Abstract
Habitat conversion is one of the major threats for biodiversity conservation and viability of natural populations. Thus, habitat disturbance alters distinct ecological processes, such as plant reproductive success and diaspore fate. In this study, we determined the effects of seasonally tropical dry forests (STDFs) conversion by anthropogenic disturbance by assessing diaspore fate of Enterolobium contortisiliquum. We compared 20 adult trees present in a STDFs preserved area and 20 adult trees present in a human-converted area. In general, diaspore fates from both areas were similar, i.e., there was no difference in the reproductive success of trees in STDFs and human-converted area. Habitat disturbance did not affect the length or width of fruits; only fruit thickness was larger in trees of STDFs habitat. None of the biometric seed measures differed between different habitat conditions. Likewise, the number of undamaged seeds, aborted seeds, pre-dispersal predated seeds, and seed production were independent of habitat conditions. Besides, we did not observe any effect of habitat disturbance on germination percentage. However, seeds from preserved STDFs germinated faster than seeds from the human-converted area. Even though the effects of human-modified habitats on the diaspore fate have already been studied, tree species exhibit different responses to habitat conversion regarding seed predation, seed dispersal, seed germination, and seedling establishment. Overall, our results show that habitat disturbance does not affect the diaspore fate of E. contortisiliquum. This study also highlights the importance of remnants trees in converted landscapes as the population’s connectors which maintain plant–animal mutualistic and antagonistic interactions that mitigate the effects of habitat disturbance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deforestation is the major threat to biodiversity conservation and the viability of natural populations (Saunders et al. 1991; Sala et al. 2000; Haddad et al. 2015). Biodiversity preservation in disturbed habitats requires an understanding of the effects of landscape change on community dynamics (Burkey 1993; Aguilar et al. 2012) since forest conversion may change species composition and abundance, thus altering ecological processes, such as mutualistic and antagonistic animal–plant interactions (Saunders et al. 1991; Aizen and Feinsinger 1994a; Debinski and Holt 2000; Emer et al. 2018, 2019; Marjakangas et al. 2019; Hooper and Ashton 2020). Habitat conversion by anthropogenic changes alters the optimal conditions for the long-term persistence of native plant species (Ashworth and Martí 2011; Galetti et al. 2013) because it affects animal populations that interact with in many stages of plants’ life cycles (Dirzo and Miranda 1991; Aizen and Feinsinger 1994b).
Due to the intimate interactions with pollinators and seed dispersers, the reproductive success of many plant species has been negatively affected by habitat conversion (Aizen and Feinsinger 1994b; Haddad et al. 2015; Browne and Karubian, 2018; Emer et al. 2018; Hooper and Ashton 2020). For instance, habitat conversion has negative consequences for plant genetic diversity (Aguilar et al. 2006, 2008; Rosas et al. 2011; Browne and Karubian 2018), since in disturbed habitats, the abundance of pollinators decreases, and selfing increases, resulting in inbreeding depression (Aguilar et al. 2006, 2008, 2012, 2019; Eckert et al. 2009; Breed et al. 2015; Broadhurst 2015). Hence, the quantity and/or quality of progenitors are reduced (Aizen and Feinsinger 1994b; Ghazoul et al. 1998; Cascante et al. 2002; Hooper and Ashton 2020). Additionally, habitat conversion negatively affects diaspore fate by reducing fruit and seed set (Ghazoul et al. 1998; Fuchs et al. 2003; Quesada et al. 2004; Kolb 2008; Hooper and Ashton 2020), reducing seed germination (Menges 1991; Bruna 1999; Cascante et al. 2002; Ashworth and Martí 2011), and decreasing seed predation (Cascante et al. 2002; Chacoff et al. 2004; Burgos et al. 2008; Herrerías-Diego et al. 2008; Mendes et al. 2016). Indeed, seed predation can be a specific antagonistic interaction, such as observed for Fabaceae family and bruchids (Coleoptera), in which about 85% of seed predation rate is caused by this insects (Johnson 1985). Due to this strong interaction, habitat alteration may affect plant demography, since landscape changes decrease the diversity and abundance of beetle species (Didham et al. 1998). However, contradictory results were found for seed predation, and no differences in predation rates by bruchines were detected in the palm Attalea humili (Andreazzi et al. 2012) located in large and small Atlantic Forest remnants. Even an increase of seed predation in fragment patches in relation to continuous forests was reported in the tree species Prunus avium and Viburnum lantana (Kollmann and Buschor 2003). Altogether, these selective pressures may determine plant reproductive success and affect population recruitment (Crawley 2000).
