Abstract
Influenza A H3N2 virus caused 1968 Hong Kong influenza pandemic, and has since been one of the most prevalent seasonal influenza viruses in global populations, representing a credible pandemic candidate in future. Previous studies have established that the hemagglutinin (HA) protein is the predominant antigen and executes receptor binding and membrane fusion. Homologous sequence analysis of all HA subtypes of influenza viruses revealed that two cysteine residues (540 and 544) are uniquely present in the transmembrane domain (TM) of HA proteins from all influenza A H3N2 viruses. However, the functions of these two cysteines have not been fully studied. Here, we generated three mutants (C540S, C544L, and 2C/SL) to investigate the effects of the two TM cysteines on the biological functions of H3 HA. We herein presented evidences that the mutations of one or two of the cysteines did not affect the proper expressions of HA proteins in cells, and more importantly all mutant H3 HAs showed decreased thermal stability but increased fusion activity in comparison with wildtype HA. Our results taken together demonstrated that the two TM cysteines are important for the biological functions of H3 HA proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influenza A virus is still a grave public health threat, causing recurrent epidemics and infrequent global pandemics, resulting in millions of human infections or even deaths [1–3]. The H3N2 virus caused 1968 Hong Kong influenza pandemic, one of the three influenza pandemics that occurred in last century including 1918 Spanish influenza (H1N1), and 1957 Asian influenza (H2N2), leading to over one million deaths all over the world [3, 4]. Since 1968, H3N2 has been one of the most prevalent seasonal influenza virus circulating in human and swine population [5, 6].
Influenza A virus is a member of the family Orthomyxoviridae and has a segmented genome of eight negative-sense ssRNA molecules encoding up to 11 viral proteins [2]. The hemagglutinin (HA) is present on the membrane surface as homotrimers [7], considered to be the predominant antigenic protein of influenza viruses [8], and closely associated with the biological characteristics of influenza viruses [9]. Among the three domains of HA protein, HA transmembrane domain (TM) has recently been reported to exert important influence on the structure and function of HA proteins more than merely anchor the protein to the membrane [10]. Influenza viruses contain HA with sequence substitutions in the TM domain, which significantly diminish the infectivity, and exhibit reduced virus–membrane fusion activity [11]. Influenza HA-mediated membrane fusion in acidic environment is a key step for the entry of enveloped viruses into cells [9, 12]. Low pH induces a conformational change in HA protein, leading to the insertion of fusion peptide into the target cell membrane, and thus triggers membrane fusion [13–15]. Growing evidences suggest that HA TM plays an important role on virus–cell membrane fusion, and changes of TM amino acid sequence may dramatically influence the fusion activity [11, 16, 17].
Our homologous sequence analysis of HA TM domain among 16 subtypes of influenza A viruses revealed that H3 HA is unique in that two cysteines (C540/544) present in its TM domain (Fig. 1). In this study, we generated three H3 HA mutants (C540S, C544L, and 2C/SL) containing mutations of one or both the two TM cysteines. Our results showed that mutations of one or two of the cysteines did not affect the proper expression of H3 HA proteins, but altered the biological functions of H3 HA proteins.
Amino acid sequence alignment of HA TM of 16 influenza A virus subtypes (H1 to H16). ECT denotes the ectodomain, TM the transmembrane domain, and CT the cytoplasmic tail. Three (3) cysteine residues (C540/544/555) in the TM of H3 HA are underlined; the numbering is based on H3 HA amino acid sequence
Materials and methods
Cell lines
Spodoptera frugiperda insect (Sf9) cells were maintained in serum-free SF900II medium (Gibco) at 28 °C. Vero is an African green monkey kidney cell line, and was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS) (Gibco) at 37 °C.
Bacmid and plasmid transfections and baculovirus infection
Transfection of recombinant bacmid DNAs into sf9 insect cells was performed using Cellfectin Reagent (Invitrogen) according to the manufacturer’s protocol. 1 μg of purified recombinant bacmid DNA was used for each transfection in a 6-well tissue culture plate.
For expressing HA proteins in Vero cells, cells were transfected with 800 ng of plasmids in a 12-well plate, using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
For baculovirus infections, sf9 cells were greater than 90 % confluent at the time of infection, and then infected with recombinant baculoviruses (rBVs) at a multiplicity of infection (MOI) of 3–5; the infection was allowed to proceed for 72 h at 28 °C.
