Abstract
Five intersecting resistivity sections have been measured in glaciofluvial deposits hosting an aquifer of regional importance situated along a heavy traffic highway in Sweden. The winter salt spreading has caused a regular salinity increase through the years. For imaging the transport of saltwater in the aquifer, the sections were measured exactly in the same location before and after winter, and interpreted using a time-lapse inverse procedure. Some auger drilling and RCPT data were available for correlation. After winter, the resistivity had generally decreased under the water table and increased above it. The decrease in resistivity in the saturated zone is interpreted as a plume of more saline groundwater created by de-icing salt from the road. The increase in the upper layer can be explained by changes in temperature and soil moisture. The study shows that time-lapse resistivity investigations has potential for imaging hydraulic pathways in complex hydrogeological environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction—background
In recent years authorities in Europe have become increasingly concerned with the protection of natural surface waters and groundwater resources, and general requirements have been set up by the European Water Framework Directive and preceded or followed by national legislations.
An important source of pollution stems from the contaminants transported by water running off from the roads. Pneumatics and fuel oils release heavy metals in relatively small amounts but regularly. Larger amounts can be released when traffic accidents occur involving vehicles transporting hazardous substances. The probability of such accidents is low, but the consequences can be serious, especially when important roads are close to vulnerable aquifers and when contaminants can spread quickly. In Sweden, it is the responsibility of the National Road Administration to take measures for protecting water resources from pollution coming from the roads. Such measures can be very costly, and careful planning involving hydrogeological studies is necessary to define priorities.
In Nordic countries, a supplementary problem arises from the salts that are spread on the roads every winter to melt snow and ice, and local increases in salt concentration have been observed at several localities (e.g. Thunqvist 2003). Such salt concentrations should be taken into consideration, even if the amounts are still moderate, since they are increasing every year. Moreover, if salt can reach the aquifer, other contaminants also can, though possibly not as quickly and in the same way as the sodium chloride. Large salt concentrations might increase the mobility of heavy metals and reduce the acid neutralising capacity and pH in surface waters (e.g. Thunqvist 2003).
Salt is easily dissolved and transported with groundwater since it reacts only little with the environment. Salt increases the conductivity of water and it should be possible to trace its transport in groundwater using geoelectrical methods. Using its geoelectrical property, one might be able to image hydrogeological paths in the subsoil as a complement to more conventional hydrogeological tests.
Such ideas have already been exploited in a number of studies and resistivity has often been used for e.g. mapping saline water intrusions (Barker 1990; Giao 2003, among many others), imaging tidal cycles (e.g. Acworth and Dasey 2001; Slater and Sandberg 2000), delineating contaminated plumes with increased chloride concentration originating from landfills (e.g. Rosqvist et al. 2003; Ogilvy et al. 2002) and for hydrogeological experiments (e.g. Slater et al. 2000).
A site in Southern Sweden has been studied, where salt water “naturally” infiltrates to the groundwater every year, stemming from the de-icing salts spread every winter.
Resistivity variations in soils and rocks
Effect of increased salt content on resistivity
It is common knowledge that resistivity in rocks and soils depends on pore water salinity. Empirical laws have been defined, but there is no general relationship covering all kinds of rocks.
For instance, Ward (1990) recalls Archie’s law (1942) expressing the resistivity of sediments devoid of clay:
where ρw is the resistivity of pore water, ϕ the porosity, S nw the fractional saturation and a and m factors depending on the rock type and on the geometry of the pores, respectively. From this empirical relation it appears that the resistivity of “clean” sediments will decrease linearly with increasing pore water conductivity.
For clay bearing sediments one usually adds a term expressing the surface conductivity due to the ions in the double layer at the surface of clay particles, like in the expression of Patnode and Wyllie or that of Waxman and Smits (1968) (cited by Ward (1990)):
where F t is the formation factor at high concentrations, B is a factor taking into account the dependence of the mobility of exchange cations on the pore water concentration, and Q the clay cation exchange capacity per unit volume.
