Abstract
Herpesviruses are enveloped DNA viruses that infect vertebrate cells. Their high potential cloning capacity and the lifelong persistence of their genomes in various host cells make them attractive platforms for vector-based therapy. In this review, we would like to highlight recent advances of three major areas of herpesvirus vector development and application: (i) oncolytic therapy, (ii) recombinant vaccines, and (iii) large capacity gene transfer vehicles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herpesviruses (HVs) are enveloped large double-stranded DNA viruses infecting vertebrate hosts with genome sizes ranging from 150 to 240 kilobase pairs (kbp). A common feature of HVs is their lifelong latency in infected hosts, associated with the maintenance of their genome in special host cells without lytic replication [1]. Their large potential capacity and long-term genome persistence in vivo make HVs attractive platforms for vector development [2]. HVs can be divided into three subfamilies based on their genetic and biological characteristics. Alpha-herpesviruses, for example herpes simplex virus 1 (HSV1), are characterized by a fast replication cycle and latency in neurons. Beta-herpesviruses, such as cytomegaloviruses (CMVs), show slow replication and latency in macrophages and endothelial cells. Gamma-herpesviruses, such as Epstein-Barr virus (EBV), also replicate slowly, induce cell transformation, and establish latency in lymphocytes [1]. HV vectors can be divided in three major groups based on their applications: (i) oncolytic viruses (OVs), (ii) vectors for recombinant vaccines, and (iii) large capacity gene transfer vehicles. Oncolytic HVs are replication-competent HSV1-derived recombinant viruses targeted to tumor cells and already in clinical use (e.g., T-VEC [3, 4]). HV-based recombinant vaccines are either replication-competent or helper-dependent vectors and currently under preclinical investigation (e.g., CMV-based HIV vaccines [5]). For gene transfer applications, mainly helper-dependent vectors based on HSV1 amplicon technology are used in experimental settings (e.g., neurobiological applications [6]).
Genetic engineering of HV vectors
The first strategy to construct recombinant HVs was based on sub-cloned viral genes, which after mutation and functional analysis, could be reintroduced into the viral genome. The HSV genome was the first to be modified in the genomic context [7, 8] followed by the establishment of the same technique for CMVs [9, 10]. In principle, a co-transfected marker gene flanked by viral sequences is introduced into the viral genome upon infection of a host cell exploiting its homologous recombination machinery. The use of selectable markers is mandatory for this approach, since recombination, which leads to the desired genetic modification, is a rare event. This technology is still in use in modification of alpha-herpesviruses, but most frequently, construction of recombinant HVs, especially for beta- and gamma-herpesviruses, nowadays is based on the bacterial artificial chromosome (BAC) technology.
Stable maintenance of very large foreign DNA was reported using a vector based on the fertility factor (F-factor) and coined BACs [11]. Due to the single copy maintenance of these amplicons, BACs show outstanding sequence integrity in appropriate E.coli strains, regardless of their size and embedded repeat regions [11,12,13]. The murine CMV was the first HV to be cloned as infectious BAC [14]. By now, all HV model genomes were cloned as BACs using essentially the same technology initially described for CMV (for review see [15]). The construction of a HV BAC starts with homologous recombination in infected cells (using a procedure similar to that depicted in Fig. 1a). Here, the plasmid sequences required for the BAC maintenance in E.coli, are introduced into the double-stranded genomes of HVs that are circularized after entering the host nucleus. For insertion into the viral genome, the plasmid contains sequences homologous to the selected viral insertion site. The recombinants are propagated further to amplify the vector containing genomes. Then the circular replication intermediates of the vector containing HV genomes can be isolated from infected host cells and transferred to E.coli where they are exclusively maintained as circular BACs. To regenerate infectious virus, the HV BAC DNA is isolated from E.coli and transfected back into permissive host cells, where HV replication can start on the circular intermediate.
