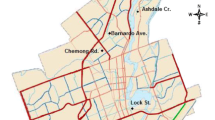
An 800-m section of one of the main highways in Sweden, the E4, located about 10 km north of Stockholm in the municipality of Sollentuna, was selected for the study (Fig. 1). The field site was chosen because the roadside environment varies along this stretch, while the variation of the external conditions, for example meteorology, traffic characteristics and salt application, are small. The road stretch comprises three different hydrogeological environments: clay soil with open grass field, silty-sandy till with sparse mixed forest (deciduous trees and Norway spruce (Picea abies)), and outwashed sandy soil (medium grained) with sparse coniferous forest (mainly Scots pine (Pinus sylvestris)). A simple inventory showed that the total stem density was about 750 ha−1 and the basal area was approx. 30 m2 ha−1. Scrub and small deciduous trees were present to a greater extent closer to the road than further away. At the forest sites, the trees begin at 7–9 m from the road, while closer to the road the vegetation consists of grass. Road-verge soil, consisting of crushed rock with a top layer of clay and silt, occur within approximately 2–3 m from the road at the forest sites. In the open field, the road-verge soil stretches to about 18 m from the road, where the long slope ends with a ditch. The difference in height between the road and the ditch is approximately 4 m in the open field, while the forest areas are rather flat.
Location of the field site. The generalized geological map from the region is based on Möller and Stålhös (1969)
The divided six-lane highway has a high traffic density, with an annual average daily traffic volume of about 90,000 vehicles with a speed limit of 110 km/h. The highway has a total width of 20 m and runs in a NW-SE orientation. Only the southwestern side of the road (the southbound lanes) was included in the field studies, because of noise barriers positioned on the other side of the road. The prevailing wind direction in the area during the winter 2003/04 was from S-SW-W and the annual precipitation was approx. 550 mm. Climatic data were obtained from the Swedish Road Administration’s Road Weather Information System (RWIS) and from SMHI (Swedish Meteorological and Hydrological Institute). Salt application data for the winter 2003/04 were obtained from the Swedish Road Administration Construction and Maintenance (the local road maintenance station) as daily deicing salt loads. The mean salt application rate on the southbound lanes was during the winter 2003/04 approx. 8 tonnes/km. The salting season in this area is mainly between November and March, and during this study period it stretched from 20 October 2003 to 11 March 2004.
2.2 Field Investigations and Chemical Analyses
Three different methods of measurement were tested: airborne deposition sampling, soil sampling and DC resistivity measurements.
2.2.1 Airborne Deposition
The accumulated airborne deposition of salt, including precipitation and splash, spray and snow ploughing from the road, was collected from 19 December 2003 to 5 April 2004, at different distances from the road, in order to reflect the total load of salt on the surroundings. The length of the sampling period comprised about 80% of the entire salting season and 85% of the total amount of salt used on the road was applied during the sampling period.
Deposition samplers were placed along two transects: one in the open field and one in the sparse coniferous forest area, at distances of 2, 10, 25 and 50 m from the road. In the forest site, deposition samplers were also placed at 5 m, and in the open field, additional deposition samplers were placed at 18 and 100 m from the road. The total deposition was collected in 5-l buckets with a funnel (∅ 195 mm) on top. Two or three deposition samplers were placed at each sampling point, about 1 m apart. The deposition samplers were collected and emptied at the end of the sampling period. In the open field, the deposition samplers were collected and replaced also on one occasion in February. Large amounts of ploughed snow made it unfeasible to empty the samplers closest to the road in the forest site at this occasion. Overflow in the samplers can therefore not be excluded.
A subsample from each deposition sampler was analysed separately for chloride concentration using a Dionex Ion Chromatograph (DX 120).