Over the last decade, many researchers investigated the effects of habitat disturbance on diaspore fate by assessing both mutualistic and antagonistic interactions (Aizen and Feinsinger 1994a, b; Ashworth and Martí 2011; Skogen et al. 2016; Morrison and Mendenhall 2020). However, different plant species have distinct responses to habitat disturbance (Chen et al. 2017; Newbold et al. 2019), and the existing evidence do not show clear patterns for the effects of habitat disturbance on plant reproductive success (Aizen and Feinsinger 1994a, b; Costin et al. 2001; Ghazoul 2005; Ashworth and Martí 2011; Skogen et al. 2016). It has been proposed that tropical trees could be more adaptable and resilient to habitat conversion as a result of their longevity, high intra-population genetic diversity, and high rates of pollen movement (White et al. 2002; Hamrick 2004; Deacon and Cavender-Bares 2015).
Most of the studies that assessed diaspore fate response under habitat disturbance have involved evergreen species, but a better understanding is needed for trees from Seasonally Tropical Dry Forests (STDFs) (but see Aizen and Feinsinger 1994a, b; Rocha and Aguilar 2001b; Ashworth and Martí 2011; Souza-Silva et al. 2015). Despite the large tropical distribution and importance of STDFs, these ecosystems are endangered by anthropogenic activities (Murphy and Lugo 1986; Janzen 1988; Sánchez-Azofeifa et al. 2005, 2009; Espírito-Santo et al. 2009; Dupin et al. 2018), with a deforestation rate of 12% in Latin America from 1980 and 2000 (Miles et al. 2006). STDFs soils are often fertile (Murphy and Lugo 1986), and the conversion of these forests into pasture and agricultural landscapes has been increasing in the last decades (Mass 1995; Espírito-Santo et al. 2009; Dupin et al. 2018; Clemente et al. 2020).
In this study, we assessed the effects of STDFs conversion into agricultural and pasture environments on diaspore fate of Enterolobium contortisiliquum tree. For this purpose, we compared fruit and seed morphometry, seed production, abortion, predation, and germination between trees from preserved and human-converted STDFs. We hypothesized that STDFs conversion would negatively affect diaspore fate, since in disturbed STDFs, individuals of E. contortisiliquum are under stressful environmental conditions. Thus, they would produce narrower fruits with less and smaller seeds. We also expect higher seed predation intensity and abortion percentages, and lower germination rates.
Methods
Study species
Enterolobium contortisiliquum (Vell.) Morong is a Neotropical leguminous tree frequently found in Brazilian STDFs (Oliveira-Filho 2006). Although reproductive studies about E. contortisiliquum do not exist, it seems that the species is pollinated by moths, hawk moths, other small nocturnal insects, and even by diurnal bees, as already registered for E. cyclocarpum, which has similar flower morphology (Janzen 1982; Rocha and Aguilar 2001a; Frankie et al. 2004; Hamrick and Apsit 2004). Flowering occurs during a short period, from September to October, while fruits ripen between June and July. The species has endozoochoric dispersal that occurs just after fruit ripening. Rodents, such as agoutis, are the seed dispersers (Moreira et al. 2015). Mature fruits and seeds of E. contortisiliquum resemble other Enterolobium species. Fruits are smooth, shiny, indehiscent, and deep brown, as in E. cyclocarpum (Janzen 1982), and seeds are hard, ovoid, and brown (Link and Costa 1995). Fruit development lasts over almost one year and seed dispersal occurs over the dry season, before the flowering period. Thus, mature fruits are resultant from the pollination of the previous year, as observed in E. cyclocarpum (Frankie et al. 2004).
