Abstract
The aim of this study was to investigate the effects of transplanted Wharton’s jelly mesenchymal stem cells (WJMSCs) of caprine umbilical cord on cutaneous wound healing process in goat. After collection of caprine pregnant uterus of mixed breed goats from abattoir, the Wharton’s jelly (WJ) of umbilical cord was harvested. The tissues were minced in ventilated flasks and explant culture method was used for separating mesenchymal stem cells (MSCs). The isolated cells were immunostained for Actin protein, histochemically assayed for the presence of alkaline phosphatase activity, and analyzed for detection of matrix receptors (CD44) and hematopoetic lineage markers (CD34), using flow cytometery. After The isolated cells, 3 × 106 MSCs were stained with BrdU and prepared for transplantation to each wound. Four 3-cm linear full thickness skin incisions were made on both sides of thoracic vertebrate of four Raeini goats (two wounds on each side). The left wounds were implanted with MSCs in 0.6 ml of Phosphate buffer saline (PBS), and the right wounds considered as control group that received 0.6 ml of PBS. The samples were taken from the wounds 7 and 12 days after the wounding, and healing process was compared histologically between the two groups. Anti-BrdU staining showed that the transplanted cells were still alive in the wound bed during the study. The histopathological study revealed that re-epithelialization was complete at days 7 in treated wounds with WJMSCs, whereas in control wound the wounds still showed incomplete epithelialization 12 days after wounding. Also, microscopic evaluation showed less inflammation, thinner granulation tissue formation with minimum scar in the treated wounds in comparison with control wounds. In conclusion, this study demonstrates the beneficial effect of caprine WJMSCs in cutaneous wound healing in goat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Optimum healing of cutaneous wound requires a well-orchestrated integration of the complex biological and molecular events of cell migration and proliferation and extracellular matrix deposition, angiogenesis and remodeling. However, this orderly progression of healing process is impaired in many chronic diseases (Wu et al. 2007). Recent reports describing the plasticity of stem cells may herald a new era in the treatment of many disorders (Badiavas and Falanga 2003). Mesenchymal stem cells (MSCs) have shown a strong propensity to ameliorate tissue damage in response to injury and disease (Phinney and Prockop 2007). MSCs have demonstrated efficacy as therapeutic vectors in animal models of skeletal defects (Horwitz et al. 2002), lung injury (Ortiz et al. 2007), kidney disease (Kunter et al. 2006), diabetes (Lee et al. 2006), myocardial infarction (Minguell and Erices 2006) and various neurogical disorders (Phinney and Isakova 2005). It is well established that MSCs produce a variety of cytokines and adhesion molecules that regulate aspects of hematopoiesis. Additionally, MSCs express transcripts encoding proteins that regulate a broad range of biological activities, including angiogenesis, wound healing, immunity, and defense as well as neural activities. MSCs promote tissue repair by secretion of factors that enhance regeneration of injured cells, stimulate proliferation and differentiation, decrease inflammatory and immune reactions. Therefore, the ability of such cells to alter the tissue microenvironment may contribute more significantly than their capacity for transdifferentiation in effecting tissue repair (Phinney and Prockop 2007). In the most pervious studies, MSCs that were used to treat skin defects were isolated from bone marrow (Sasaki et al. 2008; Wu et al. 2007; Badiavas and Falanga 2003; Stepanovic et al. 2003; Phinney and Prockop 2007). These reports have revealed that stem/progenitor cells, particularly those derived from bone marrow, significantly promote wound healing process (Phinney and Prockop 2007). Recently, it has been shown that topical administration of human umbilical cord blood MSCs in the cutaneous wound of normal (Luo et al. 2010) and diabetic mice (Tark et al. 2010) had a positive effect on wound healing.