2.2.2 Soil Samples
Soil samples were collected in November 2003 and in April 2004, in order to compare chloride content before and after the salting period. The soil was sampled along 16 parallel transects, relatively evenly distributed along the 800-m road section studied. Each transect had six sampling points located at: 1, 2, 5, 10, 25 and 50 m from the road. In the open field area, sampling points at 100 m were also included. The sampling points at 2 m were only sampled in April. Five subsamples comprising the upper 20 cm of the soil were taken at each sampling point using a soil corer (25 mm diameter). All the subsamples from the same point were pooled into a composite sample before further analysis. The sampling depth was restricted to 20 cm in order to be able to take comparative samples from the whole area, since the soil layers closest to the road were very thin. The sampling depth was also restricted in order to speed up the sampling procedure. The chloride concentrations in the composite samples were analysed using a Dionex Ion Chromatograph (DX 120). All analyses were performed on air-dried and sieved soil fractions <2 mm. The preparation of the soil samples before the analysis followed to a large extent Labadia and Buttle (1996). A soil sample of 20 g was shaken with 100 ml distilled, deionised water for 30 min on a wrist-shaker. The soil extract was then filtered (through a Munktell filter OOK) before the analysis. The chloride content in the upper 20 cm soil was determined from the chloride concentration determined (g/kg soil) and the bulk density (kg/m3) for each sample. Interpolated maps showing the spatial pattern of the chloride content in the uppermost soil were generated by ordinary kriging techniques.
2.2.3 Resistivity Measurements
Direct current (DC) resistivity measurements were carried out in June 2004, in order to reflect the distribution of deicing salt in roadside soils as a function of both depth and distance from road. The resistivity method is based on measuring the electrical potential in the ground that results from an applied direct electrical current. From this measurement, information about the average resistivity of a subsurface volume is received. As salt increases the electrical conductivity (the inverse of resistivity) of the soil water, it should be possible to trace using resistivity measurements. If the geology and water content are approximately the same along the superficial part of the sections, the resistivity values may reflect the variation in conductive components in the topsoil and soil water, mainly the distribution of deicing salt when measurements are conducted after the salting period. The subsurface volume that is measured depends on the distance between the electrodes. By altering this distance, information about the resistivity at different depths is obtained.
The measurements were carried out with an ABEM Lund Imaging System together with an ABEM Terrameter SAS 4000. Four transects, with a spacing of 5 m between transects, were measured in the three different geological environments: till, clay and sandy soil. The measurements in the sandy and till areas were made using a 20 m long multi-electrode system with a Wenner array and electrode spacing of 0.5 m. In the clay area, one 20 m transect was measured in the same way, while the three other transects, each 60 m long, were measured with an electrode spacing of 1.5 m. The first electrode was placed near the asphalt surface and transects were extended perpendicular to the road. The chosen measuring approach gave an approximate depth penetration of 0.25–3 m for the electrode spacing 0.5 m and 0.75–9 m in the clay area with the electrode spacing of 1.5 m. The apparent resistivity was inverted to true resistivity by using the commercial software Res2Dinv (Geotomo Software, 2004), and the robust inversion method. The results were presented as inverted model resistivity sections showing the variations in resistivity with depth as a function of the distance from the road. The section that was considered as most representative for each soil area was selected to illustrate the variation in resistivity. The robust inversion method was chosen as it produces models with sharp boundaries, in contrast to the smooth inversion, which generates model sections with smoothly varying resistivity. Both inversion methods were tested, but the results did not differ to a great extent.
3 Results
The overall deposition pattern, a significant decrease with distance from the road, was well reflected by the three different methods used: airborne deposition sampling, soil sampling and DC resistivity measurements. However, the rate of the decrease and the level of detail varied between the methods. Because of the chosen sampling scheme, the different methods of measurement represented different time and spatial resolution.
3.1 Airborne Deposition
The airborne deposition samples showed a decrease in the chloride deposition with distance from the road, ranging from 340 g/m2 at 2 m from the road to 0.8 g/m2 at 100 m in the open field (Fig. 2). In the sparse forested area, the mean chloride deposition was 330 g/m2 closest to the road and 2 g/m2 at 50 m. The chloride deposition was hence quite similar in the sparse forest site and the open field; no significant difference was found.