Construction of HA expression plasmid vectors
To construct the wildtype (WT) and mutant HA expression vectors using pcDNA3.0 plasmid, the full-length coding sequence of WT H3 HA (GenBank accession number FJ830855.1, submitted by our lab) or mutant HAs (designated as C540S, C544L, and 2C/SL) was amplified and then cloned into the ApaI and KpnI sites in pcDNA3.0 (Invitrogen).
Generation of recombinant HA expression bacmids and baculoviruses
A Bac-to-Bac baculovirus expression system was utilized for the generation of recombinant HA expression bacmids and baculoviruses. Initially, the WT or mutant HA genes were cloned into the pFast-Bac-Dual vector (Invitrogen, Carlsbad, CA, USA); recombinant bacmids were generated by site-specific homologous recombination following transformation of the pFast-Bac-Dual transfer plasmid containing above HA genes into E. coli DH10-Bac competent cells, which contained the AcMNPV baculovirus genome (Invitrogen). The obtained recombinant bacmids were then transfected into Sf9 cells. Transfected cells were incubated for 3 days, and the target recombinant baculoviruses (rBVs) were then harvested from the supernatant.
Western blotting
Cell lysates were separated on 10 % SDS polyacrylamide gels; the separated proteins were electrophoretically transferred to polyvinylidene difluoride membranes (Roche, 45 μm), and then detected with murine monoclonal antibody (mAb) specific for influenza H3 HA (Novus Biologicals, NB100-73161) and commercial ECL kit (Pierce). Protein loads were estimated using murine anti-β-actin mAb (Abmart, M20010).
Immunofluorescence staining
Immunofluorescence (IF) staining was performed as described previously [18]. Briefly, Sf9 cells expressing HA proteins were washed with PBS-Tween-20 (0.05 %) and fixed in 100 % pre-cooled methanol at 4 °C for 10 min, then with primary mAb specific for H3 HA (Novus Biologicals, NB100-73161), followed by Cy3-conjugated secondary antibody (PTG, 00009-1). Cells were stained with 4′-6′-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) as counterstain, and read under a fluorescence microscope (Observer Z1, Carl Zeiss, Germany).
Hemagglutination activity assay of HA suspensions
The supernatants of HA suspensions prepared by ultrasonication could be subjected to the conventional hemagglutination activity assay for influenza viruses as exemplarily described [19]. Briefly, sf9 cells, respectively, expressing WT and mutant HAs were ultrasonicated to prepare HA suspensions. Then hemagglutination activity assay was performed using 1 % chicken RBCs in U-bottom 96-well plates. The plates were incubated at room temperature for 30 min and observed for the formation of button or mat within the wells.
Erythrocyte hemolysis assay
Erythrocyte hemolysis assay was performed as described previously [20] with some modifications. Briefly, sf9 cells were first treated with 5 μg/ml TPCK-trypsin for 10 min at 37 °C before prepared to 1 × 107/ml cell suspensions in the citric acid/sodium citrate buffer solution with different pHs, which was prepared by different ratios between 0.1 M citric acid and 0.1 M sodium citrate solution, and then incubated with chicken RBCs (2 % final concentration) at room temperature for 30 min. After incubation, the chicken RBCs were removed by centrifugation (3000 rpm, 3 min), and the supernatants were transferred to an ELISA plate for NADPH content determination by optical density measurement (OD340 nm) with a Bio-Tek ELISA plate reader (Bio-Tek Instruments, Inc, Winooski, VT). Blank values were derived from sf9 cell suspensions without HA expression that underwent identical treatment.
Syncytium formation assay
Syncytium formation assay for cell–cell fusion was performed as described previously [21, 22]. Briefly, 24 h after transfection with HA expression plasmid, Vero cells were washed and treated with 5 μg/ml TPCK-trypsin as described above. To initiate cell fusion, the cell monolayers were exposed to acidic phosphate-buffered saline buffer with magnesium and calcium (PBS+) in the range of pHs for 15 min. The pH of PBS + buffer (Invitrogen) was adjusted by 0.1 M citric acid. Cells were neutralized by using cell culture medium and left at 37 °C for 4 h. Samples were then fixed in 4 % paraformaldehyde at 4 °C for 10 min, and stained with DAPI and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI). Representative fields were captured under a fluorescence microscope (Observer Z1, Carl Zeiss, Germany).