Pre-dispersal seeds of E. contortisiliquum are predated by the larvae of Merobruchus bicoloripes (Coleoptera: Bruchidae) (Pic 1930) (Link and Costa 1995; Morandini and Viana 2009). Bruchid females oviposit on or near the fruits. When the eggs hatch, the larvae enter through the pericarp and go into the seeds, where they develop. The insect completes its life cycle consuming one or more seeds and emerges from the fruit as adult (Janzen 1969). The ingestion of E. contortisiliquum pods is harmful to cattle, causing photosensitivity reactions and abortion (Bonel-Raposo et al. 2008; Costa et al. 2009; Olinda et al. 2015). As a result, most farmers cut the trees near their ranches, as the fruiting period occurs during the dry season, coincident with low forage availability for cattle. Therefore, E. contortisiliquum is under threat due to habitat conversion and selective cutting (Moreira et al. 2015).
Study area and sampling design
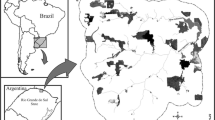
The study was conducted in northern Minas Gerais State (southeastern Brazil), in the surroundings of Lapa Grande State Park (LGSP) (ca. 16°42′S, 43°56′W), a protected area with 15.000 ha. The climate in the region is characterized by marked dry winters from May to September and rainy summers, from November to March. The predominant climate is tropical semiarid (Aw in Köppen’s classification) with average rainfall ranging from 700 to 1200 mm and average temperature among 21 and 25 °C (Antunes 1994). The vegetation of LGSP is composed of cerrado and STDFs. According to Portillo-Quintero and Sánchez-Azofeifa (2010) about 52% of Brazilian STDFs have already been converted to some sort of human activity. In northern Minas Gerais, estimates indicate that 18% of STDFs has been replaced of agriculture, silviculture, and extensive cattle ranching (Rodrigues 2000; Espírito-Santo et al. 2009; Dupin et al. 2018), which has resulted in an altered matrix with scattered trees. We studied 20 reproductive trees in a preserved STDFs area (inside LGSP limits) and 20 reproductive trees in the park surroundings, which is characterized by a converted landscape of agriculture and pasture with some scattered E. contortisiliquum trees. We measured the height and diameter at breast height (DBH) of the 40 sampled trees. The minimum distance between E. contortisiliquum individuals was 5 m. In a radius of 10 m around each studied tree, we counted the individuals of E. contortisiliquum trees and other tree species (see Supplementary Table S1).
Fruit and seed morphometry
The study was conducted during the fruiting period (July) of 2011. On each reproductive tree, we collected between 26 and 30 mature fruits surrounding the tree crown. These fruits were produced by natural pollination in the previous year. We determined the fruit morphometrics by measuring length (in the longitudinal direction), width (in the transverse direction), and thickness of the fruit pericarp using a digital caliper (mm). After that, fruits were opened, and all seeds were extracted to assess seed morphometry, as previously described for fruits. Due to the irregular shape of aborted seeds, they were excluded from morphometric measures.
Seed production
For each fruit, we counted the number of undamaged seeds, the number of aborted seeds, the number of pre-dispersal predated seeds by bruchid beetles, and the total number of seeds produced (i.e., the sum of all previous categories). Seeds without injuries were considered potentially viable (undamaged seeds). The seeds were considered aborted when exhibited irregular shape with a brown and dry endosperm (Cascante et al. 2002). Bruchid beetle damage was identified by the characteristic hole left on seeds when adult beetle emerges (Janzen 1977).