Boxplots showing the median, quartiles, and minimum and maximum values of the chloride deposition with distance from the road according to the measured total chloride deposition in the airborne deposition samplers during December 2003–April 2004, for transects in the open field and in the sparse forest area, respectively (corresponding to the clay and sandy soil area, respectively in Fig. 6). For the samples with n = 2, the boxplots show the mean along with the minimum and maximum values
The mean annual natural deposition of chloride in the Stockholm region was calculated to be 0.6 g/m2 in open fields and 1.1 g/m2 in forest areas, using monitoring data from IVL Swedish Environmental Research Institute collected within the Throughfall Monitoring Network (Nettelbladt, Westling, Akselsson, Svensson, & Hellsten, 2006). This indicates that the deicing salt to some extent influenced the salt deposition at a distance of 50 m from the road, and were close to the background values at a distance of 100 m.
The integrated amount of chloride calculated from the sampling in the containers (2–50 m from the road), represented 16% of applied salt, which was during the sampling period approximately 4.3 kg Cl/m. To estimate the total amount of airborne deposition, a mathematical model has been fitted to the deposition data. Blomqvist (1999) described the airborne spread of deicing salt by an exponential function considering both splash and spray transport mechanisms. The total deposition (D(x)) at a certain distance from the road (x) was described by the function:
where a splash and a spray is the maximal deposition rate that occurs from splash and spray, respectively, and a b is the background deposition rate. An attempt to fit the Eq. 1 to the deposition data measured in this study resulted in a value of a splash = 8,000 mg/m2/day and a spray = 120 mg/m2/day, assuming a background value (a b ) of 3 mg/m2/day. The deposition data from both the forest and the open-field site were included in the model, since the measurements did not differ much between the two sites. The model and how well it described the deposition data can be viewed in Fig. 3. The deposition rate was obtained by dividing the measured chloride deposition with the length of the sampling period in days. Assuming that the airborne deposition reaches a background value at a distance of 100 m from the road, the model estimated the total amount of airborne deposition of deicing salt (0–100 m from the road) to about 45% of applied salt (expressed as chloride). This estimation was based on the assumption that the model could be extrapolated to the road boundary. Furthermore, the model estimated that the majority of this salt spread by air and ploughing, 90%, was deposited within 10 m of the road, and about half within 2 m of the road (Fig. 4).
Cumulative distribution of the airborne chloride deposition with distance from the road calculated with the mathematical model presented in Fig. 3
3.2 Soil Chloride Content
The chloride content in the soil samples collected in April decreased with distance from the road, ranging from around 20–50 g/m2 closest to the road to about 2–5 g/m2 at a distance of 50 m (Figs. 5 and 6). This pattern was not observable in the soil samples collected in November, indicating that the chloride content and distribution in the soils in April mainly originated from deicing salt that have been spread from the road.
Boxplots showing the median, quartiles, and minimum and maximum values of the chloride content in the upper 20 cm soil with distance from the road according to the soil samples collected in April and in November, for road-verge soil (n = 15–16), sandy soil (n = 4) and till soil (*n = 3, otherwise n = 4), respectively
Simplified geological map of the field investigation site and the location of the different measurement points (left) and the chloride content in the upper 20 cm soil sampled in November 2003 (middle) and in April 2004 (right). The chloride content was interpolated by ordinary kriging techniques. The maps show the field condition along an 800-m road stretch up to a distance of 50-m from the road
There was a significant increase in the chloride content from November to April, most pronounced within 10 m from the road (Fig. 5). No boxplots were created for the clay soil since the sampling only comprised two soil samples at each distance. The mean chloride content in the clay soil in November was 9 g/m2 at 5 m, 17 g/m2 at 10 m, and 4 g/m2 at 25 and 50 m. In April, the mean chloride content was 59 g/m2 at 5 m, 20 g/m2 at 10 m, 6 g/m2 at 25 m, and 5 g/m2 at 50 m. The different soil areas showed a similar pattern. Nevertheless, the chloride content was slightly higher in the clay and till soils than in the sandy soil. The chloride content in the clay soil measured in April (1–50 m from the road) represented in average 12% of the total amount of chloride applied as deicing salt during the winter season, whereas in the sandy soil the chloride content only represented 5%. Due to the high leaching ability of chloride it was assumed that the chloride content measured in the upper part of the soil in April originated from deicing salt applied during the immediately preceding salting season. The average amount in the till soil corresponded to 8% of the applied deicer chloride. This may be due to a longer storage time for water, and consequently also for salt, in the fine-grained soils, in particular the clay soil. In the coarse-grained sandy soil, the salt can more easily be flushed further down the soil profile and hence not be captured with the sampling approach used. Naturally, this will also apply for the natural deposition of salt that infiltrates the soil.