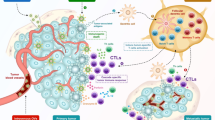
Herpesvirus (HV) vectors can be sorted in three groups. The replication-competent HV vectors maintain replication capacity in certain cells and can be propagated either by homologous recombination in the host cell (a) or by BAC technology (b). These mutations (x) can determine cell tropism and tumor targeting or affect immunomodulation. c The replication defective HV vectors can only replicate in special cell lines (comp) complementing the affected viral gene (\(\otimes\)). They are mainly propagated by BAC technology and used for expressing transgenes and antigens (gray arrow). d The helper-dependent HV vectors carrying only the packaging signal (ψ) and origin of replication (gray circle) require a helper virus for propagation. The replication-competent HV vectors are utilized mainly for oncolytic approaches; the amplicon vectors dominate the HV-based gene transfer systems, and as platform for recombinant HV vaccines, all three classes are under investigation
Once in E.coli, the HV BAC is amenable to the well-developed genetic engineering technologies applicable in bacteria. Virtually all genetic changes including deletions, modifications, and insertions ranging from one base pair to many kbp can be constructed by BAC technology (for review see [15, 16]). The most popular method to modify HV genomes in E.coli is the BAC recombineering. Recombineering is a two-step mutagenesis methodology based on the lambda phage red recombinase which allows for exact genetic manipulation. First, the mutation along with a selectable marker is introduced into the desired locus of the HV BAC. Next, the selectable marker is replaced by the mutated gene resulting in a precise mutation seamlessly introduced at the site of interest (for general overview see [17], for HV applications see [18]).
Depending on their replication capacity, HV vectors can be sorted in three clusters (Fig. 1). The replication-competent HV vectors maintain replication capacity in certain cell lines without specific complementation of viral genes (Fig. 1a, b). However, their capacity to replicate in most cell types, which are permissive for the wild-type viruses, is frequently limited either in vitro and/or in vivo. Replication-competent recombinant HVs can either be generated by homologous recombination in infected cells (Fig. 1a) or by BAC technology (Fig. 1b). The replication defective HV vectors can only replicate in special cell lines complementing the affected viral gene (Fig. 1c). These types of HV vectors lost their replication capacity in normal cells and frequently were attenuated even in complementing cell lines, and therefore they are constructed preferentially by BAC technology. The helper-dependent HV vectors (also called amplicon vectors) which avoid all viral protein coding regions, require a helper virus for their propagation (Fig. 1d). Besides the transgenes, they only carry cis elements for amplification by the viral DNA replication machinery and packaging. The replication-competent HV vectors are utilized mainly for oncolytic approaches; the amplicon vectors dominate the HV-based gene transfer systems, and as platform for recombinant HV vaccines, all three classes are under investigation.
Applications
Oncolytic recombinant HVs
Most recently, the first genetically engineered oncolytic virus (OV), the herpes simplex virus type 1 (HSV1) Imlygic® talimogene laherparepvec (T-VEC), was approved by the Food and Drug Administration (FDA) to treat advanced melanoma marking a breakthrough in oncolytic therapy [3, 4]. Several features of HSV1 make it a highly attractive platform for development of oncolytic therapies. HSV1 replication is characterized by a broad cell tropism and efficient viral propagation that naturally ceases in cytolysis [19]. It is a highly prevalent human pathogen causing a self-limited disease in immune-competent individuals. Specific and effective antiviral therapy has been available for decades [20] and can be administered either locally or systemically. Basic research provided extensive knowledge on its replication and host interaction. Its fusion machinery can be manipulated for detargeting and retargeting of viruses [21]. Genetic engineering of HSV1 is available since the early 80s, and now BAC-based technologies make it rapid and precise resulting in stable genomes [22,23,24]. Roughly 30 kbp of the 152 kbp HSV1 genome are nonessential for viral replication and thus provide ample capacity and flexibility to functionalize the virus by heterologous genes. Finally, infectious virions can be generated in high titres [25]. Since recent reviews provide comprehensive overviews of HSV1 OVs [21, 26, 27], we will focus on a few aspects relevant for future developments.
Safety of OVs based on HSV1 was the major issue during their initial engineering. To prevent systemic infection or spread of this neurotropic virus to the brain, several viral genes were mutated or deleted (for review see [26]). HSV1 genes with functions in nucleotide metabolism and DNA synthesis were mutated for selective replication in tumor cells [26]. These include UL39 encoding the large subunit of the ribonucleotide reductase (also called the infected cell protein (ICP) 6), and UL23 encoding the viral thymidine kinase making the OV insensitive to the antiviral prodrug acyclovir. The HSV1 gene RL1 encodes the neurovirulence factor ICP34.5 required for virus replication in mouse brain cells ([28] and references therein). Deletion of both copies of the RL1 gene supports tumor-specific replication [3]. In general, while effective in limiting viral replication to tumor cells, most gene deletions resulted in virus attenuation both in normal and in tumor cells. Clinical trials show that T-VEC is effective for the treatment of melanoma accompanied by mild to moderate side effects [3, 4]. Overall, however, safety of HSV1 OVs was achieved at the expense of virus potency.
