Abstract
The current experiment was performed to find the potential effect of inorganic and organic forms of zinc (Zn) on growth, intestinal histomorphology, immune response, and paraoxonase (PON1) activity in broiler. In this experiment, a total of 450 broiler chickens were assigned to four experimental and control groups. The birds received organic Zn at the rate of 50 mg/kg (OZ-50) and 60 mg/kg (OZ-60) or inorganic Zn at the rate of 50 mg/kg (IZ-50) and 60 mg/kg (IZ-60) for an experimental period of 30 days. Significantly (P < 0.05) higher feed consumption, body weight, feed conversion ratio, and production efficiency factor (PEF) were recorded in OZ-50. Similarly, antibody titer against infectious bronchitis (IB) and PON1 activity was higher (P < 0.05) in OZ-50 compared with the control group. In addition, significantly (P < 0.05) higher villus dimensions and goblet cell count were recorded for the group OZ-50 compared with other treatments. It was concluded that the organic form of Zn was superior in improving the growth, histological features of intestines, humoral response, and PON1 activity in broiler.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is a well-known essential micronutrient required for a number of biological activities in animals (Khan et al. 2011; Rahman et al. 2014; Naz et al. 2016; Shah et al. 2019). Presently, Zn has been recommended at the rate of 40 mg/kg in the feed (Naz et al. 2016). The bioavailability of Zn is affected negatively by a number of factors such as high concentration of calcium and presence of phytates in the feed leading to the deficiency of this micronutrient (Jarosz et al. 2017). Zinc is usually added in the poultry feed in the form of oxides or sulfates. These forms of Zn exhibit low bioavailability, irritate the gastrointestinal mucosa, and increase its excretion in the environment leading to the environmental pollution (Cao et al. 2002).
Generally, inorganic source of Zn is more common in poultry feeding system. However, now numerous negative effects of inorganic Zn such as less absorption, low bioavailability, and high hydrophobicity as well as oxidation processes have been reported (Min et al. 2018). Organic sources of Zn have now been increasingly popular for enhanced poultry production mostly in the form of chelates, methionine, or other complexes (Jarosz et al. 2017; Min et al. 2018). Numerous studies have reported improved findings in the form of higher feed efficiency and weight gain in broiler-fed organic form of Zn (Jahanian and Rasouli 2015; Akhavan-Salamat and Ghasemi 2019). There is continuous efforts to find a new form of organic Zn complexes with better effects. Combination of Zn with glycine is known for better bioavailability due to the improved absorption through intestinal villi, which can help to reduce the glycine (Gly) content in feed and thus the feed cost (Jarosz et al. 2017).
The published literature so far did not conclude research findings on the effect of Zn-Gly chelates and zinc sulfate (ZnSO4) on growth, immune response, intestinal histology, and paraoxonase (PON1) activity in broiler. The aim of the study was to determine the organic and inorganic form of Zn on the growth performance, intestinal mucosa, humoral immunity, and PON1 activity in broiler.
Materials and methods
Experimental animals
The study was conducted on a total of 450 broiler chicks (Ross 308). The birds were kept in a semi-intensive poultry farm in cages (1 m × 1 m) consisting of 10 birds per cage. In addition, these cages were equipped with a feeder and drinkers which were adjusted continuously with the age of the birds. All cages were kept under the same environmental condition with a continuous lighting system. During brooding period, the temperature was maintained at 34 °C, which was continuously dropped down to thermoneutral temperature of 20–22 °C. The birds were fed a mixture of appropriate feed for each particular period of rearing ad libitum as shown in Table 1 with unlimited access to water. The birds were divided into five groups: one was the control and the other four experimental groups with 90 birds each. The basal diet of the broilers in the control group was almost similar as recommended for Ross 308 along with mineral-vitamins mixture with 40 mg/kg Zn. The chickens in the other groups than the control group received Zn either in the form of inorganic form (zinc sulfate) at the rate of 50 and 60 mg/kg (IZ-50 and IZ-60) or organic form (OZ-50 and OZ-60) as Zn-Gly. Birds were routinely vaccinated against infectious diseases including infectious bronchitis (IB). The experiment was carried out for 35 days including the first week as the adaptation period. Survival rate was recorded for each group whenever occurred.
Measurements
On day 35, data on feed consumption and gain in body weight were recorded on a cage basis. These data were used to the calculate feed conversion ratio (FCR). The production efficiency factor (PEF) was computed by the method described by Abudabos et al. (2018).