Statistical analysis
Data are presented as mean ± SEM from at least three separate experiments. Unless otherwise noted, the differences between groups were analyzed using Student’s two-tailed t test when only two groups were compared or assessed by one-way analysis of variance (ANOVA) when more than two groups were compared. Differences were considered statistically significant at P < 0.05.
Accession number
Genbank Accession number for H3 HA sequence (FJ830855.1) is found at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/).
Results
Comparable in vitro expression of wildtype (WT) and mutant H3 HA proteins
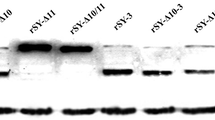
We first cloned the WT H3 HA gene from one H3N2 strain (A/swine/Guangdong/01/1998), and then generated 3 HA mutants by mutating the 2 TM cysteines either individually or in combination (designated C540S, C544L, and 2C/SL) (Fig. 2a). C540/544 was mutated in light of the prominent amino acid residues in the same positions of other HAs. WT and 3 mutant HA genes were packaged into recombinant baculoviruses (rBVs) using a Bac-to-Bac baculovirus expression system, and the ectopic expressions of WT and mutant HA proteins were analyzed by Western blot (Fig. 2b) and IF staining (Fig. 2c). The data showed that WT and 3 mutant H3 HA proteins were comparably expressed in sf9 cells (Fig. 2b), and mainly located on plasma membranes (Fig. 2c), demonstrating that the mutations of the two TM cysteines did not change the expression levels and locations of all mutant HA proteins. Similar expressions were observed in 293 T cells and MDCK cells (data not shown).
Amino acid sequences of TM domain of WT and 3 mutant H3 HAs and the expressions of HA proteins. a Amino acid sequences of TM domain WT and 3 mutant H3 HA proteins. C540S (C540 was mutated into serine); C544L (C544 into leucine); 2C/SL (C540 into serine and C544 into leucine). b Western blot of expressed WT and 3 mutant HA proteins. Actin was included as an internal control for Western blot. c Immunofluorescence staining of WT and mutant HA proteins. Cells were stained with DAPI (Blue fluorescence) as counterstain, and HA proteins were stained with Cy3-conjugated antibody (Red fluorescence). Cells infected with rBVs without HA gene are designated as NC (Color figure online)
Mutations of two TM cysteines diminished HA thermal stability
We first investigated whether mutant HAs had altered stabilities. With our limited resources, we discovered a simple but useful approach to study the stability of cell-expressed HAs by assaying the changes of HA titers of sonicated HA suspensions under changed conditions. The protein suspensions of WT, C540S, C544L, and 2C/SL HAs were incubated at indicated temperatures (37, 40, 44, 46, 48, or 52 °C), and cooled down to 25 °C (room temperature) before HA titers were measured (Fig. 3). For all HAs, their HA titers were decreased when the temperatures were increased; when the temperature reached at 52 °C, no HA titer was detected for all HAs (Fig. 3). At lower temperatures (37, 40 °C), the relative ratios of HA titers of WT and all mutant HAs were not significantly different. Interestingly, when the temperatures reached at 44, 46, or 48 °C, the relative ratios of HA titers were more revealing; at 48 °C, WT HA retained more than 60 % of its HA titers while all mutant HAs lost more than 90 % of their HA titers (P < 0.001); at 46 °C, WT HA retained nearly 80 % of its HA titers while all mutant HAs lost more than 60 % of their HA titers (P < 0.001); at 44 °C, the HA titer relative ratio of WT HA was significantly higher than that of mutant HAs with mutations of TM cysteines (C540S, C544L, and 2C/SL) (P < 0.05). No significant difference of the relative ratio of HA titer was observed among 3 mutant HAs. We also have tested different pHs, and NaCl concentrations, but found that mutations of the two cysteines had no significant effects in the tested conditions (Fig. 4). Taken together, the results demonstrated that the two TM cysteines (C540/544) contributed to the thermal stability of H3 HA.
