Abstract
Many national exposure programmes have been performed in tropical and subtropical climates during the last 50 years. However, ambitious programmes involving more than a few countries are scarce. In this paper a recently formed network of test sites is described involving 12 test sites in Asia (India, Vietnam, Thailand, Malaysia and China including Hong Kong) and four test sites in Africa (South Africa, Zambia and Zimbabwe). This effort is part of the 2001–2004 Swedish International Development Agency (SIDA) funded Programme on Regional Air Pollution in Developing Countries (RAPIDC). Corrosion attack after one (2002–2003) year of exposure (carbon steel, zinc, copper, limestone and paint coated steel) are presented together with environmental data (SO2, NO2, HNO3, O3, particles, amount and pH of precipitation, temperature and relative humidity) for all the test sites. The obtained corrosion values are substantially higher than expected for limestone, higher than expected for carbon steel and lower than expected for zinc compared to values calculated using the best available dose–response functions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atmospheric corrosion research in tropical and subtropical climates started around 1945 and results are available from several individual countries, for example Australia, Brazil, China, Cuba, India, Manila, New Zealand, Nigeria, Panama, Papua New Guinea, Philippine Islands, South Africa, Taiwan, Singapore and Vietnam (Tidblad, Mikhailov, & Kucera, 2000). However, ambitious programmes involving more than a few countries are limited to the MICAT project (Morcillo, Almeida, Rosales, et al., 1998) which includes 12 Ibero-American countries, Spain and Portugal, and the ISOCORRAG programme with more than 50 sites located in Europe, Argentina, Canada, Japan, New Zealand and USA (Knotkova, 1993). In Asia, the programme co-ordinated from Australia and involving 13 sites in Australia, Philippines, Thailand and Vietnam (Cole, 2000) and the programme co-ordinated from Japan and involving 23 sites in Japan, China, and South Korea (Maeda et al., 2001) should be mentioned. In Africa, the long-term study of Callaghan (1991) should not be left unnoticed. These programmes either have an environmental characterisation limited to SO2 as a main pollutant (Callaghan, 1991; Knotkova, 1993; Morcillo et al., 1998) or have relatively short exposure times (Cole, 2000; Maeda et al., 2001).
Partly in order to fill this gap the present project “RAPIDC/Corrosion” was initiated as a part of the 2001–2004 Swedish International Development Cooperation Agency (SIDA) funded Programme on Regional Air Pollution in Developing Countries (RAPIDC). The Programme was managed on SIDA’s behalf by the Stockholm Environment Institute (SEI). The corrosion project was co-ordinated by the Swedish Corrosion Institute (SCI). The objective of the present paper is to describe the corrosion project and to give results and conclusions after 1 year of exposure. Results will in the future be available after 2 and 4 years of exposure. Since 1 year of exposure is a relatively short time it should be stressed that the conclusions presented in this paper are preliminary in anticipation of the results after 2 and 4 years.
Another important aspect of the project, which is not described here, was transfer of knowledge on establishing test sites, exposing specimens, collection of environmental data, and methodology of developing dose–response functions using modelling and statistical treatment.
2 Materials and Methods
2.1 Rack and Test Sites
Each site was maintained by a dedicated partner who was responsible for the safety of the rack, the exposure/withdrawal of passive samplers, the collection of environmental data and the withdrawal of corrosion specimens. After exposure, the samples were returned to the Swedish Corrosion Institute for evaluation of corrosion attack. Table 1 shows a list of test sites including the responsible organisations and starting date of exposure. The network of test sites consists of six partners (12 sites) in Asia and three partners (4 sites) in Africa.
At each test site a rack was erected supporting the materials samples and passive samplers (Fig. 1). The rack should preferably be situated in an open space, preferably on a roof, and it contains three distinct elements: (1) flat metal/painted samples (a–d) and their support consisting of a wooden frame; (2) stone samples and their support consisting of a carousel (e); (3) passive samplers and their support and sheltering consisting of two metal discs (f).