Blood sampling
Blood samples were collected from 1 bird/cage randomly from the brachial vein into heparinized tubes, centrifuged (2700×g), and stored at frozen temperature (− 20 °C).
Intestinal sample collection
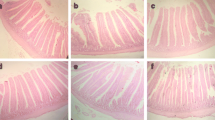
After bleeding, the birds were killed for sampling of intestines. The ileum was identified and a small piece was removed and flushed with cold BPS. Samples were routinely processed and stained with hematoxylin and eosin (H&E) and observed at × 10 under the microscope attached with a digital camera for villus height, villus width, crypt depth, and goblet cells.
Paraoxonase-1 activity (PON1)
The PON1 activity in blood samples was found using a spectrophotometer (IRMECO Model U2020) by the method described by Safiullah et al. (2019). Briefly, 350 μl of calcium chloride (2 mmol), Tris-base buffer (0.1 M, pH 8.0), and 3 μl of paraoxon were mixed and measured at 503 nm after minutes of incubation.
Humoral immunity
Antibody titer against infectious bronchitis (IB) was determined as described by Zia ur Rehman et al. (2017).
Statistical analysis
Data was analyzed by a one-way ANOVA (SPSS 21.0 (SPSS Inc., Chicago, IL) followed by Tukey’s test to find the significant difference between treatment means. Significance was declared at P < 0.05.
Results
The results of feed consumption, body weight, FCR, and PEF in broiler in the control and treatment groups are shown in Table 2. The results showed that feed consumption was significantly (P < 0.05) higher in the treatment groups in comparison with the control. In addition, feed intake was significantly (P < 0.05) high in OZ-50 compared with all the other treatments. Similarly, body weight was also significantly (P < 0.05) higher in OZ-50 showing the efficacy of organic Zn. Interestingly, the OZ-60 had a lower effect on feed intake and weight gain in broiler compared with OZ-50. The FCR was also significantly (P < 0.05) low in OZ-50. On the other hand, the PEF was significantly (P < 0.05) improved in the treatment groups compared with the control with no significant difference (P > 0.05) in organic and inorganic forms of Zn.
The effect of the two forms of Zn on survival rate, antibody titer, and PON1 of broiler is given in Table 3. No significant difference was found in the survival rate in birds in the control and treatment groups. Mean antibody titer against IB virus was significantly (P < 0.05) higher in OZ-50 compared with all other treatments. Similarly, PON1 was also significantly (P < 0.05) high in the treatment groups.
The effect of dietary supplementation of Zn in the organic and inorganic form is given in Table 4. Villus height, width, depth, and goblet cells were significantly (P < 0.05) high in OZ-50 compared with the control and all other treatments.
Discussion
In the current study, growth performance in the form of mean feed intake, weight gain, and feed efficiency were significantly higher in broilers in the treatment groups compared with the control. It was also clear that the organic form of Zn was also superior in its effect compared with the inorganic form. In addition, there was a dose-dependent effect in both types of Zn. The organic form of Zn is a chelating agent that increases the absorptive and utilization capacity of feed in the body and declines excretion of essential nutrients from the body. The superior effect of organic Zn may be due to the higher bioavailability of Zn in the blood causing enhanced digestibility of nutrients (Star et al. 2012). The use of Zn-Gly complex has a beneficial impact on the growth and immune response (Jarosz et al. 2017). The higher performance in Zn-treated birds has also been attributed to the positive effect of Zn on the digestive enzymes’ secretion (Naz et al. 2016). The organic form of Zn is more environment friendly and remains in the gut for a long time resulting in improved performance.
Similarly, in the current experiment, villus dimensions and goblet cells count in the ileum were significantly affected by the type of Zn supplementation. The outcome of the organic form of Zn was superior in comparison with the inorganic Zn on the histology of ileum. Improved villus dimensions have already been reported by different groups of researchers in response to organic and inorganic sources of Zn in broiler (Payne et al. 2006; Hu et al. 2013; Levkut et al. 2017; Shah et al. 2019). In the present study, the improved dimensions of villus may be linked with increased absorptive surface and higher proliferation of crypt cells of the villus due to the supplementation of Zn. In addition, the number of goblet cells increased in the experimental groups that could be attributed to mucin gene regulation, secretion of bioactive factors, and differentiation and improvement in host immunity (Shah et al. 2019) leading to the higher performance in the Zn-treated birds.