Seed germination
To verify if the habitat disturbance affects the percentage and the time of seed germination, we collected a sample of 10 seeds per tree from each habitat condition (200 seeds per habitat). Seeds used in this experiment were undamaged, as described above. These 400 seeds were subjected to scarification by carefully sanding the seed cover to break seed dormancy. After that, seeds were placed in Petri dishes covered with a sheet of filter paper and moistened with distilled water. Then, seeds were incubated in a germination chamber (B.O.D. type) under 12 h photoperiod with controlled temperature of 25 °C on dark and 30 °C on the light. All Petri dishes were observed at 24 h intervals for 30 days and seeds were considered germinated once the radicle protrusion was observed. Mean germination time (MGT) was obtained by the equation: MGT = ∑(n × d)/N, where n is the number of seeds germinated on each day, d is the number of days from the beginning of the test, and N is the total number of seeds germinated at the end of the experiment (Ellis and Roberts 1981).
Data analyses
To test how habitat disturbance affects fruit and seed morphometrics, we used generalized linear mixed model (GLMM) with Gaussian error distribution and lmer function for R (lme4 package; Bates et al. 2015). We built separate models for each response variable. Our models included habitat condition (preserved SDTF or converted area) as the predictor variable with a fixed effect and sampled tree as a random effect. The response variables were (1) length, (2) width, and (3) thickness of fruits and seeds.
To determine the effect of habitat disturbance on seed production, we used GLMM models with Binomial error distribution and logit link function and glmer function (lme4 package; Bates et al. 2015). Our models also included habitat condition (preserved STDF area or converted landscape) as a fixed effect and tree as a random effect. In these models, the response variables were the proportion of (1) undamaged seeds; (2) aborted seeds; (3) pre-dispersal predated seeds; and (4) seeds production.
The effect of habitat disturbance on seed germination was evaluated by comparing germination time and seed germination percentage during 30 days between habitat conditions. We calculated the mean germination time (MGT) following Labouriau (1983) for each condition. The germination speed was evaluated with a regression analysis using Weibull parametric survival distribution and survival function. The number of germinated seeds was tested through a GLM with Poisson error distribution and the response variable was the habitat condition. All analyses were performed in software R version 3.4.2 (R Development Core Team 2017).
Results
Fruit and seed morphometry
A total of 598 fruits were sampled in each habitat (1,196 in total) and 10,495 seeds were obtained in STDFs area, whereas 10,686 seeds were sampled in human-converted habitat (21,181 seeds in total). All fruits and seeds were used for morphometric analysis (including undamaged and pre-dispersal predated seeds). Habitat condition did not affect fruit length (X2 = 0.10, df = 1, p = 0.75) or fruit width (X2 = 2.50, df = 1, p = 0.11). However, fruit thickness was larger in trees from preserved STDF (X2 = 5.40, df = 1, p = 0.02). In contrast, none of the seeds’ morphometric measures differed between habitat condition (X2 = 0.07, df = 1, p = 0.79 for seed length, X2 = 0.81, df = 1, p = 0.37 for seed width, and X2 = 0.31, df = 1, p = 0.58 for seed thickness) (Table 1).
Seed production and seed predation
There was no difference in seed production between habitat conditions. Likewise, the number of undamaged seeds (X2 = 0.03, df = 1, p = 0.87), aborted seeds (X2 = 1.19, df = 1, p = 0.27), predated seeds (X2 = 0.51, df = 1, p = 0.48), and total seed production (X2 = 0.14, df = 1, p = 0.71) were independent of habitat condition (Table 2, Fig. 1). Trees from both habitat conditions produced an average of 18 seeds per fruit, of which 80–82% were undamaged or potentially viable seeds, 8–9% were aborted seeds, and 9–11% were predated (Fig. 1).