The highest variation in chloride content measured in April along the continuous road stretch was found 5–15 m from the road (Fig. 6). Within this distance, neither the soil type nor the topography varied to a great extent, except for the slope in the clay area. The tree barrier is situated within this distance, which may indicate the influence of vegetation type on the chloride deposition and distribution.
3.3 Resistivity Measurements
The DC resistivity measurements were made on a more detailed scale, since the measurement spacing was set to 0.5 m. Therefore, this method detected features like ditches and drainage systems, as a marked decrease in the resistivity values at a distance of 2–4 m from the road in the sandy soil and till areas and at 17–18 m in the clay area (Fig. 7) The lower resistivity values in the ditches and drainage systems were most likely a result of higher water content and salinity, compared to the natural soil layers.
An increase in resistivity values with distance from road was clearly apparent in the topsoil of the sandy soil and till areas (Fig. 7a and b). In this study, soil water content was only measured in the upper part of the soil. The water content in the upper 20-cm till and sand soil measured in June varied only a few percent with distance from the road (18–17% in till and 14–12% in sandy soil at 5 and 25 m from the road, respectively). The clay content in the upper 20-cm soil was higher within 2 m from the road than at further distances, as the soil closest to the road consisted of roadside-verge soil instead of the natural geology. These rather small variations in water content and geology could not alone explain the change in resistivity with distance from road. The transition to higher resistivity values in the topsoil corresponded rather well with the position of the tree barrier in the till and sandy soil areas.
The measurements also provided information of the variation in resistivity with depth. The sections in sandy soil clearly showed a lower resistivity beneath a depth of 1.5–2 m, probably representing the groundwater level (Fig. 7a). The difference between the four sections measured in the sandy soil was small. The sections in till, on the other hand, showed a much more complex pattern (Fig. 7b). No groundwater level could be given due to the superficial bedrock surface, which partly rose up to the ground surface along the transects. Hence, the high resistivity values at depth in this area reflected the bedrock. The significant variation in resistivity in the upper part of the sections partly reflected the natural variation due to the heterogeneous hydrogeological conditions of till. Several boulders in the till probably affected the local resistivity. A higher amount of fine particles such as silt was found in the till area than in the sandy area, giving local low resistivity areas along the sections in till. However, all sections in till had a clear low resistivity zone in the topsoil in the vicinity of the road.
The clay area also showed lower resistivity values within a distance of 10 m from the road (Fig. 7c). At greater distances (>20 m), the natural low resistivity values of the clay soil exceeded any impact of the increased salinity because of deicing salt deposition.
4 Discussion
Despite the differences in the three methods, each of them identified the impact from the deicing salt (expressed as chloride) and, indirectly, the deposition pattern. The three different methods studied: direct sampling of the total deposition in containers, analysis of soil samples, and DC resistivity measurements, reflected different sampling strategies that cannot directly be compared, but are instead complementary.