In the current study, PON1 concentration in the treatment groups improved irrespective of the nature of supplemental Zn. Previously improved PON1 activity was reported in broiler under the supplementation of different compounds such as selenium, vitamin E, l-carnitine, ginger, and synthetic γ-aminobutyric acid (Khan et al. 2014; Chand et al. 2016; Zia ur Rehman et al. 2017; Safiullah et al. 2019). PON1 is known as an important antioxidant enzyme and its concentration is correlated with vitamin C (Khan et al. 2014). Zinc is well known as an important part of the antioxidant system of the body in addition to the protective effect of protein, immune cells, and enzymes through the suppression of free radicals (Naz et al. 2016). Zinc is also known for the stabilizing role of lipid peroxidation through neutralizing free radicals. In addition, Zn is also an important part of superoxide dismutase, which defends the body against injury of the free radicals (Rahman et al. 2014) which might have resulted in the higher blood concentration of PON1.
In the current study, the organic and inorganic forms of Zn improved the antibody titer against IB. Again, a higher antibody titer was observed in OZ-50. Zinc is well known for activation of antibody production and engulfing foreign bodies (Khan et al. 2011). In the organic form, Zn has also been known for the improvement of IgA and IgG (Jarosz et al. 2017). Further, Jarosz et al. (2017) reported that Zn-Gly is involved in the potentiation of the immune system through the production of inflammatory cytokines and increased expression of immunoglobulins compared with the inorganic form of Zn in broiler.
Conclusion
From the findings of the present study, it was concluded that the organic form of Zn was superior in improving the growth, histological features of intestines, humoral response, and PON1 activity in broiler.
References
Abudabos A.M., Alyemni A.H., Dafalla, YM. and Khan R.U., 2018. The effect of phytogenics on growth traits, blood biochemical and intestinal histology in broiler chickens exposed to Clostridium perfringens challenge. Journal of Applied Animal Research, 46, 691-695. https://www.tandfonline.com/doi/full/10.1080/09712119.2017.1383258. Accessed 25 March 2019
Akhavan-Salamat H. and Ghasemi HA., 2019. Effect of different sources and contents of zinc on growth performance, carcass characteristics, humoral immunity and antioxidant status of broiler chickens exposed to high environmental temperatures. Livestock Science, 223, 76-83. https://www.sciencedirect.com/science/article/abs/pii/S1871141319303452. Accessed 20 April 2019
Cao J., Henry P.R., Davis S.R., Cousins R.J., Miles R.D., Littell R.C. and Ammerman C.B., 2002. Relative bioavailability of organic zinc sources based on tissue zinc and metallothionein in chicks fed conventional dietary zinc concentrations. Animal Feed Science and Technology, 101, 161–170. https://www.sciencedirect.com/science/article/pii/S0377840102000512. Accessed 16 April 2019
Chand N., Muhammad S., Khan R.U., Alhidary I.A. and Zia ur Rahman 2016. Ameliorative effect of synthetic γ-aminobutyric acid (GABA) on performance traits, antioxidant status and immune response in broiler exposed to cyclic heat stress. Environmental Science and Pollution Research, 23,23930–23935. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s11356-016-7604-2. Accessed 14 March 2019
Hu C.H., Qian Z.C., Song J., Luan Z.S. and Zuo A.Y., 2013. Effects of zinc oxide-montmorillonite hybrid on growth performance, intestinal structure, and function of broiler chicken. Poultry Science, 92, 143–50. https://academic.oup.com/ps/article/92/1/143/1553796 Access 19 April 2019
Jahanian R. and Rasouli E., 2015. Effects of dietary substitution of zinc-methionine for inorganic zinc sources on growth performance, tissue zinc accumulation and some blood parameters in broiler chicks. Journal of Animal Physiology and Animal Nutrition, 99, 50–58. https://onlinelibrary.wiley.com/doi/abs/10.1111/jpn.12213. Accessed 8 March 2019
Jarosz Ł., Marek A., Grądzki Z., Kwiecień M., Żylińska B. and Kaczmarek B., 2017. Effect of feed supplementation with zinc glycine chelate and zinc sulfate on cytokine and immunoglobulin gene expression profiles in chicken intestinal tissue. Poultry Science 96, 4224-4235. https://academic.oup.com/ps/article/96/12/4224/4349810. Accessed 3 April 2019