More recent engineering strategies aim at developing highly tumor-selective OVs while at the same time retaining their full oncolytic potential. HSV1 virions gain access to the host cells by a multistep process involving a set of viral transmembrane proteins [29, 30]. The essential glycoprotein (g) D interacts with one of its host receptors nectin-1, herpesvirus entry mediator (HVEM), or modified heparan sulfates, thereby determining HSV1 tropism to neuronal and epithelial cells. The conserved gH/gL and gB together mediate fusion of the virion envelope with the host membrane. Receptor binding of gD results in a conformational change thought to initiate a cascade of intermolecular signaling to gH/gL subsequently transferred to gB. Several strategies have been exploited to detarget HSV1 virions from their natural receptors and retarget them to cancer-specific surface proteins such as HER-2 (human epidermal growth factor receptor 2) overexpressed in breast, ovarian, and other cancers or the IL-13 Receptor 2α expressed in glioblastoma (for review see [21]). Heterologous ligands like HER-2-specific single-chain fragment variable (scFv) antibodies were successfully introduced into the N-terminus of gD coupled with additional engineering of the natural targeting sites. Therefore, retargeting of HSV1 to several carcinomas that overexpress HER-2 was achieved. Recent evidence indicates that gH and gB are also amenable to retargeting strategies challenging the current view of the cascade-like signaling during virion entry [31, 32]. HER-2-specific scFv engineered into the gH enabled HER-2-specific targeting and cis-activation of the fusion event [31]. Activation occurs in the presence of a mutant gD deleted for its nectin-1/HVEM binding site. Thus, oncolytic HSV1 could potentially be retargeted to more than one cancer receptor, e.g., by engineering of gD AND gH, thereby addressing tumors with low-level expression of HER-2, or that potentially develop resistance to HER-2-based targeting. Most importantly, gH-based retargeting maintained wild-type lytic potency of the therapeutic virus [31].
Virus infections are counterbalanced by a variety of innate immune mechanisms. Initially, the innate immunity, which involves the induction of an antiviral state in the infected cell and production of a pro-inflammatory milieu limiting the spread of the pathogen, was viewed as a barrier to efficient oncolytic virus therapy [33, 34]. On the other hand, it became clear that OV therapy has the potential to synergize with the adaptive immune responses elicited at the tumor site in order to result in effective tumor elimination.
Several HSV1 proteins including ICP47 and UL49.5 inhibit the cellular peptide transporter TAP to prevent the presentation of antigenic peptides by the MHC class I pathway and consequently evade the T cell response. To enhance the tumor-specific immune response, US12/ICP47 was deleted from tumor-targeted HSV1 [3]. In the absence of TAP inhibitors, peptides presented in HSV1-infected tumor cells are recognized more efficiently and should activate not only virus- but also tumor-specific effector T cells [33, 35]. However, early evasion of innate immunity by OVs is necessary for robust replication in order to mount a successful oncolysis. This allows for sufficient release of tumor antigens to elicit an effective anti-tumoral immune attack.
OV-induced inflammation of the tumor microenvironment is accompanied by the release of various immune-stimulating cytokines. To enhance the expression and concentration of immune-stimulating host factors at the site of OV activity, cytokine encoding transgenes were inserted into OVs [26, 34, 36]. Recombinant OV-driven cytokine expression increased the recruitment of immune cells to the infected tumors significantly improving their therapeutic efficacy [26]. T-VEC, the first oncolytic virus approved by the FDA, encodes the granulocyte-macrophage colony-stimulating factor (GM-CSF) [3]. Phase III clinical trials using T-VEC showed anti-tumor effects upon treatment of melanoma [4, 37], suggesting that GM-CSF contributes to the therapeutic outcome while detailed analysis is lacking. Following injection of melanoma lesions, distant uninjected lesions were decreased in size supporting a systemic immunotherapeutic effect of T-VEC [38].