2.2 Characterisation of the Environment
Passive sampling was performed on all sites for the gaseous pollutants SO2, NO2, O3 and HNO3 (Ferm, De Santis, & Varotsos, 2005), and for particulate matter (Ferm, Watt, O’Hanlon, De Santis, & Varotsos, 2006). Sampling was performed on a bi-monthly basis i.e., samplers were exchanged each second month. The total sampling period was one year making in total six bi-monthly sampling periods. The starting date of the first sampling period varied from site to site and coincided with the starting date of the exposure of specimens given in Table 1. All exposure periods for passive samplers were consecutive so that the end of a sampling period marked the start of a new sampling period.
Complementary data on temperature, relative humidity, amount of rain and its pH were collected by the partners at a nearby environmental station. These data were reported to the Swedish Corrosion Institute on a monthly basis.
2.3 Sample Preparation and Evaluation of Corrosion Attack
For each of the materials and exposure periods, a set of three identical samples was prepared and exposed and these are referred to as the ‘triplicates.’ At each site a total of nine samples were exposed in the summer of 2002 for each material and this means that there are three sets of triplicates and that in total the samples are sufficient for three exposure periods. At present date withdrawals have been made after 1 and 2 years of exposure and a withdrawal after 4 years of exposure is planned in the summer of 2006. The samples exposed for 2 years are currently evaluated.
All flat samples, carbon steel (Dc 04, SS – EN 10 130), zinc (Z1 – DIN EN 1179), copper (Cu DHP, SS 5015) and painted steel were cut to dimensions 100 × 150 mm2 as suggested by ISO 9226. The thickness of all flat samples was 1 mm except for steel, which had a thickness of 2 mm. Carbon steel, zinc and copper were degreased and weighed prior to exposure. Steel was painted with two layers of alkyd (90 μm): (1) Conseal Primer 50 μm, fast drying alkyd based primer; (2) Ultra Topcoat 40 μm, quick drying and glossy acrylic modified alkyd topcoat and then edge protected using Vinyguard Silvergrey 88 from Jotun, Norway.
After exposure the visual impression of all flat samples was recorded by photography. The corrosion attack of the metal samples was evaluated with 10 min. consecutive pickling using Clarkes solution; 20 g Sb2O3 and 60 g SnCl2x2H2O and 1,000 ml concentrated HCl (ρ = 1.19 g ml−1) for steel, 250 g glycine (NH2CH2COOH) and distilled water to make 1,000 ml, saturated solution for zinc and 50 g amidosulfonic acid (sulfamic acid) and distilled water to make 1,000 ml for copper. Painted steel was evaluated by visual examination of the spread of corrosion attack in both directions from the 1 mm cut but expressed as the average spread in one direction following ASTM D 1654-79a.
Portland limestone specimens of dimensions 50 × 50 × 8 mm3 were obtained from the Building Research Establishment Ltd, United Kingdom, where also the corrosion attack was evaluated as mass change during exposure. The mass change was then recalculated to surface recession.
3 Results and Discussion
As mentioned in the introduction, 1 year of exposure is a relatively short time and therefore it is not worthwhile at this stage to perform a detailed statistical analysis and development of dose–response functions. This analysis will be performed when data after 2 and 4 years of exposure are available. The focus is instead on presenting the data set and its characteristics and to make an estimate on the magnitude of the effects compared to those predicted by existing dose–response functions.
3.1 Environmental Data
The environmental data are given in Table 2. Compared to European values the values in Table 2 are similar except for temperature, amount of precipitation and SO2 concentration, where the values are generally higher.
The SO2 values are lower than 20 μg m−3 except for four extreme sites: Phrapradaeng, Chongqing, Tie Shan Ping and Kitwe. Worth noting is that the site Tie Shan Ping is a rural site situated close to Chongqing. The site Kitwe is located in the copper belt area in the northern part of Zambia. Other extreme sites worth mentioning are those in Malaysia: Kuala Lumpur and Tanah Rata. Kuala Lumpur has the highest HNO3 values in the network probably due to a combination of the high NO2 emissions, the high temperature, and the humid conditions. Tanah Rata is the cleanest site in the network. It has the same precipitation level as Kuala Lumpur but a much lower temperature and is situated in the Cameron highlands.