Khan R.U., Nikousefat Z., Javdani M., Tufarelli V. and Laudadio V. 2011. Zinc-induced molting: production and physiology. World’s Poultry Science Journal, 67, 497-506. https://www.cambridge.org/core/journals/world-s-poultry-science-journal/article/zincinduced-moulting-production-and-physiology/A79140CE542DB70A2EB86DAC177E7DE0. Accessed 11 Feb 2019
Khan, R.U., Rahman, Z.U., Javed I. and Muhammad F., 2014. Serum antioxidants and trace minerals as influenced by vitamins, protein and probiotics in male broiler breeders. Journal of Applied Animal Research, 42(3), 249-255. https://www.tandfonline.com/doi/full/10.1080/09712119.2013.822815. Accessed 25 April 2019
Levkut M., Husáková E., Bobíková K., Karaffová V., Levkutová M., Ivanišinová O., Grešáková Ľ., Čobanová K., Reiterová K. and Levkut M., 2017. Inorganic or organic zinc and MUC-2, IgA, IL-17, TGF-β4 gene expression and sIgA secretion in broiler chickens. Food and Agriculture Immunology, 28(5), 801-811. https://www.tandfonline.com/doi/full/10.1080/09540105.2017.1313202. Accessed 2 May, 2019
Min, Y.N., Liu, F.X., Qi X., Ji S., Cui L., Wang Z.P. and Gao Y.P. 2018. Effects of organic zinc on tibia quality, mineral deposit, and metallothionein expression level of aged hens. Poultry Science, 99, 366-372. https://academic.oup.com/ps/article/98/1/366/5088951. Accessed 23 March 2019
Naz S., Idris M., Khalique M.A., Alhidary I.A., Abdelrahman M.M., Khan R.U., Chand N., Farooq U. and Ahmad S. 2016. The activity and use of zinc in poultry diet. World’s Poultry Science Journal, 72, 159–167. https://www.cambridge.org/core/journals/world-s-poultry-science-journal/article/activity-and-use-of-zinc-in-poultry-diets/BCEC224438E5B666E0993C0D729D44AA. Accessed 2 April 2019
Payne R.L., Bidner T.D., Fakler T.M. and Southern L.L., 2006. Growth and intestinal morphology of pigs from sows fed two zinc sources during gestation and lactation. Journal of Animal Science, 84(8), 2141-2149. https://academic.oup.com/jas/article-abstract/84/8/2141/4777380. Accessed 29 April 2019
Rahman, H., Qureshi, M.S. and Khan, R.U., 2014. Influence of dietary zinc on semen traits and seminal plasma antioxidant enzymes and trace minerals of Beetal bucks. Reproduction in Domestic Animals 48(6), 1004–1007. https://onlinelibrary.wiley.com/doi/abs/10.1111/rda.12422. Accessed 12 Feb 2019
Shah M., Zaneb H., Masood S., Khan R.U., Ashraf S., Sikandar A., Rehman H.F. and Rehman H.U., 2019. Effect of dietary supplementation of zinc and multi-microbe probiotic on growth traits and alteration of intestinal architecture in broiler. Probiotic Antimicrob Proteins. (in press) doi https://doi.org/10.1007/s12602-018-9424-9. https://www.ncbi.nlm.nih.gov/pubmed/29680883 Acessed 19 Feb 2019
Safiullah, Chand N., Khan R.U., Naz S., Ahmad M. and Gul S., 2019. Effect of ginger (ZingiberofficinaleRoscoe) and organic selenium on growth dynamics, blood melanodialdehyde and paraoxonase in broilers exposed to heat stress. Journal of Applied Animal Research, 47, 212–216. https://www.tandfonline.com/doi/full/10.1080/09712119.2019.1608211. Accessed 12 Jan 2019
Star L., Van der Klis J.D., Rapp C. and Ward T.L. 2012. Bioavailability of organic and inorganic zinc sources in male broilers. Poultry Science, 91(12), 3115–3120. https://academic.oup.com/ps/article/91/12/3115/1568653. Accessed 29 Feb 2019
Zia ur Rehman, Chand N and Khan R.U. 2017. The effect of vitamin E, L-carnitine and ginger on production traits, immune response and antioxidant status in two broiler strains exposed to chronic heat stress. Environmental Science Pollution Research, 24, 26851–26857. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s11356-017-0304-8. Accessed 3 April 2019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chand, N., Zahirullah, Khan, R.U. et al. Zinc source modulates zootechnical characteristics, intestinal features, humoral response, and paraoxonase (PON1) activity in broilers. Trop Anim Health Prod 52, 511–515 (2020). https://doi.org/10.1007/s11250-019-02036-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-019-02036-4