Airborne deposition measurements give information on the instant deposition, i.e. the amount of deicing salt deposited in the containers during the sampling period. Hence, it varies due to the length of the sampling period and the actual weather situation. The results are affected by the construction and shape of the samplers and their local position in relation, not only to distance from the road, but also to prevailing wind direction and altitude (Blomqvist & Johansson, 1999; Gustafsson and Hallgren Larsson, 2000). However, many of these effects are considered to balance each other out over a longer sampling period, which in this study included the main part of the salting season. Sampling in the immediate vicinity of the road, where the deposition is highest, was difficult due to snow ploughing. Losses due to the sampling technique cannot be excluded. In budget calculations concerning airborne spread, the recovery of salt applied on the road is usually less than 60% (e.g. Blomqvist & Johansson, 1999). The integrated amount of chloride retrieved with the airborne deposition measurements conducted within 2–50 m from the road represented about 16% of the salt applied on the road. This is a value that agrees well with other studies (e.g. Pedersen & Fostad, 1996).
To estimate the total amount of airborne deposition, an exponential function described by Blomqvist (1999) was successfully fitted to the deposition data. Hence, the model seems to be applicable for these open-field and sparse-forested (Scots pine) areas. This model includes both splash and spray, which emphasize that both transport mechanisms are important to consider if we want to evaluate the salt deposition with distance from road. With this model we calculated that, during the sampling period, approximately 45% of applied deicer chloride on the road was spread by airborne transport mechanisms, which in this study also includes the contribution from snow ploughing. This result represents an extrapolation of the measurements within 2 m distance of the road, which makes the calculated amount uncertain. About half of the airborne spread was estimated to be deposited within this distance (2 m of the road) and the major part, about 90%, within 10 m of the road. This was also confirmed by the soil sampling and resistivity measurements, which both showed the highest impact within 10 m distance. The airborne deposition seemed to be close to the background levels at 100 m from the road, which is in agreement with the results of Viskari et al. (1997) and Blomqvist and Johansson (1999).
No clear effect of a tree barrier could be detected in the airborne deposition samples. The roadside trees were expected to act as a barrier to salty spray from the road, which may be indicated by a more dramatic decrease in chloride concentration with distance at the forest site than at the open-field site (Hautala et al., 1995). The lack of difference between the forest and the open-field site may be due to measuring errors or to the fact that the forest was too sparse to capture a significant amount of the salt spray. Nevertheless, both the soil sampling and the resistivity measurements demonstrated a high variability within the zone where the tree barrier was located.
Soil sampling gives the bulk seasonal accumulation of deposited salts and the variation in the deposition with time cannot be determined. The results are dependent on the amount of chloride leaching, and are therefore influenced by the soil sampling depth and the heterogeneity of the ground. In strongly heterogeneous soils, a large number of sampling sites and several samples at each site are necessary in order to obtain a realistic picture of the distribution pattern, which is time-consuming fieldwork. The soil salinity analysis, however, may be important for an effect analysis. The difference between the long-term application of deicing salt and the total amount of chloride in the soils will be due to either transport to groundwater or surface water or to uptake in vegetation. The soil sampling method gives therefore important information on the different responses to environmental changes for different hydrogeological environments.
The soils sampled after the salting season reflected a distribution pattern of chloride not detected in the soils sampled before the salting season. This indicates that the main part of the chloride from deicing salt application is transported further down the soil profile and not stored in the upper part of the soil from one winter season to another. The chloride content measured in November was however higher than the estimated background value. The leaching of chloride from the upper part of the soil depends to a large extent on the amount of rainfall during autumn (Pedersen & Fostad, 1996; Pedersen et al., 2000). Even if the amount of leaching is unknown, the soil chloride analysis showed that the clay soil had the highest ability to store chloride among the soils measured. This reflected the larger water storage ability and poorer drainage ability of fine-grained soils than of the more coarse-grained soils.