It rejoins Archie’s law for Q=0, that is when there is no clay in the sediment, and it implies that the slope of the curve for the dependence of the rock’s resistivity will be steepest at higher pore water salinity. Such a general conclusion can also be drawn from the expression defined by Sen et al. (1988). One can also cite P.D. Chinh (2000).
Taylor and Barker (2002) recently published an experimental study on Triassic sandstones, more or less consolidated and with varying clay content. They measured the resistivity of four types of saturated samples for varying electrolyte resistivity. They observed that the slope is steepest for lower electrolyte resistivity, and that the samples with the highest clay content show the narrowest range for the resistivity decrease. As may be expected, a change in the pore water resistivity will have less effect on the resistivity of a clay bearing sediment. Consistently, Barker (1990) had noted that mapping saltwater intrusions is more difficult in sediments with higher clay content.
Temperature
For the temperature, one can consider the general relation given in Ward (1990), after Keller and Frischknecht (1966):
where t is the temperature in °C and α is the temperature coefficient of resistivity. Ward (1990) gives α ≈ 0.025 per °C. This implies a linear relationship in a log-log scale. However, this relation is based on the idea that the rock resistivity will vary linearly with the pore water resistivity, which is not always the case. Consequently, it should be considered with caution for large temperature variations.
Water content
Higher saturation will produce a decrease of the rock’s resistivity. Like for the pore water salinity, several empirical laws have been defined and generalisation is difficult. One can cite Archie’s law (1) for “clean” sediments.
Taylor and Barker (2002) have measured the resistivity of four different types of triassic sandstones under desaturation. The resistivity does not decrease linearly, and its drop is maximum in the samples with the lowest clay content: up to 250% total variation against 15–30% in clayey or shaly sandstones. Consequently, the response to variations in water content will be more perceptible in “clean” sediments.
All these properties are consequences of the general mechanisms of conduction in rocks, which are still inaccurately understood but usually considered as a balance between electrolytic and surface conduction phenomena. The above considerations can be expressed by the general idea that electrolytic conduction will be comparatively more important in sediments with low clay content, and therefore resistivity will vary more with pore water salinity and saturation level in such materials.
The results obtained on sandstones should be transposable to unconsolidated glaciofluvial sediments, although maybe not in every detail. In such sediments, the granulometric clay fraction does not always correspond to mineralogical clays, and this can cause some difference. Furthermore, pore connectivity can be expected to be better.
However, while it is possible to separate the effects of different parameters in a laboratory experiment, this is usually not possible in the field, and the observed resistivity changes are due to conjugate effects. The aim of the interpreter is to distinguish those that are predominant, but this is not always an easy task.
Bergaåsen—site description
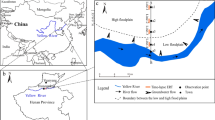
The Bergaåsen site, close to Ljungby in Southern Sweden (see Fig. 1) was chosen because at this locality, the heavy traffic highway E4 is close to an aquifer of regional importance, located in the glaciofluvial sediments that stretch between the highway and the river Lagan. A regular increase of salt concentration in the groundwater has been observed in recent years. It is concentrated in a 250 m wide band along the road where concentrations have exceeded 100 mg l−1. Along the river, concentrations are less high, but still between 50 and 100 mg l−1. The normal concentration of Cl in the area is between 5 and 10 mg l−1 in the shallow groundwater, corresponding to conductivities below 15 mS m−1 (H. Bruch, Mark&Vatten AB, personal communication). According to the criteria defined by the Swedish Environmental Protection Agency (SEPA 1999), the risk of corrosion of pipes is enhanced above 100 mg l−1, and taste can be affected for chloride concentrations above 300 mg l−1. In and around the studied area, chloride concentrations have been measured in a few wells and the highest concentrations are observed between a few metres and 8-m depth, sometimes even 10-m depth. They are very localised in a usually 2-m thick strata at most and decrease rapidly underneath (Mark&Vatten AB, personal communication).