Recent studies have indicated other source of MSCs in Wharton’s jelly, a gelatinous connective tissue from umbilical cord (Mitchell et al. 2003). Wharton’s jelly Mesenchymal cells possess stem cell properties. These cells could be induced to differentiate into osteogenic, chondrogenic, adipogenic, myogenic, and neuron like cells in vitro. It has also been found that the transformed human MSCs of Wharton’s jelly (WJMSCs) survive in different organs of rat after transplantation without the need for immunological suppression, suggesting that WJMSCs might be a good stem cell source for transplantation (Yang et al. 2008). Although there are a lot of studies about positive effects of transplanted stem cells on cutaneous wound healing, to the authors’ knowledge, there is no documented data about the role of WJMSCs as a new source of stem cells on skin wound’s repair. In this study, the effects of transplantation of WJMSCs of caprine umbilical cord on first intention cutaneous wound healing in goat were evaluated.
Material and methods
All experimental protocols were approved by the Research Ethic Committee of the Kerman Neuroscience Research Center of Kerman, Iran. All chemicals except those otherwise indicated were purchased from Sigma-Aldrich Company (St. Louis, MO, USA).
Isolation and culture of MSCs from caprine Wharton’s jelly
WJMSCs of caprine umbilical cord were isolated using the method described previously by Babaei et al. (2008). Briefly, Four Uteri of pregnant mixed goats, in last two months of pregnancy, were collected from abattoir and transported within two hours to the laboratory. Umbilical cords were obtained from the late-gestation fetuses and placed in sterile phosphate buffer saline (PBS, composition in mM: 140 NaCl; 2 KCl; 1.5 KH2PO4; 15 Na2HPO4) supplemented with 2 μg/mL amphotericin B (Bristol-Myers Squibb), 200 IU/mL penicillin and 200 μg/mL streptomycin. Umbilical cord segments, 5 cm long, were cut longitudinally and then, the umbilical cord artery and veins were wiped off. The Wharton’s Jelly of umbilical cord was cut into 2 × 2 mm2 segments. 8–10 segments were transferred to each 35 mm disposable Falcon culture dish (Becton Dickinson & Company Franklin lakes) containing 1 mL of cell culture medium (α-MEM; Alpha modification of Minimum Essential Medium Eagle) supplemented with 20% fetal bovine serum (FBS, Gibco), 2 μg/mL amphotericin B, 200 IU/mL penicillin, and 200 μg/mL streptomycin and maintained at 37°C in a humidified atmosphere of 5% CO2. Adherent Wharton’s jelly fragments were observed 24 h after plating and their cell culture mediums was filled up to 3 mL. Jelly explants were removed from dish cultures 5 days after plating and the adherent cells were cultured for at least 5 more days and the medium was refreshed every 72 h. The adhered cells were dissociated with 0.5 g/l trypsin + 1.0 mM EDTA in PBS. Cells were subcultured in a 250 mL Falcon flask (Becton Dickinson & Company Franklin lakes) and denoted as passage 1.
Immunocytochemistry
Isolated Wharton’s jelly cells were immunostained for detection of α-SMA (mouse monoclonal Clone 1A4; Sigma, A2547) in their cytoplasms. Isolated cells were seeded over a glass slide and allowed to grow up to 48 h. After culturing, growth medium was removed and slides were washed with PBS and were fixed in 4% paraformaldehyde for 5 min at 4°C. After a subsequent rinse with PBS, slides were blocked with 10% normal goat serum for 30 min in humidified chamber at room temperature and washed with PBS. Then the slides were incubated with primary antibody for 60 min, washed three times with PBS and incubated with the secondary antibody (goat anti-mouse IgG conjugated to horseradishperoxidase) for 60 min at room temperature. Afterwards, the cells were stained with 3,3’-diaminobenzidine (DAB) and the brown precipitate product was considered as a positive reaction to α-SMA. Then images were taken by an inverted microscope (Olympus, IX71, Japan) equipped with a digital color camera (DP72, Olympus, Japan).
Alkaline phosphatase assay
The method of Alkaline phosphatase (AP) Assay has been described in the previous study by Babaei et al. (2008). Briefly, the isolated cells were grown on a 35 mm culture dish for several days until colony formation and the medium was refreshed every 72 h. AP activity was detected by using an AP Kit (Sigma-Aldrich Chemie GmbH, Germany, Catalog No. 86-1) according to the manufactures instruction. A dark red reaction product following 15 min of exposure to alkaline dye mixture confirmed AP activity. As a positive control, a blood smear from patient with pyogenic leukocytosis was prepared and stained.