3.2 Corrosion Data
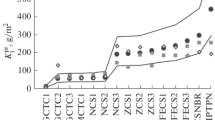
The corrosion data are given in Table 3. The analysis of painted steel and limestone are non-destructive while the analysis (pickling) of the metals are destructive and this is the reason why one of each of the triplicate metal samples (no. 3) have been saved for further analysis by other methods. As can be seen from Table 3 the difference between no. 1 and no. 2 for the metals is generally very low, in the range of 5%.
Regarding zinc one should note the slightly higher corrosion values for zinc at the Tie Shan Ping test site compared to Chongqing, which has a much higher SO2 value, and also the very high values observed in Kitwe. For limestone, the high values in Johannesburg deserves to be mentioned, considering the pollution situation.
3.3 Correlation Between Environmental and Corrosion Data
Table 4 shows the rank correlations between the corrosion and selected environmental parameters. The environmental parameter that has the highest correlation to most of the parameters is the amount of \( \rm SO^{{2 - }}_{4} \) analysed from the particulate deposited matter. It should be mentioned that \( \rm SO^{{2 - }}_{4} \) is not necessarily deposited as sulphate but may also be the reaction product of other particulate deposits, probably CaCO3, and gaseous SO2.
Other parameters with high correlation are SO2 for carbon steel, pH for zinc and SO2, pH and HNO3 for limestone. Nitric acid is a highly corrosive gas, even compared to SO2, as has been shown in recent laboratory experiments for copper (Samie, Tidblad, Kucera, & Leygraf, 2005).
3.4 Comparison of Observed and Predicted Data
Since it is not possible to develop dose–response functions based on the data presented in Table 2 and 3 due to the short exposure time it is instead worthwhile to use existing dose–response functions and compare the predicted values with the experimental values. Functions have recently been developed for the multi-pollutant situation within the EU 5FP project Model for multi-pollutant impact and assessment of threshold levels for cultural heritage (MULTI-ASSESS). These functions are also presented in a paper within this special volume of Water, Air and Soil Pollution dedicated to the Acid Rain 2005 conference (Kucera et. al., 2005). However, due to the relatively high measured SO2 levels it is more correct to use functions for the SO2-dominating situation developed within the International co-operative programme on effects on materials including historic and cultural monuments (ICP Materials). Functions from this program are available for zinc, copper and limestone but not for carbon steel (Tidblad et al., 2001),
where ML is the mass loss after 1 year of exposure in g/m2, R is the surface recession after 1 year of exposure in μm, [SO2] is the SO2 concentration in μg/m3, [O3] is the O3 concentration in μg/m3, Rh is the relative humidity in %, T is the temperature in °C, Rain is the amount of precipitation in mm/year and [H+] is the hydrogen ion concentration in precipitation in mg/l. For carbon steel a function developed within ISO TC 156 Corrosion of metals and alloys has been used (Mikhailov, Tidblad, & Kucera, 2004)
where D Cl is the chloride deposition in mg m−2 day−1. The results of applying these functions are shown in Table 5 together with the observed values. The values are given in a common unit (μm) for all materials by using the density of carbon steel (7.8 g cm−3), zinc (7.14 g cm−3) and copper (8.93 g cm−3). For carbon steel, copper and limestone the predicted values are lower but for zinc the observed values are lower. One explanation of the low zinc values can be that this material is particularly sensitive to the starting conditions of the exposure. It is worth noting the substantial difference between observed and predicted values for Portland limestone. For copper the comparison is misleading since the 1-year values cannot be predicted by the proposed equation (R 2 = 0.27). Therefore, the 2- and 4-year results will be very valuable in the assessment of corrosion rates in the RAPIDC network compared to results from other networks in Europe.
4 Conclusions
A network of organisations and corrosion test sites have been formed with six partners (12 sites) in Asia and three partners (4 sites) in Africa and an exposure programme was started in May–August 2002. Complete results of an extensive environmental characterisation and corrosion data of painted steel, carbon steel, zinc, copper and limestone after one year of exposure in the network have been presented and the conclusions below are preliminary in anticipation of results after 2 and 4 years of exposure. SO2 pollution is the most decisive factor but in addition a correlation to pH has been found for zinc and limestone and a correlation to HNO3 has been found for limestone. Based on best available dose–response functions the corrosion values are higher than expected except for zinc where the values are lower than expected. For limestone the values are substantially higher than expected. For painted steel and copper 1 year of exposure is too short and a comparison can be misleading. Therefore, future studies will include a complete evaluation of the 2- and 4-year results that will be very valuable in the assessment of corrosion rates in the RAPIDC network compared to results from other networks in Europe.