In many cases, tumors represent a microenvironment that escapes the attack and control by the immune system. Tumors may dampen their response to immune effectors, prevent the presentation of cancer antigens, and inhibit invading immune effector cells [39]. Tumors achieve resistance to the immune system by co-opting immune checkpoints that play an essential role in establishing and maintaining self-tolerance. Immune checkpoint inhibitors have entered the clinical stage with very promising results. Full success of immune checkpoint inhibitors, however, requires a pre-existing anti-tumor immune response in the patient. Thus, OVs seem to be the ideal partner of immune checkpoint inhibitors: OV infection leads to release of tumor antigens and attraction of immune cells to the tumor environment that in the presence of immune checkpoint inhibitors can be unleashed to full activity [37]. Most importantly, tumor-specific antigens released by OVs have the potential to induce a specific immune response by the patient’s immune system. This way, OV-driven tumor vaccination may be reached without previous knowledge of the tumor genetics. Promising clinical results were gained for treatment of melanoma by combining T-VEC with different immune checkpoint inhibitors [37]. Co-application of T-VEC with the immune checkpoint inhibitor ipilimumab appeared to have greater efficacy in advanced melanoma than either therapy alone [40]. Future strategies will likely go beyond the mere co-application of these two treatment modalities by “arming” OVs to deliver immune checkpoint inhibitors to the site of virus replication and oncolysis [4, 36, 41].
T-VEC and its approval by the FDA set the stage for the broad application of oncolytic immunotherapy. Meanwhile more potent and highly tumor-specific HSV1 OVs are in development supported by modular BAC engineering platforms. High-throughput screening approaches [42] will likely reveal novel strategies to enhance OV selectivity, potency, immunogenicity, and tumor penetration aiming at systemic and long-lasting therapeutic effects.
HV-based recombinant vaccine platforms: a new type of immunization
Effective vaccines have to stimulate the adaptive immunity of an individual for its long-lasting protection against a wild-type pathogen without the risk of an infectious disease. Therefore, standard procedures in vaccinology are either the application of immunogenic subunits or of attenuated or inactivated pathogens. However, vaccines based on these procedures are not always protective. This induced approaches based on rational design of recombinant vaccine vectors that present heterologous antigens. For development of recombinant HV vaccine platforms, the most frequently used technology applies replication-competent vectors, which are genetically attenuated but able to initiate limited replication. Alternatively, helper-dependent vectors which can only initiate productive infection in the presence of trans-complementation are used. Here, the antigen is inserted into the HV vector as a separate transcription unit, normally leading to overexpression of the heterologous antigen after vector entry.
In the last decades, an impressive amount of work was published on different approaches to generate HV-based recombinant vaccines (for recent review see [43,44,45]). Therefore, here, we concentrate on current developments, which reshaped our expectations about recombinant vaccines in general. Recombinant vaccines are currently designed to induce immune responses which mimic the natural immunity, because it is well accepted that convalescent infections induce the best immunity. This however may not hold true, if recombinant CMVs are used as vaccine vectors: Replication-competent CMV vectors can induce an immune response which is both quantitatively and qualitatively more powerful than the natural infection by the pathogen of interest. First, it was shown using recombinant murine CMV expressing nucleoprotein (NP) of influenza A virus that in contrast to the influenza virus infection itself or expression of the NP by a recombinant vaccinia vector, the recombinant MCMV will induce a relatively low T cell response. However, the T cell memory, instead of shrinking as it is usual for the other vaccinations, expands after applying the antigen in the context of MCMV [46]. This CMV-induced memory inflation is due to the change in antigen presentation induced by the viral immune evasins and now could be transferred to other vector platforms too ([47]; for recent review see [48]). Secondly, recent work based on the rhesus CMV (rhCMV) model showed that the cellular immune response induced by special rhCMV vectors differs also qualitatively from the normal one. Recombinant rhCMV expressing simian immunodeficiency virus (SIV) antigens induced a protective immunity against an SIV challenge with unusually good efficacy [5]. Detailed analysis of the T cell response upon vaccination with recombinant rhCMV vectors revealed the induction of a broad range of T cells specific for normally subdominant epitopes. This was in sharp contrast to the normal immune response or antigen delivery with other vectors where fewer immunodominant epitopes were recognized. Moreover, the induced T cells did not match the classical restriction rules: both MHC class I or II epitopes were recognized by CD8 T cells, which normally only recognize class I restricted epitopes [5, 49]. Interestingly, this unusual immune response required a special set of mutations in the vector backbone, influencing cell tropism and antigen presentation. CMVs are thought to be highly species-specific in vivo. Therefore, one should expect that CMV vectors may not work as efficiently in other species due to the well-tuned species-specific interactions, which are required for immune modulation. However, it may not apply to infection of closely related species as it was shown that a rhCMV-derived vector, after repair of some of its genetic defects, was able to mount a cellular immune response in Cynomolgus monkeys [50]. This may allow for rational design of recombinant CMVs, which can be used in cross-primate applications.