Map showing the location of the investigated area, the distribution of the glaciofluvial deposits and the chloride concentration in the groundwater (adapted from Bruch 2000)
The aquifer is known to be vulnerable due to the high permeability of the glaciofluvial deposits in some sites (up to 0.01 m2 s−1 in the coarsest sediments, Mark&Vatten AB, personal communication). They are far from being homogeneous and investigations prior to the road construction have revealed localised caps of gravels and coarse sand as potential infiltration windows. In some places, it has been estimated that run-off water from the road reaches the groundwater table in only one hour (Vägverket Konsult 2001). At other locations, localised clay deposits may offer a relative protection.
The city of Växjö (75,000 inhabitants) has planned to use the Bergaåsen aquifer as a complementary resource. Extensive pumping tests and analyses have been conducted over the last few years. They have shown that the water is of high quality except for the salt concentration, which is increasing slowly but regularly. The natural resource is important, about 250 l s−1 can be pumped, and it can be supplemented by artificial infiltration (Mark&Vatten 2000). However, it is very sensitive to contamination from the highway E4 and several protection measures have therefore been discussed as E4 is one of the heaviest traffic routes in Sweden, and 25% of the traffic is due to goods transport. Moreover, salt has to be strewn on the road from November to the middle of March every year for traffic safety.
The aim of our project was to image hydrogeological paths using salt as a tracer by applying geoelectrical techniques before and after winter. Such site conditions are rather common in Nordic countries, and the results could be very relevant for other local studies.
Site investigations
Geoelectrical measurements
Five geoelectrical sections have been measured on the glaciofluvial deposits in an area known to be particularly vulnerable (see Figs. 2, 3). Four parallel 80 m long sections running from highway E4 and perpendicular to it have been measured and designated BET01, BET02, BET03 and BET04 from south to north. They intersect the 400 m long section BEL01 along a small road running parallel to E4. All lines are situated in the area most influenced by the road, where salinities above 100 mg l−1 have been measured.
The measurements were performed by Scandiaconsult in October 2001 and April 2002 using the ABEM Lund Imaging System and the Wenner and Schlumberger arrays. The minimum electrode spacing was 2 m for the long line BEL01 and 1 m for the four crossing lines.
The electrodes could not remain in place between the two measurement series, but their locations were recorded with extra care and very often, old electrode holes were found and reused. Precise measurements of their positions and altitudes were taken and used in the interpretation.
One important point concerning the site conditions is the very high contact resistances in the first layer of drained sandy sediments. These however did not prevent the acquisition of resistivity data.
Interpretation of resistivity data
For the interpretation, the commercial software Res2Dinv (Loke 1996–2004) and the so-called robust inversion method were used. The minimization of a mixed L1-norm was done as an iteratively re-weighted least-squares algorithm, similar to that proposed by Farquharson and Oldenburg (1998). Robust constraints were introduced on both the data and the parameters.
This method is less sensitive to large outliers in the data, but these can easily be removed from the data prior to the inversion, at least when using a traditional array, and if the data density is sufficiently large. It is also possible to reduce their influence by using weighting matrices. The main advantage of the robust inversion is that it can produce models with sharp boundaries separating zones of relatively constant resistivity unlike the usual diffuse layer limits obtained with the L2-norm (Loke et al. 2003). Consequently, it accounts more easily for larger contrasts of resistivity. Such models are often found to represent geological reality better and that is why the “blocky method” is often preferred.
For estimating the changes between measurements made at two different times, the programme provides different options for the “time-lapse inversion”, which define the constraints between successive time steps (Loke 1999; Chambers et al. 2004). The aim is that the result should be more representative of actual changes in the underground and less affected by noise, due for example to changes in the contact between the electrodes and the ground. Several possibilities exist. In each case, the inversion result for the first time step is taken as a reference model. The model for the second step can be bound to the first one by a least-squares constraint, a robust constraint or they can also be unbound. Consistently, the two time steps have been inverted simultaneously, since the noise level is of the same order for both of them. We have chosen a robust constrain between the second and first models for the results we present hereafter, but the other constraints have also been tried out. The results are quite similar in all cases, except for the deepest parts of the BEL01 section: which show more undulations on the pictures obtained with least-squares methods. Nevertheless, the results can be considered with good confidence, at least down to 10 m interpreted depth on the line BEL01.