Seed germination
We did not observe any effect of habitat disturbance on germination percentage (an average of 85% for seeds from preserved STDF and 87% for seeds from human-converted area, p = 0.78). However, seeds from trees located at preserved STDFs germinated almost twice faster (MGT = 2.4 ± 0.85 days), than seeds from trees in the human-converted area (MGT = 4.09 ± 0.63 days; p = 0.003). Thus, in preserved STDFs, 80% of seeds germinated after ca. 10 days, while in the human-converted area, seeds took ca. 17 days to germinate (Fig. 2).
Discussion
In general, our findings suggest that habitat disturbance did not affect Enterolobium contortisiliquum diaspore fate. Only fruit thickness was larger in trees from preserved STDFs as well as its germination speed was higher. However, the majority of studied traits did not indicate a negative effect of landscape conversion on the reproductive success of this tree species. In fact, not all tree species are impacted by land conversion in the same way (Henle et al. 2004; Winfree et al. 2011).
The diaspore fate influences plant dispersal and establishment (Westoby et al. 1996; Leishman et al. 2000; Moles and Westoby 2004). As seeds have morphological traits in response to the environmental conditions (Vázquez-Yanes and Orozco-Segovia 1993), we expected that fruit and seed morphometries formed in the human-converted area would be smaller and narrower. Anthropogenic habitat conversion modify local climatic conditions, and these areas become warmer and drier than natural habitats (Britter and Hanna 2003; Frishkoff et al. 2015; Senior et al. 2017). Consequently, trees in human-converted area would grow under stressful conditions when compared to trees of preserved area. However, STDFs conversion into pasture and agriculture only negatively affected fruit thickness. Thus, environmental conditions of the human-converted area (i.e., high incidence of radiation and extremes of temperature and humidity) may not be stressors for E. contortisiliquum. As observed for other STDF plant species, E. contortisiliquum may have strategies to cope with drought through better efficiency in water use, allowing them to have physiological and morphological adjustments at high temperatures and less water availability (Pineda-García et al. 2013; Lohbeck et al. 2015).
Our results also indicated that the number of seeds per fruit of E. contortisiliquum was similar among habitats. If habitat disturbance affects the number of pollinia deposited in stigmas, as suggested by Aizen and Feinsinger (1994a), it would be expected more fruits and seed set in an undisturbed area. However, we did not observe any effect of habitat disturbance on seed production per fruit. This result may be related to pollinators’ capacity to transfer a sufficient pollen charge to maintain the same level of seed production, regardless of the habitat condition, as discussed below. Despite a lower density of trees in the converted habitat, trees may not suffer from pollen limitation or gene flow. Many studies highlight the ecological value of remnant trees in converted landscapes to increase population connectivity (Manning et al. 2006; Breed et al. 2011). Thus, E. contortisiliquum populations from both habitats may be well connected by gene flow, which promotes similar progeny.
Although we did not study pollen gene flow or progeny relatedness, our results suggest that pollination is not constrained by habitat conversion, as undamaged and aborted seeds were similar in both habitat conditions, as well as fruit and seed set. The disturbed area is surrounding the LGSP; thus, pollinators may be dispersing among patches, favoring more compatible crosses between unrelated trees. Despite the lack of knowledge on E. contortisiliquum pollinators, flowers are apparently pollinated by moths (Moreira et al. 2015), as observed for the congeneric species E. cyclocarpum (Frankie et al. 2004; Hamrick and Apsit 2004), and moths can visit many trees during a foraging route (Haber and Frankie 1989), promoting gene flow between different areas. Considering that approximately 20% of the progeny produced by the congeneric species E. cyclocarpum is self-fertilized (Rocha and Aguilar 2001b), an alternative explanation is that E. contortisiliquum would promote self-pollination, which would produce a similar number of seeds among habitats.