Besides its antigen-specific immunity, CMV is also able to induce natural killer cell-mediated cross-protection against other pathogens [51] which can be utilized for immunization against hard-to-target pathogens [52]. Shaping the NK response by recombinant CMV vectors can also improve the adaptive cellular immune response and presents a new approach for rational design of CMV-based recombinant vaccines [53].
EBV-transformed B cells can efficiently induce virus-specific T cells in vitro. Based on these observations, a replication-incompetent EBV-based vector platform was developed to deliver heterologous antigens in order to induce specific T cells in vitro for immune monitoring and cell-based immunotherapy. This EBV vector system consists, on one hand, of a packaging cell line carrying a modified EBV BAC, which encodes all genetic information for production of EBV particles, while the genome cannot be encapsidated. On the other hand, a transfer vector (called mini-EBV) is required, which lacks more than half of the EBV genome rendering it incompetent for lytic replication but capable of B cell transformation [54]. This system was efficiently applied to generate HCMV-specific T cell clones including a large variety of previously unknown specificities [54,55,56]. A newer generation of this vector platform was also reported, which lacked transforming activity, but retained its unparalleled B cell transducing capacity [57].
Gene transfer applications
HV vectors are special among viral vectors because their potential transgene capacity (>100 kbp) allows for delivery of complete genomic genes or even loci. The most advanced high-capacity HV vector systems are based on the HSV1 amplicon technology. Besides their exceptional capacity, amplicon vector particles induce minimal toxicity upon transduction due to the lack of viral genes operating in the target cells. In addition, unlike other viral vectors, HSV1-based amplicons can target a wide range of cell types and cell lines in vitro. In vivo, in gene therapy settings, HSV1 amplicons are especially useful for gene transfer to neurons and other epithelial cells, which are naturally infected by the wild-type virus (for recent review see [6, 44]).
Transgenes are provided in high-capacity BAC vectors equipped with amplicon sequences called infectious BAC (iBAC) and packaged into HSV1 virus-like particles by the amplicon technology [58]. Most recently, using the iBAC technology, the first comprehensive human genomic amplicon vector library was generated by packaging a complete genomic BAC library into an HSV1 amplicon. This resulted in an amplicon preparation carrying the representative set of genomic genes [59]. After transduction of this library to target cells with specific functional defects, the library can be screened for clones with induced phenotypic changes.
The capacity and safety of the amplicon vectors allowed the design of a new vaccine production strategy. All genetic information, which is required for formation of rotavirus virus-like particles (RVLP) can be integrated into an HSV1-based amplicon vector. Then, instead of applying the required multiple expression plasmids with co-transfections, VPLs can be produced after transduction of a single dose of the recombinant amplicon vector [60]. This way, complex subunit vaccines could be produced with simple and efficient protocols.
References
A.J. Davison, Overview of classification. In Human Herpesviruses: Biology, Therapy and Immunoprophylaxis, ed. by A. Arvin, G. Campadelli-Fiume, E. Mocarski, P. S. Moore, B. Roizman, R. Whitley, et al. (Cambridge University Press; 2007)
C.E. Thomas, A. Ehrhardt, M.A. Kay, Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 4(5), 346–358 (2003). doi:10.1038/nrg1066.
B.L. Liu, M. Robinson, Z.Q. Han, R.H. Branston, C. English, P. Reay et al., ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 10(4), 292–303 (2003). doi:10.1038/sj.gt.3301885.
R.H. Andtbacka, H.L. Kaufman, F. Collichio, T. Amatruda, N. Senzer, J. Chesney et al., Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33(25), 2780–2788 (2015). doi:10.1200/JCO.2014.58.3377.
S.G. Hansen, M. Piatak Jr., A.B. Ventura, C.M. Hughes, R.M. Gilbride, J.C. Ford et al., Immune clearance of highly pathogenic SIV infection. Nature 502(7469), 100–104 (2013). doi:10.1038/nature12519.
D. Jerusalinsky, M.V. Baez, A.L. Epstein, Herpes simplex virus type 1-based amplicon vectors for fundamental research in neurosciences and gene therapy of neurological diseases. J. Physiol. Paris 106(1–2), 2–11 (2012). doi:10.1016/j.jphysparis.2011.11.003.
E.S. Mocarski, L.E. Post, B. Roizman, Molecular engineering of the herpes simplex virus genome: insertion of a second L-S junction into the genome causes additional genome inversions. Cell 22(1 Pt 1), 243–255 (1980).
J.R. Smiley, Construction in vitro and rescue of a thymidine kinase-deficient deletion mutation of herpes simplex virus. Nature 285(5763), 333–335 (1980).