References
Callaghan, B. G. (1991). Atmospheric corrosion testing in southern Africa: results of a twenty year national exposure programme. Division of Materials Science and Technology, GAcsir 450H6025*9101, Scientia Publishers, CSIR, pp. 75.
Cole, I. S. (2000). Mechanisms of atmospheric corrosion in tropical environments. ASTM STP 1399. In S. W. Dean, G. Hernandez-Duque Delgadillo & J. B. Bushman (Eds.), American Society for Testing and Materials. West Conshohocken, PA.
Ferm, M., De Santis, F., & Varotsos, C. (2005). Nitric acid measurements in connection with corrosion studies. Atmospheric Environment, 39, 6664–6672.
Ferm, M., Watt, J., O’Hanlon, S., De Santis, F., & Varotsos, C. (2006). Deposition Measurement of Particulate Matter in connection with Corrosion Studies. Analytical and Bioanalytical Chemistry. (in press)
Knotkova, D. (1993). Atmospheric corrosivity classification. Results of the international testing program ISOCORRAG,” corrosion control for low-cost reliability. In: 12th international corrosion congress, vol. 2 (pp. 561–568). Houston, Texas: Progress Industries Plant Operations, NACE International.
Kucera, V., Tidblad, J., Kreislova, K., Knotkova, D., Faller, M., Reiss, D., et al., (2005). The UN/ECE ICP materials multi-pollutant exposure on effects on materials including historic and cultural monuments. Acid Rain. (in press)
Maeda, Y., Moriocka, J., Tsujino, Y., Satoh, Y., Xiaodan, Z., Mizoguchi, T., et al. (2001). Materials damage caused by acidic air pollution in East Asia. Water, Air and Soil Pollution, 130, 141–150.
Mikhailov, A. A., Tidblad, J., & Kucera, V. (2004). The classification system of ISO 9223 standard and the dose–response functions assessing the corrosivity of outdoor atmospheres. Protection of Metals, 40(6), 541–550.
Morcillo, M., Almeida, E. M., Rosales, B. M., et al. (Eds.) (1998). Functiones de Dano (Dosis/Respuesta) de la Corrosion Atmospherica en Iberoamerica, Corrosion y Proteccion de Metales en las Atmosferas de Iberoamerica, Programma CYTED, Madrid, Spain, pp. 629–660.
Samie, F., Tidblad, J., Kucera, V., & Leygraf, C. (2005). Atmospheric corrosion effects of HNO3. Method development and initial results on laboratory exposed copper. Atmospheric Environment, 39/38, 7362–7373.
Tidblad, J., Kucera, V., Mikhailov, A. A., Henriksen, J., Kreislova, K., Yates, T., et al. (2001). UN/ECE ICP Materials. Dose–response functions on dry and wet deposition effects after 8 years of exposure. Water, Air and Soil Pollution, 130, 1457–1462.
Tidblad, J. Mikhailov, A., & Kucera, V. (2000). Acid deposition effects on materials in subtropical and tropical climates. Data compilation and temperate climate comparison. SCI Report 2000:8E, Swedish Corrosion Institute, Stockholm, Sweden.
Acknowledgement
The Swedish International Development Cooperation Agency (SIDA) is acknowledged for financial support. We are also grateful to Martin Ferm of the IVL Swedish Environmental Research Institute for valuable discussions about the results of the passive samplers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tidblad, J., Kucera, V., Samie, F. et al. Exposure Programme on Atmospheric Corrosion Effects of Acidifying Pollutants in Tropical and Subtropical Climates. Water Air Soil Pollut: Focus 7, 241–247 (2007). https://doi.org/10.1007/s11267-006-9078-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11267-006-9078-6