Analysis of the thermal resistance of WT and mutant HAs. Three batches of WT, C540S, C544L, and 2C/SL HA suspensions prepared from Sf9 cells were incubated at the temperatures of 37, 40, 44, 46, 48, and 52 °C, and then HA titers were measured as described. The relative ratio was calculated as the percentage of the remaining HA titer after incubation over the starting HA titer for each batch; then the mean relative ratios were calculated based on three batches. Columns, mean relative ratio; bars, SEM; * denotes P < 0.05, *** denotes P < 0.001, compared with all mutant HAs
Analysis of the effects of pH and NaCl on WT, C540S, C544L, and 2C/SL HA titers. The prepared proteins suspensions of WT, C540S, C544L, and 2C/SL HAs were incubated at different pHs (5, 6, and 7) or different NaCl concentrations (0.1, 0.2, and 0.4 M) during HA titer assays. The relative ratio was calculated as the percentage of the remaining HA titer after incubation over the starting HA titer for each batch; then the mean relative ratios were calculated based on three batches. Columns, mean relative ratio; bars, SEM
Mutations of TM cysteines increased HA fusion activity
Amino acid sequence changes of HA TM may dramatically influence the fusion activity, thus we employed both erythrocytes hemolysis assay and syncytium formation assay to evaluate the effects of mutations of the two TM cysteines on HA fusion activity. Sf9 cells expressing respective HAs were first treated with 5 μg/ml trypsin before prepared to cell suspensions in buffers with pH of 5.0, 5.2, 5.4, or 7.4, then incubated with chicken RBCs to induce erythrocytes hemolysis, and then the released NADPH contents were measured at OD340 nm, which finally converted to the amount of total lysed erythrocytes according to the established standard curve (Fig. 5a). The data showed that the fusion activity of WT HA was significantly lower than that of all 3 mutant HAs at pH 5.0 (P < 0.01), pH 5.2 (P < 0.05), and pH 5.4 (P < 0.001), but no obvious differences among 3 mutant HAs.
Analysis of fusion activity of WT and mutant HAs. a The amount of lysed erythrocytes was determined by optical density measurement (OD340 nm) at indicated pH by the erythrocytes hemolysis assay. The experiments were repeated at least three times. Columns, mean of three independent experiments done in duplicate; bars, SEM. At pH 5.0, ** denotes P < 0.01, compared with all mutants; at pH 5.2, * denotes P < 0.05, compared with all mutants; at pH 5.4, ***, P < 0.001, compared with all mutants. b Representative photographs of the syncytium formations for WT and mutant C540S, C544L, and 2C/SL HAs at indicated pHs
The enhanced fusion activity of mutant HAs was further verified by syncytium formation assay. The expression levels of WT and mutant HAs in Vero cells were comparable (data not shown). The results showed that at pH 5.0, 5.2, and 5.4, WT and mutant C540S, C544L, and 2C/SL HAs exhibited extensive cell–cell fusions, but the syncytium size of WT was smaller than mutant HAs (Fig. 5b). In summary, our data demonstrated that mutations of the two TM cysteines increased the fusion activity of H3 HA.
Discussion
The influenza HA TM domain plays an important role in HA structure and functions. In this study, we investigated the effects of mutations of one or two of the cysteines (C540 and C544) in the TM of H3 HA proteins on the biological functions of H3 HA proteins. Our results showed that mutant H3 HAs with mutations of one or two of the TM cysteines (C540/544) could be expressed properly in cells, and more importantly exhibited lower thermal resistance and enhanced fusion activity in comparison with WT H3 HA proteins.
HA executes many critical functions of influenza viruses that are constantly exposed to environmental factors during their spread [9]. Since the literature as far as we knew lacked a simple assay for studying of HA stability in cell expression systems, we adopted the conventional hemagglutination activity assay to use the changes of HA titer of HA suspensions as the indicative of HA stability. Our results demonstrated that mutations of the two TM cysteines caused significant reduction of H3 HA thermal stability, implying that TM cysteines contributed to the thermal stability of H3 HA.