The high deposition closest to the road measured by airborne deposition sampling was not captured in the soil samples, indicating that a transport of chloride to greater depths in the soil profile had already occurred. This could be enhanced by the fact that the amount of snow closest to the road was higher due to additional snow from snow ploughing. The chloride was assumed to be retained in the liquid portion of the snowpack, which leads to a high chloride content in the first portion of the snowmelt. A vast amount of water may then flush the chloride down to deeper levels during later meltwater fluxes. Horizontal transport of salt may also have occurred as a result of surface runoff or superficial horizontal water flow during snowmelt, which may contribute to a different distribution pattern in the soil than for airborne deposition. Nevertheless, as with deposition, the chloride content in the soil samples seemed to decrease exponentially with distance from road. This was most pronounced in the sandy soil.
Measurements of resistivity did not give any direct information on chloride distribution. The measured resistivity is a combined measure of the resistivity of the soil particles and soil water resistivity, which in turn depends on the ion composition of the soil water, the TDS (total dissolved solids) content. Natural variation in resistivity due to varying geological conditions may be as high as 104 ohmm in Swedish terrain (Aaltonen & Olofsson, 2002). In a clay-free soil, it is possible to roughly calculate the soil water resistivity using Archie’s law, an empirical equation formulated in the 1940s (Reynolds, 1997). The lack of information regarding water content at depth in this study made it impossible to quantify the salinity from the sections without great uncertainty. A clear pattern with lower resistivity close to the road and increasing values with distance from road was however shown. The resistivity measurements also reflected the heterogeneity of the soil in the roadside environment, which could be difficult to obtain with soil sampling. Since the measurements are rather rapid, inexpensive and non-destructive, this method could be useful for long-term monitoring of the impact of salt on the roadside environment. Leroux and Dahlin (2006) showed that time-lapse resistivity investigations have potential for imaging saltwater transport and pathways in complex hydrogeological environments. Aaltonen and Olofsson (2002) developed a system for long-term monitoring of groundwater chemical changes over time with DC resistivity measurements. With long-term monitoring, both the spatial and temporal variation may be assessed.
5 Conclusions
A comparison between the three sampling methods used in this study indicated a similar lateral spread pattern despite the great differences in the nature of the measurements. The different methods complemented each other in a useful way. The accumulated airborne deposition represented the chloride load deposited on ground in the roadside environment. The soil sampling and analysis gave an indication of the response of different soils to the infiltrating chloride. Finally, the resistivity measurements represented a non-invasive method that provides a spatial distributed picture of the subsurface, showing an integrated value of the variation in geology, water content and salinity. When differences in geology and water content were small, the change in resistivity was assumed to reflect the distribution of chloride from deicing salt.
Both splash and spray were identified as important transport mechanisms in order to describe the airborne deposition. An exponential function that includes these two transport mechanisms described successfully the airborne deposition with distance from road. This model may prove useful to assess the environmental impact of deicing salt in the roadside environment of a highway, in particular at non- or sparse-forested areas.
The resistivity measurements showed that the salinity conditions of the till area investigated were much more complex than the other soil areas due to high hydraulic heterogeneity and thin soils with outcropping bedrock.
Different methods of measurement that represent different time and spatial resolutions should be combined in order to make a reliable description of the salt deposition pattern and the impact on the roadside environment. It is essential to identify the different pathways taken by deicing salt and its ultimate fate if impact on the roadside environment is to be predicted with accuracy. Continuous monitoring of the spread and transport of deicing salt would provide valuable information in this regard.
References
Aaltonen, J., & Olofsson, B. (2002). Direct current (DC) resistivity measurements in long-term groundwater monitoring programmes. Environmental Geology, 41, 662–671.
Ahmed, A. M., & Sulaiman, W. N. (2001). Evaluation of groundwater and soil pollution in a landfill area using electrical resistivity imaging survey. Environmental Management, 28(5), 655–663.
Bäckman, L., & Folkesson, L. (1995). Saltpåverkan på vegetation, grundvatten och mark utmed E20 och Rv 48 i Skaraborgs län 1994. VTI meddelande, 775–1995 (in Swedish).
Bartosová, A., & Novotny, V. (1999). Model of spring runoff quantity and quality for urban watersheds. Water Science and Technology, 39(12), 249–256.