Geotechnical investigations
As part of this project, Scandiaconsult performed a number of auger drillings and CPT with resistivity (RCPT) measurements (Daniel et al. 2003) at the intersections of the measured resistivity lines, three on line BET03 and one on line BET04. The locations of the geotechnical drillings and geophysical lines are plotted in Fig. 3.
The materials found were mainly sands and coarse sands until 4 to 6 m depth in all the drillings except in the one at the intersection between the lines BET03 and BEL01. Sandy gravels were found at the intersection with BET01 and silty sands, intercalated with coarse sands, were found between 6.5 and 8 m depth at the intersections with lines BET01 and BET02. The intersection with line BET03 is the only point were some 50 cm of layered clay were found at 2 m depth. At this location, 0.4 m thick silty sand was found above the clay, and the rest was sand. Farther away on line BET03, only sands (coarse to medium grained) were found between the ground surface and 4 m depth. The drillings showed very high spatial variability in the glaciofluvial deposits, which is quite expected for that kind of sediments.
Groundwater table was found at 143–144 m above sea level. It is known to be almost horizontal and to slope gently towards the river in this very restricted area. It is clear from the RCPT soundings, shown in Fig. 4, that the resistivity decreases dramatically under the groundwater table in the coarse sediments, from over 10,000 Ω m to a few hundred. Only at the intersection with line BET03 was the groundwater level less clearly determined, due probably to capillary rise in the finer sediments. At this point a decrease in resistivity under the groundwater level was observed as well, but less clearly, from over 400 to less than 200 Ω m.
Measurement of the conductivity in the groundwater
A number of groundwater conductivity measurements and pumping tests were made by the consultants Mark&Vatten Ingenjörerna AB, in the whole area, and these served as a basis for establishing the groundwater flow and salinity maps on a large scale. Only one of the observation wells is situated directly in the area of the resistivity measurements (see location on Fig. 2), where chloride concentration and groundwater conductivity were measured (Table 1 gives the values measured on 13 October 2000). The salinity varies with depth and reaches a maximum between 4.5 and 5.5 m. This characteristic has been observed in many wells in the area—the salinity is maximum at an intermediate depth. Calculating a regression line for these two parameters, we find that a chloride concentration increase of 100% will cause a conductivity increase of 34% in the groundwater. Depending on the lithology that determines different formation factors, we should expect a similar increase in the sediment conductivity with salinity if they contain little or no clay, otherwise a smaller increase. More precise laboratory study is required but it is likely that the same increase in the groundwater conductivity would induce different responses in the various sediments found in the area.
However, such a sign reversal of the conductivity gradient was not observed in any of the RCPT profiles (Fig. 4), where the resistivity seems to remain low at depth. Several explanations are possible. The RCPT drillings might not have been deep enough to observe this trend reversal. The surroundings of the observation well might be characterised by particular and very localised hydrogeological conditions, where shallow groundwater is relatively separated from deeper water. It is, however, unlikely that such localised conditions exist, since chloride concentration at an intermediate level has been observed at several locations in the area: it must be quite general in this aquifer. It is also possible that chloride had migrated deeper into the aquifer between October 2000 and April 2002, reaching earlier levels. The distribution of chloride in depth may also vary seasonally.
Meteorological measurements
During 2001 and 2002, the precipitation, the atmospheric temperature and the amounts of salt spread were recorded in the area. The annual temperature variation is important, from 12°C to −15°C on an average. When the measurements were made in October 2001, the temperature was around 10°C following a warmer period, whereas the temperature was around 5°C in April 2002 following a colder period of several months with temperatures around 0°C.
Around 14 kg m−1 of salt were spread on that section of road during the winter of 2001/2002, and the precipitations amounted to 77 mm between the two resistivity measurements. The general hydrological balance is not known.