Previous researches have extensively studied the effects of the TM of viral envelope proteins on protein structures and functions as well as viral activities. The three hydrophobic clusters of four residues in TM1 of L protein of hepatitis B virus (HBV) were showed to be necessary for infectivity [23]; TM of E2 glycoprotein of sindbis virus when deleted altered virus infectivity, stability, and host range [24]. The involvement of TM in viral glycoprotein fusion activity has been shown in several viruses, including F protein of paramyxovirus [25], Newcastle disease virus (NDV) fusion protein [26], vesicular stomatitis virus (VSV) G protein [27], and influenza HA [11, 17]. Here we showed that when H3 HA TM cysteines (C540/544) were mutated, the resultant mutant HAs (C540S, C544L, and 2C/SL) manifested significantly higher fusion activity than WT HA, providing definitive evidence that HA fusion activity could be influenced by specific residues in HA TM.
It is plausible to assume that the effects of TM cysteines (C540/544) on HA fusion activity might be unique because they are only present in H3 HA; whether the corresponding residues in other subtype HAs have similar effects on their HA fusion activity needs further studies. A single mutation in TM with increased HA fusion activity had been shown in Measles virus (MV) fusion (F) protein of which the mutation of a central TM region leucine (L507A) was more fusogenic than the unmodified F protein [28]. However, one previous study showed that a point mutation (G520L, corresponding to S539 in H3 numbering) in H2 HA TM inhibited HA fusion activity, but another point mutation (G530W, corresponding to G549 in H3 numbering) had no effect on HA fusion activity [29]. Another study showed that point mutations within HA TM including W530/533, S539, and G549 did not affect HA fusion activity [17]. All these suggested that it would be premature at this moment to generalize the functions of TM amino acid residues in HA fusion activity.
For the cell model utilized in this study, we were aware that the Sf9 cells were not the natural host cells of influenza viruses. Nonetheless, the insect sf9 cell was a well-established cell model for influenza virus-like particles (VLPs) vaccine production [30–32]; in addition, the sf9 cells-expressed influenza HA could normally mediate membrane fusion in acidic pH environment [33, 34], which indicated that sf9 cell is an appropriate model for HA structure and function investigation. Thus, we hold that the use the sf9 cells in our assays should not affect the authenticity and importance of our results presented in this study.
In conclusion, we have provided concrete evidences showing that the two cysteines (C540 and C544) in the TM of H3 HA contributed to H3 HA thermal stability and fusion activity. Our results shall command future interesting inquiries; for example, whether the effects of these two TM cysteines on HA proteins are position-dependent; how the mutations of these 2 TM cysteines will affect the biological functions of recombinant H3N2 viruses.
References
R. Salomon, R.G. Webster, Cell 136, 402–410 (2009)
R.A. Medina, A. Garcia-Sastre, Nat. Rev. Microbiol. 9, 590–603 (2011)
G. Neumann, T. Noda, Y. Kawaoka, Nature 459, 931–939 (2009)
J.K. Taubenberger, J.C. Kash, Cell Host Microbe 7, 440–451 (2010)