Bernhardt-Römermann, M., Kirchner, M., Kudernatsch, T., Jakobi, G., & Fischer, A. (2006). Changed vegetation composition in coniferous forests near motorways in Southern Germany: The effects of traffic-borne pollution. Environmental Pollution, 143(3), 572–581. DOI:http://dx.doi.org/10.1016/j.envpol.2005.10.046.
Blomqvist, G. (1999). Air-borne transport of de-icing salt and damage to pine and spruce trees in a roadside environment. Licentiate thesis TRITA-AMI-LIC 2044, Division of Land and Water Resources, Department of Civil and Environmental Engineering, Royal Institute of Technology, Stockholm, 25 pp.
Blomqvist, G. (2001). De-icing salt and the roadside environment: Air-borne exposure, damage to Norway spruce and system monitoring. Dissertation TRITA-AMI-PHD 1041, Division of Land and Water Resources, Department of Civil and Environmental Engineering, Royal Institute of Technology, Stockholm, 32 pp.
Blomqvist, G., & Johansson, E.-L. (1999). Airborne spreading and deposition of de-icing salt – A case study. Science of the Total Environment, 235, 161–168.
Buttle, J. M., & Labadia, C. F. (1999). Deicing salt accumulation and loss in highway snowbanks. Journal of Environmental Quality, 28(1), 155–164.
Foos, A. (2003). Spatial distribution of road salt contamination of natural springs and seeps, Cuyahoga Falls, Ohio, USA. Environmental Geology, 44, 14–19.
Fostad, O., & Pedersen, P. A. (2000). Container-grown tree seedling responses to sodium chloride applications in different substrates. Environmental Pollution, 109, 203–210.
Geotomo Software (2004). Manual for RES2DINV. Geotomo Software, Penang, Malaysia, 133 pp (http://www.geoelectrical.com).
Godwin, K. S., Hafner, S. D., & Buff, M. F. (2003). Long-term trends in sodium and chloride in the Mohawk River, New York: The effect of fifty years of road-salt application. Environmental Pollution, 124, 273–281.
Gustafsson, M., & Blomqvist, G. (2004). Modeling exposure of roadside environment to airborne salt – Case study. Transport Research Circular E-C063: Snow Removal and Ice Control Technology, 296–306.
Gustafsson, M. E. R., & Hallgren, L. E. (2000). Spatial and temporal patterns of chloride deposition in Southern Sweden. Water, Air, and Soil Pollution, 124(3–4), 345–369.
Hautala, E. L., Rekilä, R., Tarhanen, J., & Ruuskanen, J. (1995). Deposition of motor vehicle emissions and winter maintenance along roadside assessed by snow analyses. Environmental Pollution, 87, 45–49.
Howard, K. (1998). Monitoring the impact of road maintenance chemicals on groundwater. In T. Nystén, T. Suokko (Eds.), Deicing and dustbinding – Risk to aquifers. Proceedings of an International symposium, Helsinki, Finland. Nordic Hydrological Programme. NHP report 43, 51–62.
Kayama, M., Quoreshi, A. M., Kitaoka, S., Kitahashi, Y., Sakamoto, Y., Mauyama, Y., et al. (2003). Effects of deicing salt on the vitality and health of two spruce species, Picea abies Karst., and Picea glehnii Masters planted along roadsides in northern Japan. Environmental Pollution, 124, 127–137.
Koryak, M., Stafford, L. J., Reilly, R. J., & Magnuson, P. M. (2001). Highway deicing salt runoff events and major ion concentrations along a small urban stream. Journal of Freshwater Ecology, 16(1), 125–134.
Labadia, C. F., & Buttle, J. M. (1996). Road salt accumulation in highway snow banks and transport through the unsaturated zone of the Oak Ridges Moraine, southern Ontario. Hydrological Processes, 10, 1575–1589.
Leroux, V., & Dahlin, T. (2006). Time-lapse resistivity investigations for imaging saltwater transport in glaciofluvial deposits. Environmental Geology, 49(3), 347–358.