Results and interpretation
Sections from October 2001
On most of the lines (BET01, BET02, BET04 on Fig. 6 (a, b, d), and BEL01 on Fig. 5), a first extremely resistive layer is visible above much less resistive deposits. It can be identified as the dry sandy sediments above the saturated levels, and the inverted resistivity values lie in the same range as the measured RCPT data. The resistive layer takes the main part of sections BET01 and BET02, whereas the underlying conductive layer appears more clearly on section BET04. The altitudes on the western part of the lines BET01 and BET02 are a little higher, and most of the investigated material is situated above the groundwater table. The resistivities in the underlying layer are smaller on line BET04, which could indicate that the groundwater salinity is higher there, but since the other lines were not investigated so deep in the aquifer, comparison is difficult. Some differences in the resistivity in the aquifer appear on the long line BEL01. The higher resistivities on lines BET01 and BET02 can reflect the locally thicker dry sediments above the aquifer, since the altitude is around 2 m more on these lines. The resistive body appearing at depth on line BEL01 around BET01 and BET02 can either be a 3D artefact due to the small relief or the actual presence of more resistive sediments at depth. It can also be due to the reduced salinity at that depth. The drillings at the intersections between these lines reach only 8 m depth, and the chloride content in the groundwater was measured only in the observation well.
The line BET03 (Fig. 6c) is different. An initial resistive layer above the groundwater table clearly appears only on the western part of the line. Around 10–20 m coordinates, the inverted resistivities values are between 100 and 180 Ω m, less than on the other sections, and the first superficial layer appears only faintly. It can be explained by the clay sediments that have been found at this location—one can expect a smaller resistivity contrast between saturated and non-saturated levels in such materials, and the transition is also usually less clear due to capillary rise. It is also possible that the salinity is more at this location, but there is no data yet available on this. Farther away on the line, the resistivities are high again, most probably because the geological material is different—only sands have been found at coordinate x=60 m.
When gathering all the sections as pictures with the commercial program Rockworks we get a very consistent view (Fig. 7). The surroundings of line BET03 stand out against the rest, probably due to the clay deposits more conductive than sands, and this might favour local salt accumulation by slowing down the groundwater flow. The more resistive parts at depth on the western parts of lines BET03, BET02 and BET01 could be explained by the presence of sands and gravels and/or by decreased salinity in the groundwater. There is unfortunately not enough data available for testing this hypothesis.
Relative changes observed in April 2002
The large dynamics of the inverted resistivity values make it difficult to see differences directly on the inverted sections, so we present sections of relative differences between April 2002 and October 2001, defined as:
where ρ1 and ρ2 are the inverted resistivity values from October 2001 and April 2002 respectively.
On all the lines (see Figs. 8, 9), we can observe a substantial increase of the resistivity in the first layer. This is probably due to the lower temperature in the ground after winter, possibly in combination with lower soil moisture content. Under this layer the resistivity has decreased in the saturated layer, and it could be imputed to salinity increase.
The relative interpreted differences in resistivity are very high. For instance the resistivity increase in the superficial layer implies a temperature drop of 20°C if we apply the law (3) cited above, hence a decrease in moisture content is needed to explain the increase in resistivity unless the ground were still partially frozen in April 2002. It may be possible that contrasts have been exaggerated by the time-lapse inversion procedure, but variations of the same range were observed in the apparent resistivity.
The line BET03 stands out once again among the others: On the eastern part of the line, the resistivity seems to increase also with depth, even if more moderately. It is possible that in the clayey sediments, the effect of salinity increase has been less pronounced or counterbalanced by temperature effects. Farther west on the line, the resistivity decreases under the groundwater table in the sandy sediments, like on the other sections.
On the southernmost part of line BEL01, the resistivity seems to increase again with depth. It could be an artefact of the inversion procedure or even a three-dimensional effect, but it could also be that the resistivity has increased in the sediments with depth. The salinity might not have increased, or at least not significantly in the groundwater under 10 m depth, since increased salinities have generally been observed in the area, concentrated at intermediate depths. This may have induced a different balance between temperature and salinity effects, resulting in decreasing resistivity at depth.