C.A. Russell, T.C. Jones, I.G. Barr, N.J. Cox, R.J. Garten, V. Gregory, I.D. Gust, A.W. Hampson, A.J. Hay, A.C. Hurt, J.C. de Jong, A. Kelso, A.I. Klimov, T. Kageyama, N. Komadina, A.S. Lapedes, Y.P. Lin, A. Mosterin, M. Obuchi, T. Odagiri, A.D. Osterhaus, G.F. Rimmelzwaan, M.W. Shaw, E. Skepner, K. Stohr, M. Tashiro, R.A. Fouchier, D.J. Smith, Science 320, 340–346 (2008)
J. Bahl, M.I. Nelson, K.H. Chan, R. Chen, D. Vijaykrishna, R.A. Halpin, T.B. Stockwell, X. Lin, D.E. Wentworth, E. Ghedin, Y. Guan, J.S. Peiris, S. Riley, A. Rambaut, E.C. Holmes, G.J. Smith, Proc. Natl. Acad. Sci. USA. 108, 19359–19364 (2011)
Y.G. Yu, D.S. King, Y.K. Shin, Science 266, 274–276 (1994)
S.J. Gamblin, J.J. Skehel, J. Biol. Chem. 285, 28403–28409 (2010)
J.J. Skehel, D.C. Wiley, Annu. Rev. Biochem. 69, 531–569 (2000)
D.K. Chang, S.F. Cheng, E.A. Kantchev, C.H. Lin, Y.T. Liu, BMC Biol. 6, 2 (2008)
M. Takeda, G.P. Leser, C.J. Russell, R.A. Lamb, Proc. Natl. Acad. Sci. USA. 100, 14610–14617 (2003)
D.L. Floyd, J.R. Ragains, J.J. Skehel, S.C. Harrison, A.M. van Oijen, Proc. Natl. Acad. Sci. USA. 105, 15382–15387 (2008)
P. Bonnafous, T. Stegmann, J. Biol. Chem. 275, 6160–6166 (2000)
X. Han, J.H. Bushweller, D.S. Cafiso, L.K. Tamm, Nat. Struct. Biol. 8, 715–720 (2001)
T. Stegmann, J.M. White, A. Helenius, EMBO J. 9, 4231–4241 (1990)
Z.N. Li, B.J. Lee, W.A. Langley, K.C. Bradley, R.J. Russell, D.A. Steinhauer, J. Virol. 82, 6337–6348 (2008)
R.T. Armstrong, A.S. Kushnir, J.M. White, J. Cell Biol. 151, 425–437 (2000)
M. Hagedorn, E.M. Neuhaus, T. Soldati, Methods. Mol. Biol. 346, 327–338 (2006)
M.L. Killian, Methods. Mol. Biol. 436, 47–52 (2008)
T.T. Wang, G.S. Tan, R. Hai, N. Pica, E. Petersen, T.M. Moran, P. Palese, PLoS Pathog. 6, e1000796 (2010)
M.L. Reed, H.L. Yen, R.M. DuBois, O.A. Bridges, R. Salomon, R.G. Webster, C.J. Russell, J. Virol. 83, 3568–3580 (2009)
R. Xu, I.A. Wilson, J. Virol. 85, 5172–5182 (2011)
C. Lepere-Douard, M. Trotard, J. Le Seyec, P. Gripon, J. Virol. 83, 11819–11829 (2009)
R. Hernandez, C. Sinodis, M. Horton, D. Ferreira, C. Yang, D.T. Brown, J. Virol. 77, 12710–12719 (2003)
M.L. Bissonnette, J.E. Donald, W.F. DeGrado, T.S. Jardetzky, R.A. Lamb, J. Mol. Biol. 386, 14–36 (2009)
K.A. Gravel, L.W. McGinnes, J. Reitter, T.G. Morrison, J. Virol. 85, 3486–3497 (2011)
D.Z. Cleverley, J. Lenard, Proc. Natl. Acad. Sci. USA. 95, 3425–3430 (1998)
M.D. Muhlebach, V.H. Leonard, R. Cattaneo, J. Virol. 82, 11437–11445 (2008)
G.B. Melikyan, S. Lin, M.G. Roth, F.S. Cohen, Mol. Biol. Cell 10, 1821–1836 (1999)
R.A. Bright, D.M. Carter, S. Daniluk, F.R. Toapanta, A. Ahmad, V. Gavrilov, M. Massare, P. Pushko, N. Mytle, T. Rowe, G. Smith, T.M. Ross, Vaccine 25, 3871–3878 (2007)
P. Pushko, T. Kort, M. Nathan, M.B. Pearce, G. Smith, T.M. Tumpey, Vaccine 28, 4771–4776 (2010)
T.M. Ross, K. Mahmood, C.J. Crevar, K. Schneider-Ohrum, P.M. Heaton, R.A. Bright, PLoS One 4, e6032 (2009)
I. Plonsky, D.H. Kingsley, A. Rashtian, P.S. Blank, J. Zimmerberg, Biol. Cell 100, 377–386 (2008)
S. Biswas, S.R. Yin, P.S. Blank, J. Zimmerberg, J. Gen. Physiol. 131, 503–513 (2008)
Acknowledgments
The authors would like to thank Dr. George Dacai Liu for critical review and revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, S., Zhou, J., Liu, K. et al. Mutations of two transmembrane cysteines of hemagglutinin (HA) from influenza A H3N2 virus affect HA thermal stability and fusion activity. Virus Genes 47, 20–26 (2013). https://doi.org/10.1007/s11262-013-0924-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-013-0924-0