Mason, C. F., Norton, S. A., Fernandez, I. J., & Katz, L. E. (1999). Deconstruction of the chemical effects of road salt on stream water chemistry. Journal of Environmental Quality, 28(1), 82–91.
Möller, H., & Stålhös, G. (1969). Geological map Stockholm NW, 1:50 000. Geological Survey of Sweden (SGU) Ae 2.
Nettelbladt, A., Westling, O., Akselsson, C., Svensson, A., & Hellsten, S. (2006). Luftföroreningar i skogliga provytor – Resultat till och med September 2005. IVL Report B 1682, IVL Swedish Environmental Research Institute (in Swedish, English summary).
Nowroozi, A. A., Horrocks, S. B., & Henderson, P. (1999). Saltwater intrusion into the freshwater aquifer in the eastern shore of Virginia: A reconnaissance electrical resistivity survey. Journal of Applied Geophysics, 42, 1–22.
Nystén, T. (1998). Transport processes of road salt in quaternary formations. In T. Nystén, T. Suokko (Eds.), Deicing and dustbinding – Risk to aquifers. Proceedings of an international symposium, Helsinki, Finland. Nordic Hydrological Programme. NHP report 43, 31–40.
Pedersen, L. B., Randrup, T. B., & Ingerslev, M. (2000). Effects of road distance and protective measures on deicing NaCl deposition and soil solution chemistry in planted median strips. Journal of Arboriculture, 26(5), 238–245.
Pedersen, P. A., & Fostad, O. (1996). Effects of deicing salt on soil, water and vegetation. Part I: studies of soil and vegetation. MITRA report 01/96, The Agricultural University of Norway (NLH), Dept. of Plant Sciences, (in Norwegian, English summary).
Reynolds, J. M. (1997). An introduction to applied and environmental geophysics (pp 418–466). New York: Wiley.
Tabbagh, A., Dabas, M., Hesse, A., & Panissod, C. (2000). Soil resistivity: A non-invasive tool to map soil structure horizonation. Geoderma, 97, 393–404.
Thunqvist, E.-L. (2003). Estimating chloride concentration in surface water and groundwater due to deicing salt application. PHD thesis TRITA-LWR-PHD 1006, Department of Land and Water Resources Engineering, Royal Institute of Technology, Stockholm, 2003, 21 pp.
Thunqvist, E.-L. (2004). Regional increase of mean chloride concentration in water due to the application of deicing salt. Science of the Total Environment, 325, 29–37.
Viskari, E.-L., & Kärenlampi, L. (2000). Roadside Scots pine as an indicator of deicing salt use – A comparative study from two consecutive winters. Water, Air and Soil Pollution, 122, 405–419.
Viskari, E.-L., Rekilä, R., Roy, S., Lehto, O., Ruuskanen, J., & Kärenlampi, L. (1997). Airborne pollutants along a roadside: Assessment using snow analyses and moss bags. Environmental Pollution, 97, 153–160.
Acknowledgements
This project was funded by the Swedish Road Administration (SRA) through the Centre for Research and Education in Operation and Maintenance of Infrastructure (CDU). Many people at the Dept of Land and Water Resources Engineering, Royal Institute of Technology (KTH) have contributed to this work. Thanks to Bertil Nilsson, Mohammad-Reza Katuzi and Karin Palmqvist Larsson for the help with the soil samples and chloride analysis. Per-Erik Jansson is also greatly acknowledged for his commitment and valuable ideas and guidance during this work. Thanks also to Ann-Catrine Norrström for comments on how to perform the soil sampling and analysis. Finally, Taina Nysten at the Finnish Environment Institute is acknowledged for valuable comments on an early draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lundmark, A., Olofsson, B. Chloride Deposition and Distribution in Soils Along a Deiced Highway – Assessment Using Different Methods of Measurement. Water Air Soil Pollut 182, 173–185 (2007). https://doi.org/10.1007/s11270-006-9330-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-006-9330-8