Flow cytometry
The isolated cells were prepared at a concentration of 1 × 105 cells/ml in MEM with 10% FBS incubated for 15 min at 4°C with a 1:9 dilution of normal goat serum in PBS to block nonspecific binding of the primary antibody. Then cells were labeled with antibodies against FITC-conjugated anti-CD44 and FITC-conjugated anti-CD34 (Chemicon; USA) for one hour. The cells were washed with 2% FBS in PBS. Cells were acquired using FACS Calibur (BD = Becton Dickinson, USA) and analyzed using WinMDI Cell Quest Software (BD Biosciences, USA).
Labeling and preparation of cells for transplantation to the wound
For tracking the transplanted cells in the skin wounds, the cells were prelabeled with bromodeoxyuridine (BrdU; Chemicon-Millipore, Temecula, CA) before the transplantation was carried out. Briefly, the stem cells were incubated with 10 μM of BrdU in the culture medium for 48 h in a humidified 37°C incubator with 5% CO2 in the air. To evaluate the efficacy of BrdU incorporation, the treated cells were incubated with 2 N HCl for 30 min. followed by incubation with the primary antibody against BrdU (abcam, USA, 1:40) overnight at 4°C. The cells were then washed three times in PBS and were incubated with fluorescent Alexa-fluor® 647 goat anti-mouse secondary antibody (Molecular probes, USA, 1:400)
After characterization and labeling of the mesenchymal cells isolated from the caprine Wharton’s jelly, 3 × 106 live cells in 600 μl PBS was prepared to transplant to each wound.
Animals and wound model
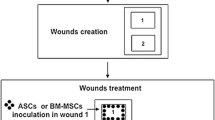
Four adult male Raeini goats with body weights ranging from 18.5–22 kg were used in the trial. The animals were housed in a goat pen and maintained on grass (hay) supplemented with concentrate. Drinkable water was made freely available. Just before the commencement of the experiment, the goats were judged to be in good health based on clinical and hematological evaluation. Four wounds were designed for each goat, two wounds on each side of the midline on dorsal surface of the back region.
The goats were sedated by Intramuscular administration of Xylazine hydrochloride, 0.05 mg/kg, and then placed on dorsal recumbency. In brief, after hair removal from the dorsal surface of thoracic regions, surgical sites were prepared aseptically for the wounding. Four 3-cm linear full thickness cutaneous incisional wounds were created on the dorsal part of thoracic region, on both sides. After accurate hemostasis, cell injections were carried out in the wounds. In this study, left wounds were considered as a treatment group, received 3 million cells in 600 μl PBS, and right wounds received only 600 μl PBS, without any cells. The mesenchymal cells and/or PBS were injected intradermally around the wound at three injection sites and also subcuticulary in wound bed at three injection sites (the total volume of six injections was 600 μl per wound). After cell transplantation, the wounds were sutured with 3–0 nylon (Naylon, Tebkeihan, Iran) in a simple interrupted pattern. All wounds were closed with two sutures.
Histological examination
For microscopic studies of wound healing process, tissue specimens were taken 7 and 12 days after surgery, from the cranial and caudal wounds, respectively, in both sides. The obtained samples from the wound beds and underlying tissue surrounded by margin of normal skin were fixed in 10% buffered formalin. The samples were embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E) for light microscopy. Histological study was in a blinded fashion. Each slide was evaluated for re-epithelialization, dermal cellularity, granulation tissue formation and angiogenesis.
To identify the transplanted cells in wound bed, Four-μm thick slices were deparaffined by standard procedure and then treated with 2 N HCL for 30 min at room temperature. The samples were incubated with the primary antibody against BrdU (abcam, USA, 1:40) overnight at 4°C. After several washing with PBS, the samples were incubated with fluorescent Alexa-fluor® 647 goat anti-mouse secondary antibody (Molecular probes, USA, 1:400) for 1 h. After three washes in PBS, the slides were mounted with glycerol and the images were taken by an Olympus IX71 inverted fluorescent microscope equipped with a DP72 digital color camera (Olympus, Japan).