Seed predation of E. contortisiliquum was not affected by habitat disturbance, and both habitat conditions exhibited similar percentage of pre-dispersal predated seeds. It is possible that habitat disturbance did not depress M. bicoloripes population and/or the matrix surrounding the studied areas did not constrain predator dispersal and, consequently, maintain the same pattern of seed attack. As pointed out by Aguilar et al. (2012), the persistence of bruchid insects on fragmented habitats may be due to the tight evolutionary relationship with Fabaceae tree species and the ability of bruchid beetles to remain nearby adult trees throughout their life cycle, despite the environmental condition. Despite fruit and seed predation is a process that affects plant reproductive success (Schupp 1988), the effects of habitat conversion on this antagonistic interaction have not been well studied (Herrerías-Diego et al. 2008), and tree species exhibit different responses (Cascante et al. 2002; Chacoff et al. 2004; Herrerías-Diego et al. 2008; Aguilar et al. 2012).
We found that the time of seed germination in E. contortisiliquum is affected by habitat disturbance. The mean germination time was significantly lower for seeds from trees from preserved STDFs than for seeds from trees from the human-converted area. The faster germination of seeds in the preserved habitat could be an important strategy to avoid seed viability loss due to deterioration and microorganism attack. An alternative explanation is that soil and/or humidity are different among habitats assuming that the human-modified area is warmer and drier (Britter and Hanna 2003; Frishkoff et al. 2015; Senior et al. 2017). Agreeing with our results, progeny from the congeneric species E. cyclocarpum trees in preserved STDFs area took less time to germinate than trees scattered in pastures (Rocha and Aguilar 2001b). Although the mean time of seed germination in the preserved habitat was almost two days faster, seeds obtained from trees of both habitat conditions showed the same germination rate. It is expected that seeds produced by scattered trees would suffer more inbreeding (Nason and Hamrick 1997) and, consequently, a reduction in germination percentage (Bruna 1999). As assumed before, it is possible that there is no pollen limitation, and pollinators could be promoting pollen flow and exogamic crosses between unrelated trees, reducing inbreeding depression and pollen limitation effects in scattered trees, which would result in a similar percentage of germination.
For many years, researchers have evaluated the effects of habitat disturbance on tree reproduction (Nason and Hamrick 1997; Herrerías-Diego et al. 2006, 2008; Aguilar et al. 2019). However, not all tropical trees species are impacted by habitat disturbance in the same way (Quesada et al. 2004; Herrerías-Diego et al. 2006; Neal et al. 2010). Despite our results were obtained in a single site and only once, we highlight the absence of negative habitat conversion effects on E. contortisiliquum’s diaspores fate in this study. Although, habitat disturbance may cause negative effects on plant reproduction, the long life-span of trees may make them resilient to immediate disturbance (Wilcock and Neiland 2002; Neal et al. 2010) and buffer populations against stochastic events. From a conservation biology perspective, E. contortisiliquum trees scattered in altered habitats of tropical dry forests may serve as important stepping stones for pollinator movement, ensuring gene flow and connecting populations, rather than being considered a living dead (Janzen 1986). Local preservation of these trees is essential to the maintenance of mutualistic and antagonistic interactions important to the biodiversity of tropical ecosystems.
Data availability
Not applicable.
Code availability
Not applicable.