R.R. Spaete, E.S. Mocarski, Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc. Nat. Acad. Sci. USA 84(20), 7213–7217 (1987)
W.C. Manning, E.S. Mocarski, Insertional mutagenesis of the murine cytomegalovirus genome: one prominent alpha gene (ie2) is dispensable for growth. Virology 167(2), 477–484 (1988)
H. Shizuya, B. Birren, U.J. Kim, V. Mancino, T. Slepak, Y. Tachiiri et al., Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc. Nat. Acad. Sci. USA 89(18), 8794–8797 (1992)
Q. Tao, H.B. Zhang, Cloning and stable maintenance of DNA fragments over 300 kb in Escherichia coli with conventional plasmid-based vectors. Nucl. Acids Res. 26(21), 4901–4909 (1998)
G.A. Smith, L.W. Enquist, Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. J. Virol 73(8), 6405–6414 (1999)
M. Messerle, I. Crnkovic, W. Hammerschmidt, H. Ziegler, U.H. Koszinowski, Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc. Nat. Acad. Sci. USA 94(26), 14759–14763 (1997)
W. Brune, M. Messerle, U.H. Koszinowski, Forward with BACs: new tools for herpesvirus genomics. Trends Genet. 16(6), 254–259 (2000).
Z. Ruzsics, U.H. Koszinowski, Mutagenesis of the cytomegalovirus genome. Curr. Top. Microbiol. Immunol. 325, 41–61 (2008).
K. Narayanan, Q. Chen, Bacterial artificial chromosome mutagenesis using recombineering. J. Biomed. Biotechnol. 2011, 971296 (2011). doi:10.1155/2011/971296.
B.K. Tischer, G.A. Smith, N. Osterrieder, En passant mutagenesis: a two step markerless red recombination system. Methods Mol. Biol. 634, 421–430 (2010). doi:10.1007/978-1-60761-652-8_30.
A. Arvin, G. Campadelli-Fiume, E. Mocarski, P.S. Moore, B. Roizman, et al. (eds.), Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis (Cambridge University Press, Cambridge, 2007)
G.B. Elion, The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother 12(Suppl B), 9–17 (1983)
G. Campadelli-Fiume, B. Petrovic, V. Leoni, T. Gianni, E. Avitabile, C. Casiraghi et al., Retargeting strategies for oncolytic herpes simplex viruses. Viruses. 8(3), 63 (2016). doi:10.3390/v8030063.
M. Nygardas, H. Paavilainen, N. Muther, C.H. Nagel, M. Roytta, B. Sodeik et al., A herpes simplex virus-derived replicative vector expressing LIF limits experimental demyelinating disease and modulates autoimmunity. PLoS ONE 8(5), e64200 (2013). doi:10.1371/journal.pone.0064200.
C.H. Nagel, A. Pohlmann, B. Sodeik, Construction and characterization of bacterial artificial chromosomes (BACs) containing herpes simplex virus full-length genomes. Methods Mol. Biol. 1144, 43–62 (2014). doi:10.1007/978-1-4939-0428-0_4.
C. Funk, M. Ott, V. Raschbichler, C.H. Nagel, A. Binz, B. Sodeik et al., The herpes simplex virus protein pUL31 escorts nucleocapsids to sites of nuclear egress, a process coordinated by its N-terminal domain. PLoS Pathog. 11(6), e1004957 (2015). doi:10.1371/journal.ppat.1004957.
G. Ungerechts, S. Bossow, B. Leuchs, P.S. Holm, J. Rommelaere, M. Coffey et al., Moving oncolytic viruses into the clinic: clinical-grade production, purification, and characterization of diverse oncolytic viruses. Mol. Ther. Methods Clin. Dev. 3, 16018 (2016). doi:10.1038/mtm.2016.18.
N.A. Sokolowski, H. Rizos, R.J. Diefenbach, Oncolytic virotherapy using herpes simplex virus: how far have we come? Oncol. Virother. 4, 207–219 (2015). doi:10.2147/OV.S66086.
P.R. Buijs, J.H. Verhagen, C.H. van Eijck, B.G. van den Hoogen, Oncolytic viruses: from bench to bedside with a focus on safety. Hum. Vaccin. Immunother. 11(7), 1573–1584 (2015). doi:10.1080/21645515.2015.1037058.
D.R. Wilcox, R. Longnecker, The herpes simplex virus neurovirulence factor gamma34.5: revealing virus-host interactions. PLoS Pathog. 12(3), e1005449 (2016). doi:10.1371/journal.ppat.1005449.