Results
Characterization of MSCs of caprine Wharton’s jelly
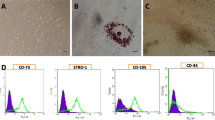
Most of isolated cells from caprine Wharton’s jelly matrix explants displayed fusiform or spindle-form cells with a prominent nucleus and extensive cytoplasmic processes. These cells reached a good confluency after about 10 days so they were subcultured and considered as passage one. Confluent cells were arranged in parallel arrays. As Wharton’s jelly cells reached considerable confluency, colonies of cells began to form (Fig. 1).
Immunocytochemical analysis was performed to test the expression of Actin protein. Cells with brown filaments in cytoplasm were considered as α-SMA Positive cells. (Fig. 2).
In the current study, isolated cell colonies formed in the culture exhibited positive AP activity. The reaction was very intense at the border of colonies.
Flow cytometry analysis of WJMSCs showed that they were negative for CD34 and positive for CD44 (Fig. 3).
Microscopic study
To identify transplanted WJMSCs in the wound, the cells were prelabeled with BrdU. In our pilot study, bromodeoxyruidine staining of MSCs demonstrated that nearly 80% of cells were labeled with BrdU. The transplanted MSCs were localized within the granulation tissue that was confirmed by means of immunohistochemical staining with monoclonal antibodies against BrdU (Fig. 4).
Histopathological evaluation of wounds on day 7, disclosed enhanced re-epithelialization in MSCs-treated wounds (complete epithelialization in all 4 treated wounds) in comparison with wounds in control group (incomplete epithelialization in all 4 non-treated wounds). Inflammatory cell infiltration in non-treated wounds was more severe than the treated wounds. Unlike treated wounds, ulcer was observed in all non-treated wounds obviously (Figs. 5 and 6).
On day 12, in non-treated wounds, more but incomplete re-epithelialization and thick granulation tissue was observed (Fig. 7), whereas, in treated wounds intact skin with complete re-epithelialization, no inflammation, and thin granulation tissue were seen. Totally, histopathological study clearly confirmed that the wound healing process in non-treated wounds was significantly in earlier phases compared with the WJMSCs-treated wounds.
According to the microscopic study, transplanted WJMSCs survived in the cutaneous wound bed and significantly promoted healing process.
Discussion
The present study investigates the efficacy of transplantation of mesenchymal stem cells that were isolated from caprine Wharton’s jelly on incisional cutaneous wound healing in goats. In recent years, great successes have been achieved in accelerating the capacity for wound healing. Several studies have indicated a contribution of MSCs to reconstituting skin in cutaneous wounds (Fu and Li 2009). Animal transplantation studies have shown that MSCs are able to differentiate into cells of the residing tissue, repair tissue damaged by trauma or disease and partially restore normal function. (Yoshikawa et al. 2008; Sasaki et al. 2008) MSCs not only participate in the regeneration of tissue of mesenchymal lineage such as intervertebral disc cartilage (Crevensten et al. 2004), bone (Arinzeh et al. 2003), and cardiomyocytes (Fukuda 2002), but also appear to differentiate into cells derived from other embryonic layers, including skin (Sasaki et al. 2008). In the most pervious studies, MSCs were isolated from bone marrow and other tissue such as adipose tissue, nerve tissue, umbilical cord blood and dermis (Kim et al. 2007; Dai et al. 2007; Perng et al. 2006; Shih et al. 2005; Jones et al. 2002; Luo et al. 2010; Tark et al. 2010). These experiments demonstrated the plasticity of MSCs and their potential usefulness in complex tissue repair and regeneration and in cell therapy (Fu and Li 2009).