When plotting all the relative different sections together (Fig. 10), we again obtain a very consistent picture, which seems to indicate salinity increase with depth below sections BET01 and BET02, and below BET04. These sections do not, however, reach as deep as BEL01. The surroundings of line BET03 show an increase in resistivity that might be due to a different result of combined effects in the clayey sediments, possibly associated with less salinity increase.
Discussion and conclusions
The time-lapse results have shown clear resistivity changes in the underground at the Bergaåsen site before and after the salting season. They most probably can be attributed to salinity changes and as reflecting the hydrogeological situation. However, temperature effects are superimposed and it is likely that different geological materials exhibit different resistivity changes for the same salinity, saturation or temperature increase or decrease, so that more information would be needed for a more precise interpretation. Similar difficulties were experienced by e.g. Rein et al. (2004), and Aaltonen (2001) has noted large seasonal variations of resistivity in Swedish soils (68% of the mean value in sandy soils). However, she has mostly studied finer-grained soils, which, apart from a few exceptions, reach their highest resistivity in the autumn, and the fluctuations of the water table also play a role. Better knowledge of the properties of the different material’s or direct measurements of salinity and temperature in the ground would certainly be useful.
It would have been interesting to measure sections more times between autumn and spring, especially during alternating snow and cold episodes when more salt is spread, and during milder periods. We might have seen salt transport in progress and a general trend along the winter instead of the result of a whole season’s accumulation. Unfortunately, economic considerations precluded such monitoring.
Some questions remain concerning the interpreted resistivity variations observed. Their predominant cause is unclear and probably varies from one lithological formation to another; it is also possible that artefacts were created by the interpretation, hence variations exaggerated or inappropriately placed them. However, variations of the same range were observed in the apparent resistivity.
This study gives an example of the method’s applicability in the field, but it also clearly shows that comparison with relevant information of a different nature would be required to get the best of the geoelectrical imaging, especially in a complex lithology with relatively small salinity changes over a year.
Induced polarisation might constitute an interesting complementary method in such a case, since it might make it possible to distinguish between the effects of increased salinity and increased fine contents in resistivity decrease. However, it would have to overcome the high grounding resistances at the site, also sufficient depth has to be reached for the investigation, which is sometimes difficult.
References
Aaltonen J (2001) Seasonal resistivity variations in some different swedish soils. Eur J Environ Eng Geophys 6:33–45
Acworth I, Dasey G (2001) Electrical imaging of a tidal creek using combined land and underwater electrodes – an example from Hat Head, NSW, Australia. In: Proceedings of the 7th EEGS meeting, Birmingham, pp 192–193
Archie GE (1942) The electrical resistivity log as an aid in determining some reservoirs characteristics. Trans Am Inst Min Metal And Petr Eng 146:54–62
Barker RD (1990) Investigation of groundwater salinity by geophysical methods. In: Ward SH (ed) Geotechnical and environmental geophysics, vol II. Society of Exploration Geophysicists, Tulsa, OK, pp 201–211
Bruch H (2000) Bergaåsen vid Hallsjö, utredning om vägarnas och trafikens påverkan på grundvattnet, Mark&Vatten Ingeniörena AB, commissioned by the technical services of the Växjö District. (In Swedish)
Chambers JE, Loke MH, Ogilvy RD, Meldrum PI (2004) Noninvasive monitoring of DNAPL migration through a saturated porous medium using electrical impedance tomography. J Contam Hydrol 68:1–22