C. Krummenacher, A. Carfi, R.J. Eisenberg, G.H. Cohen, Entry of herpesviruses into cells: the enigma variations. Adv. Exp. Med. Biol. 790, 178–195 (2013). doi:10.1007/978-1-4614-7651-1_10.
E.E. Heldwein, gH/gL supercomplexes at early stages of herpesvirus entry. Curr. Opin. Virol. 18, 1–8 (2016). doi:10.1016/j.coviro.2016.01.010.
V. Gatta, B. Petrovic, G. Campadelli-Fiume, The engineering of a novel ligand in gH confers to HSV an expanded tropism independent of gD activation by its receptors. PLoS Pathog. 11(5), e1004907 (2015). doi:10.1371/journal.ppat.1004907.
B. Petrovic, T. Gianni, V. Gatta, G. Campadelli-Fiume, Insertion of a ligand to HER2 in gB retargets HSV tropism and obviates the need for activation of the other entry glycoproteins. PLoS Pathog. 13(4), e1006352 (2017). doi:10.1371/journal.ppat.1006352.
C.A. Alvarez-Breckenridge, B.D. Choi, C.M. Suryadevara, E.A. Chiocca, Potentiating oncolytic viral therapy through an understanding of the initial immune responses to oncolytic viral infection. Curr Opin Virol. 13, 25–32 (2015). doi:10.1016/j.coviro.2015.03.015.
A. Melcher, K. Parato, C.M. Rooney, J.C. Bell, Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol. Ther. 19(6), 1008–1016 (2011). doi:10.1038/mt.2011.65.
A. Pourchet, S.R. Fuhrmann, K.A. Pilones, S. Demaria, A.B. Frey, M. Mulvey et al., CD8(+) T-cell immune evasion enables oncolytic virus immunotherapy. EBioMedicine. 5, 59–67 (2016). doi:10.1016/j.ebiom.2016.01.022
B.D. Lichty, C.J. Breitbach, D.F. Stojdl, J.C. Bell, Going viral with cancer immunotherapy. Nat. Rev. Cancer 14(8), 559–567 (2014). doi:10.1038/nrc3770.
R.S. Coffin, From virotherapy to oncolytic immunotherapy: where are we now? Curr. Opin. Virol. 13, 93–100 (2015). doi:10.1016/j.coviro.2015.06.005.
R.H. Andtbacka, M. Ross, I. Puzanov, M. Milhem, F. Collichio, K.A. Delman et al., Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM phase III clinical trial. Ann. Surg. Oncol. 23(13), 4169–4177 (2016). doi:10.1245/s10434-016-5286-0.
D.M. Pardoll, The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12(4), 252–264 (2012). doi:10.1038/nrc3239.
I. Puzanov, M.M. Milhem, D. Minor, O. Hamid, A. Li, L. Chen et al., Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J. Clin. Oncol. 34(22), 2619–2626 (2016). doi:10.1200/JCO.2016.67.1529.
S.J. Russell, K.W. Peng, J.C. Bell, Oncolytic virotherapy. Nat. Biotechnol. 30(7), 658–670 (2012). doi:10.1038/nbt.2287.
K.J. Allan, D.F. Stojdl, S.L. Swift, High-throughput screening to enhance oncolytic virus immunotherapy. Oncolytic Virother. 5, 15–25 (2016). doi:10.2147/OV.S66217.
B. Dong, D.S. Zarlenga, X. Ren, An overview of live attenuated recombinant pseudorabies viruses for use as novel vaccines. J. Immunol. Res. 2014, 824630 (2014). doi:10.1155/2014/824630.
P. Marconi, R. Argnani, A.L. Epstein, R. Manservigi, HSV as a vector in vaccine development and gene therapy. Adv. Exp. Med. Biol. 655, 118–144 (2009). doi:10.1007/978-1-4419-1132-2_10.
D. Watanabe, Medical application of herpes simplex virus. J. Dermatol. Sci. 57(2), 75–82 (2010). doi:10.1016/j.jdermsci.2009.10.014.
U. Karrer, M. Wagner, S. Sierro, A. Oxenius, H. Hengel, T. Dumrese et al., Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J. Virol. 78(5), 2255–2264 (2004)
B. Bolinger, S. Sims, G. O’Hara, C. de Lara, E. Tchilian, S. Firner et al., A new model for CD8+ T cell memory inflation based upon a recombinant adenoviral vector. J. Immunol. 190(8), 4162–4174 (2013). doi:10.4049/jimmunol.1202665.