In the current study, MSCs were isolated from Wharton’s jelly of caprine umbilical cord. The Wharton’s jelly of the umbilical cord contains mucoid connective tissue and fibroblast like cells (Wang et al. 2004). In pervious studies Wharton’s jelly cells were successfully isolated from human and procaine umbilical cord tissue and explanted as primary culture (Mitchell et al. 2003; Wang et al. 2004). It has been confirmed that multipotent MSCs exist in the Wharton’s jelly of the human umbilical cord and can be isolated easily (Meyer et al. 2008). Mesenchymal stromal cells isolated from Wharton’s jelly have been induced to form bone, cartilage, adipose, and neuron-like cells (Troyer and Weiss 2008; Yang et al. 2008). In a preliminary study, Babaei isolated MSCs from Wharton’s jelly of caprine umbilical cord. He stated that the isolated cells from Wharton’s jelly show stem cells behaviors (Babaei et al. 2008).
The morphology of the heterogenous population of Wharton’s jelly cells isolated from explants included, mesenchymal-like cells with a fusiform or stellete appearance and individual round cells. The cells are able to form colonies after confluency stage in dish culture (Mitchell et al. 2003). The Wharton’s jelly contains colony-stimulating activity and growth factors IGF-1, EGF and TGFα (Wang et al. 2004). The classical assay used to identify MSCs is the colony forming unit assay that identifies adherent spindle shaped cells that proliferate to form colonies (Sasaki et al. 2008). Wharton’s jelly was shown to be composed of smooth-muscle-actin-positive and myofibroblast-like stromal cells (Kobayashi et al. 1998; Mitchell et al. 2003). Another nonspecific test for approving stem cells is expression of AP enzyme. AP is an enzyme that is long-known to be expressed in embryonic stem cells as well as primordial germ cells (MacGregor et al. 1995). In the pervious study, Babaei et al. (2008) showed that colonies formed by WJMSCs of caprine umbilical cord exhibited AP activity in vitro. In an in vitro study, AP activity was also detected in matrix cells colonies of pig umbilical cord (Carlin et al. 2006).
Base on the flow cytometric analysis to determine various superficial cell markers in human MSCs isolated from Wharton’s jelly, it has been suggested that these stromal cells are similar to MSCs. Human MSCs of Wharton’s jelly express matrix receptors CD44 but not hematopoietic lineage marker CD34 (Wang et al. 2004; Troyer and Weiss 2008). It has been stated that canine bone marrow-derived mesenchymal stem cells were positive for CD44 and negative for CD34. The negative results obtained for CD34 demonstrated that cells of hematopoietic origin were excluded during the WJMSC culture. The positive results obtained for CD44 emphasized that the isolated cells were MSCS (Jung et al. 2009).
In the present study, based on the cells characteristics and behaviors in culture medium and also according to the imunohistochemical staining and flow cytometric analysis, the isolated mesenchymal cells from caprine Wharton’s jelly were mesenchymal stem cells. The isolated cells in our study were fusiform or spindle-form cells and were able to form cell colony in culture. These cells showed a positive reaction to immunohistochemical staining of α-actin smooth muscle and alkaline phosphate. The flow cytometric analysis showed that some of the isolated cells were positive for CD44 and most of them were negative for CD34.
The Wharton’s jelly cells are non-immunogenic on the first injection into the allogenic recipients. However, repeated injection of Wharton’s jelly Cells produced an immunogenic response (Troyer and Weiss 2008). In this study we used allogenic WJMSCs transplantation; donors were mixed breed goats and recipients were Raeini goats, and the BrdU staining of labeled cells confirmed that the transplanted cells were still present in the wound bed. To date, there is no research about the effects of MSCs of Wharton’s jelly on cutaneous wound healing, but the beneficial effects of this type of MSCs were approved in various experimental disorders. Yang’s revealed the beneficial effects of human MSCs isolated from Wharton’s jelly after complete transaction of the rat spinal cord. He stated that transplanted cells promote the regeneration of corticospinal fibers and locomotors recovery in the rat. He also stated that the transformed Human WJMSCs in the rat were still viable 4 months after transplantation without need for immunological suppression, suggesting that these cells might be a good stem cell source for transplantation (Yang et al. 2008). Lund et al. (2007)) administered WJ-Cs into the eyes of a rodent model of retinal disease. They reported that the WJ-Cs exhibited the best histological evidence of photoreceptor rescue (Lund et al. 2007). Human WJ-Cs ameliorates apomorphine-induced behavioral deficits in a hemiparkinsonian rat model (Weiss et al. 2006). In other studies the positive effects of WJ-MSC for treatment of stroke (Borlongan et al. 2004) or myocardial infarction (Grinnemo et al. 2004) were confirmed.