Chinh PD (2000) Electrical properties of sedimentary rocks having interconnected water-saturated pore spaces. Geophysics 65(4):1093–1097
Daniel CR, Campanella RG, Howie JA, Giacheti HL (2003) Specific depth cone resistivity measurements to determine soil engineering properties. J Environ Eng Geophys 8(1):15–22
Farquharson CG, Oldenburg DW (1998) Non-linear inversion using general measures of data misfit and model structure. Geophys J Int 134:213–227
Giao PH (2003) A comparative study of different electric imaging configurations in investigations of a fresh-saline water interface. In: Proceedings of the 9th EEGS meeting, Prague, pp 0–079
Loke MH (1996–2004) Manual for Res2Dinv, available at http://www.geoelectrical.com
Loke MH (1999) Time-lapse resistivity imaging inversion. In: Proceedings of the 5th EEGS meeting, Budapest, Hungary, 6–9 September, Em1
Loke MH, Acworth I, Dahlin T (2003) A comparison of smooth and blocky inversion methods in 3D electrical imaging surveys. Eplor Geophys 34(3):182–187
Mark&Vatten AB (2000) Technical report “Bergaåsen vid Hallsjö, utredning om vägarnas och trafikens påverkan på grundvattnet” (11 pages + maps, in Swedish)
Ogilvy R, Meldrum P, Chambers J, Williams G (2002) The use of 3D electrical resistivity tomography to characterise waste and leachate distribution within a closed landfill. Thriplow UK JEEG 7(1):11–18
Rein A, Hoffmann R, Dietrich P (2004) Influence of natural time-dependent variations of electrical conductivity on DC resistivity measurements. J Hydrol 285:215–232
Rosqvist H, Dahlin T, Fourie A, Röhrs L, Bengtsson A, Larsson M (2003) Mapping of leachate plumes at two landfill sites in South Africa using geoelectrical imaging techniques. In: Proceedings Sardinia 2003, 9th international waste management and landfill symposium, S. Margherita di Pula, Cagliari, Italy, 6–10 October 2003
Sen PN, Goode PA, Sibbit A (1988) Electrical conduction in clay bearing sandstones at low and high salinities. J Appl Phys 63(10):4832–4840
SEPA (1999) (Swedish Environmental Protection Agency) Environmental quality criteria groundwater, report 5051
Slater LD, Sandberg SK (2000) Resistivity and induced polarization monitoring of salt transport under natural hydraulic gradients. Geophysics 65(2):408–420
Slater L, Binley AM, Daily W, Johnson R (2000) Cross-hole electrical imaging of a controlled saline tracer injection. J Appl Geophys 44:85–102
Taylor S, Barker R (2002) Resistivity of partially saturated Triassic sandstone. Geophys Prospect 50:603–613
Thunqvist EL Johansson (2003) Estimating chloride concentration in surface water and groundwater due to deicing salt application. Ph. D. thesis, Kungl. Tekniska Högskola, Stockholm
Vägverket Konsult (2001) Väg E4 – Bergaåsen-vattenskydd. Teknisk utredning (April 2001), report No. 732063. (in Swedish), Vägverket region Sydöst, Jönköping
Ward (1990) Resistivity and induced polarization methods. In: Investigations in geophysics No. 5: Geotechnical and Environmental Geophysics, volume I: review and tutorial, Society of Exploration Geophysicists, pp 147–189
Waxman MH, Smits LJM (1968) Electrical conductivities in oil-bearing shaly sands. Trans Soc Pet Eng 243:107–115
Acknowledgements
We thank Scandiaconsult, the Road Administration of Sweden and Mark och Vatten Ingenjörerna AB for sharing information with us and allowing us to use their results. We would like to thank particularly Jörgen Brorsson (Ramböll-Scandiaconsult), Agne Gunnarson (Swedish Road Administration), Lars-Göran Svensson (BGV-Konsult) and Hans Bruch (Mark & Vatten Ingenjörerna AB). Anna-Karin Jönsson helped in gathering drilling information for the site. Virginie Leroux was partly financed by a European Commission Marie Curie grant, contract number N°EVK1-CT-2000-50004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leroux, V., Dahlin, T. Time-lapse resistivity investigations for imaging saltwater transport in glaciofluvial deposits. Environ Geol 49, 347–358 (2006). https://doi.org/10.1007/s00254-005-0070-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-005-0070-7