G.A. O’Hara, S.P. Welten, P. Klenerman, R. Arens, Memory T cell inflation: understanding cause and effect. Trends Immunol. 33(2), 84–90 (2012). doi:10.1016/j.it.2011.11.005.
S.G. Hansen, H.L. Wu, B.J. Burwitz, C.M. Hughes, K.B. Hammond, A.B. Ventura et al., Broadly targeted CD8(+) T cell responses restricted by major histocompatibility complex E. Science 351(6274), 714–720 (2016). doi:10.1126/science.aac9475.
B.J. Burwitz, D. Malouli, B.N. Bimber, J.S. Reed, A.B. Ventura, M.H. Hancock et al., Cross-species rhesus cytomegalovirus infection of cynomolgus macaques. PLoS Pathog. 12(11), e1006014 (2016). doi:10.1371/journal.ppat.1006014.
E.S. Barton, D.W. White, J.S. Cathelyn, K.A. Brett-McClellan, M. Engle, M.S. Diamond et al., Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447(7142), 326–329 (2007). doi:10.1038/nature05762.
P.C. Beverley, Z. Ruzsics, A. Hey, C. Hutchings, S. Boos, B. Bolinger et al., A novel murine cytomegalovirus vaccine vector protects against Mycobacterium tuberculosis. J Immunol. 193(5), 2306–2316 (2014). doi:10.4049/jimmunol.1302523.
I. Brizic, T. Lenac Rovis, A. Krmpotic, S. Jonjic, MCMV avoidance of recognition and control by NK cells. Semin Immunopathol. 36(6), 641–650 (2014). doi:10.1007/s00281-014-0441-9.
A. Moosmann, N. Khan, M. Cobbold, C. Zentz, H.J. Delecluse, G. Hollweck et al., B cells immortalized by a mini-Epstein-Barr virus encoding a foreign antigen efficiently reactivate specific cytotoxic T cells. Blood 100(5), 1755–1764 (2002).
S. Ameres, X. Liang, M. Wiesner, J. Mautner, A. Moosmann, A diverse repertoire of CD4 T cells targets the immediate-early 1 protein of human cytomegalovirus. Front. Immunol. 6, 598 (2015). doi:10.3389/fimmu.2015.00598.
M. Wiesner, C. Zentz, M.H. Hammer, M. Cobbold, F. Kern, H.J. Kolb et al., Selection of CMV-specific CD8+ and CD4+ T cells by mini-EBV-transformed B cell lines. Eur. J. Immunol. 35(7), 2110–2121 (2005). doi:10.1002/eji.200425936.
E. Hettich, A. Janz, R. Zeidler, D. Pich, E. Hellebrand, B. Weissflog et al., Genetic design of an optimized packaging cell line for gene vectors transducing human B cells. Gene Ther. 13(10), 844–856 (2006). doi:10.1038/sj.gt.3302714.
A. Gimenez-Cassina, R. Wade-Martins, S. Gomez-Sebastian, J.C. Corona, F. Lim, J. Diaz-Nido, Infectious delivery and long-term persistence of transgene expression in the brain by a 135-kb iBAC-FXN genomic DNA expression vector. Gene Ther. 18(10), 1015–1019 (2011). doi:10.1038/gt.2011.45.
M.M. Lufino, P.A. Edser, M.A. Quail, S. Rice, D.J. Adams, R. Wade-Martins, The infectious BAC genomic DNA expression library: a high capacity vector system for functional genomics. Sci. Rep. 6, 28644 (2016). doi:10.1038/srep28644.
A.F. Meier, M. Suter, E.M. Schraner, B.M. Humbel, K. Tobler, M. Ackermann et al., Transfer of anti-rotavirus antibodies during pregnancy and in milk following maternal vaccination with a herpes simplex virus type-1 amplicon vector. Int. J. Mol. Sci. (2017). doi:10.3390/ijms18020431
Funding
This study was funded by the Deutsches Zentrum für Infektionsforschung (DZIF TTU-IICH-708.2 to A.R. and Z.R).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Edited by Anja Ehrhardt and Florian Kreppel.
Rights and permissions
About this article
Cite this article
Bailer, S.M., Funk, C., Riedl, A. et al. Herpesviral vectors and their application in oncolytic therapy, vaccination, and gene transfer. Virus Genes 53, 741–748 (2017). https://doi.org/10.1007/s11262-017-1482-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-017-1482-7