A lot of studies confirmed those bone marrows MSCs are useful in cutaneous wound healing process. Bone marrow MSCs repair epithelium in vitro through differentiation and fusion (Spees et al. 2003). It has been demonstrated that MSCs prompted cutaneous wound repair via differentiation into multiple skin cell types including; keratinocytes, endothelial cells, pericytes, and monocytes (Sasaki et al. 2008). Wu suggested that bone marrow engrafted in cutaneous wound of diabetic rat completed re-epithelialization after 7 days (Wu et al. 2007). MSCs have also been reported to differentiate into various epithelial cell types such as; skin epithelial cells, after systemic administration in vivo (Nakagawa et al. 2005). Luo et al. (2010) showed that human umbilical cord MSCs could differentiate into keratinocyte in the cutaneous wound tissue of mice, when it was administered locally on the fresh wounds (Luo et al. 2010). Our study showed that epithelialization was complete on day 7 or earlier in the treated wounds with WJMSCs, whereas in the control wound (without treatment), the wounds still showed incomplete epithelialization 12 days after wounding. Also microscopic evaluation showed minimum inflammation and thin granulation tissue formation with minimum scar in the MSCs treated wounds, which indicates a better wound healing process in the treatment group.
In conclusion, this study demonstrates the beneficial effect of caprine WJMSCs in cutaneous wound healing in goat. Administration of WJMSCs may present novel therapeutic methods in the treatment of cutaneous wound, especially in chronic wounds and other conditions.
References
Arinzeh TL, Peter SJ, Archambault MP, van den Bos C, Gordon S, Kraus K, Smith A, Kadiyala S (2003) Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am 85-A:1927–1935
Babaei H, Moshrefi M, Golchin M, Nematollahi-Mahani SN (2008) Assess the pluripotency of caprine umbilical cord Wharton’s jelly mesenchymal cells by RT-PCR analysis of early transcription factor nanog. Iran J Vet Surg 3:57–65
Badiavas EV, Falanga V (2003) Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol 139:510–516
Borlongan CV, Hadman M, Sanberg CD, Sanberg PR (2004) Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 35:2385–2389
Carlin R, Davis D, Weiss M, Schultz B, Troyer D (2006) Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol 4:1–13
Crevensten G, Walsh AJ, Ananthakrishnan D, Page P, Wahba GM, Lotz JC, Berven S (2004) Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng 32:430–434
Dai Y, Li J, Li J, Dai G, Mu H, Wu Q, Hu K, Cao Q (2007) Skin epithelial cells in mice from umbilical cord blood mesenchymal stem cells. Burns 33:418–428
Fu X, Li H (2009) Mesenchymal stem cells and skin wound repair and regeneration: possibilities and questions. Cell Tissue Res 335:317–321
Fukuda K (2002) Molecular characterization of regenerated cardiomyocytes derived from adult mesenchymal stem cells. Congenit Anom Kyoto 42:1–9
Grinnemo KH, Mansson A, Dellgren G, Klingberg D, Wardell E, Drvota V, Tammik C, Holgersson J, Ringdén O, Sylvén C, Le Blanc K (2004) Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infracted rat myocardium. J Thorac Cardiovasc Surg 127:1293–1300
Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Mull L, Hofmann T (2002) Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA 99:8932–8937
Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, Markham AF, Jack A, Emery P, McGonagle D (2002) Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum 46:3349–3360
Jung DI, Ha J, Kang BT, Kim JW, Fu SQ, Lee JH, Woo EJ, Park HM (2009) A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci 285:67–77
Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS (2007) Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 48:15–24
Kobayashi K, Kubota T, Aso T (1998) Study on myofibroblast differentiation in the stromal cells of Wharton’s jelly-expression and localization of smoot muscle actin. Early Hum Dev 51:223–233
Kunter U, Rong S, Djuric Z, Boor P, Müller-Newen G, Yu D, Floege J (2006) Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol 17:2202–2212
Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ (2006) Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA 103:17438–17443
Lund RD, Wang S, Lu B, Girman S, Holmes T, Sauvé Y, Messina DJ, Harris IR, Kihm AJ, Harmon AM, Chin FY, Gosiewska A, Mistry SK (2007) Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 25:602–611
Luo G, Cheng W, He W, Wang X, Tan J, Fitzgerald M, Li X, Wu J (2010) promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair Regen 18:506–513
MacGregor G, Zambrowicz BP, Soriano P (1995) Tissue non-specific alkaline phosphatase is expressed in both embryonic and extraembryonic lineages during mouse embryogenesis but is not required for migration of primordial germ cells. Development 121:1487–1496
Meyer T, Pfeiforth A, Hocht B (2008) Isolation and characterization of mesenchymal stem cells in Wharton’s jelly of the human umbilical cord: potent cells for cell-based therapies in paediatric surgery? Eur Surg 40:239–244
Minguell JJ, Erices A (2006) Mesenchymal stem cells and the treatment of cardiac disease. Exp Biol Med (Maywood) 231:39–49
Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-easa K, Hildreth T, Troyer D (2003) Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells 21:50–60
Nakagawa H, Akita S, Fukui M, Fujii T, Akino K (2005) Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol 153:29–36
Ortiz LA, DuTreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG (2007) Interleukin 1 receptor antagonist mediates the anti-inflammatory and anti-fibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 104:11002–11007
Perng CK, Ku HH, Chiou SH, Chen IL, Tsai FT, Yang YP, Chang KY, Kao CL (2006) Evaluation of wound healing effect on skindefect nude mice by using human dermis-derived mesenchymal stem cells. Transplant Proc 38:3086–3087
Phinney DG, Isakova I (2005) Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des 11:1255–1265
Phinney DG, Prockop DJ (2007) Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair— current view. Stem Cells 25:2896–2902
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H (2008) Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 180:2581–2587
Shih DT, Lee DC, Chen SC, Tsai RY, Huang CT, Tsai CC, Shen EY, Chiu WT (2005) Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells 23:1012–1020
Spees JL, Olson SD, Ylostalo J, Patrick JL, Smith J, Perry A, Peister A, Wang MY, Propckop DJ (2003) Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA 100:2397–2402
Stepanovic V, Awad O, Jiao C, Dunnwald M, Schatteman GC (2003) Leprdb diabetic bone marrow cells inhibit skin wound vascularization but promote wound healing. Circ Res 92:1–7
Tark KC, Hong JW, Kim YS, Hahn SB, Lee WJ, Lew DH (2010) Effects of human cord mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg 65:565–572
Troyer DL, Weiss ML (2008) Wharton’s jelly-drived cells are a primitive stromal cell population. Stem Cells 26:591–599
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC (2004) Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 22:1330–1337
Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D (2006) Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 24:781–792
Wu Y, Chen L, Scott PG, Tredget EE (2007) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25:2648–2654
Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS (2008) Transplantation of human umbilical mesenchymal stem cells from Whartons jelly after complete transection of the rat spinal cord. PLoS ONE 3:1–11
Yoshikawa T, Mitsuno H, Nonaka I, Sen Y, Kawanishi K, Inada Y, Takakura Y, Okuchi K, Nonomura A (2008) Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg 121:860–877
Acknowledgments
This study was supported financially by the Research Council of Veterinary College, Kerman Shahid Bahonar University, for which the authors are most grateful.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azari, O., Babaei, H., Derakhshanfar, A. et al. Effects of transplanted mesenchymal stem cells isolated from Wharton’s jelly of caprine umbilical cord on cutaneous wound healing; histopathological evaluation. Vet Res Commun 35, 211–222 (2011). https://doi.org/10.1007/s11259-011-9464-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-011-9464-z