References
Aguilar R, Ashworth L, Galetto L, Aizen MA (2006) Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecol Lett 9:968–980
Aguilar R, Quesada M, Ashworth L, Herrerias-Diego Y, Lobo J (2008) Genetic consequences of habitat fragmentation in plant populations: susceptible signals in plant traits and methodological approaches. Mol Ecol 17:5177–5188
Aguilar R, Ashworth L, Calviño A, Quesada M (2012) What is left after sex in fragmented habitats? Assessing the quantity and quality of progeny in the endemic tree Prosopis caldenia (Fabaceae). Biol Conserv 152:81–89
Aguilar R et al (2019) Habitat fragmentation reduces plant progeny quality: a global synthesis. Ecol Lett 22:1163–1173
Aizen MA, Feinsinger P (1994a) Forest fragmentation, pollination, and plant reproduction in a Chaco Dry Forest, Argentina. Ecol 75:330–351
Aizen MA, Feinsinger P (1994b) Habitat fragmentation, native insect pollinators, and feral honey bees in Argentine “Chaco Serrano.” Ecol Appl 7:378–392
Andreazzi CS, Pimenta CS, Pires AS, Fernandez FA, Oliveira-Santos LG, Menezes JF (2012) Increased productivity and reduced seed predation favor a large-seeded palm in small Atlantic forest fragments. Biotropica 44:237–245
Antunes FZ (1994) Caracterização climática – Caatinga do estado de Minas Gerais. Informe Agropecuário 17:15–19
Ashworth L, Martí M (2011) Forest fragmentation and seed germination of native species from the Chaco Serrano Forest. Biotropica 43:496–503
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 67:1–48
Bonel-Raposo J, Riet-Correa F, Guim TN, Schuch ID, Grecco FN, Fernandes CG (2008) Intoxicação aguda e abortos em cobaias pelas favas de Enterolobium contortisiliquum (Leg. Mimosoideae). Pesq Vet Bras 28:593–596
Breed MF, Ottewell KM, Gardner MG, Lowe AJ (2011) Clarifying climate change adaptation responses for scattered trees in modified landscapes. J Appl Ecol 48:637–641
Breed MF, Ottewell KM, Gardner MG, Marklund MHK, Dormontt EE, Lowe AJ (2015) Mating patterns and pollinator mobility are critical traits in forest fragmentation genetics. Hered 115:108–114
Britter R, Hanna S (2003) Flow and dispersion in urban canopies. Annu Rev Fluid Mech 35:469–496
Broadhurst L (2015) Pollen dispersal in fragmented populations of the dioecious wind pollinated tree, Allocasuarina verticillata (Drooping Sheoak, Drooping She-Oak; Allocasuarinaceae). PLoS ONE 10(3):e0119498. https://doi.org/10.1371/journal.pone.0119498
Browne L, Karubian J (2018) Habitat loss and fragmentation reduce effective gene flow by disrupting seed dispersal in a neotropical palm. Mol Ecol 27:3055–3069
Bruna E (1999) Seed germination in rainforest fragments Nat 402:139
Burgos A, Grez A, Bustamante RO (2008) Seed production, pre-dispersal seed predation and germination of Nothofagus glauca (Nothofagaceae) in a temperate fragmented forest in Chile. Forest Ecol Manag 255:1226–1233
Burkey T (1993) Edge effects in seed and egg predation at two Neotropical rainforest sites. Biol Conserv 66:139–143
Cascante A, Quesada M, Lobo JA, Fuchs EA (2002) Effects of dry forest fragmentation on the reproductive success and genetic structure of the tree Samanea saman. Conserv Biol 16:137–147
Chacoff N, Morales JM, Vaquera MP (2004) Efectos de la fragmentación sobre la aborción y depredación de semillas en el Chaco Serrano. Biotropica 36:109–117
Chen Q, Tomlinson KW, Cao L, Wang B (2017) Effects of fragmentation on the seed predation and dispersal by rodents differ among species with different seed size. Integr Zool 12:468–476
Clemente CMS, Espíritos-Santo MM, Leite ME (2020) Estimates of deforestation avoided by protected areas: a case study in Brazilian tropical dry forests and Cerrado. Landsc Res 45:470–483
Costa RLD, Marini A, Tanaka D, Berndt A, Andrade FME (2009) Um caso de intoxicação de bovinos por Enterolobium contortisiliquum (Timboril) no Brasil. Archivos de Zootecnia 58:313–316
Costin BJ, Morgan JW, Young AG (2001) Reproductive success does not decline in fragmented populations of Leucochrysum albicans subsp. albicans var. tricolor (Asteraceae). Biol Conserv 98:273–284
Crawley MJ (2000) Seed predators and plant population dynamics. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities. CAB International, Wallingford, pp 167–182
Deacon NJ, Cavender-Bares J (2015) Limited pollen dispersal contributes to population genetic structure but not local adaptation in Quercus oleoides forests of Costa Rica. PLoS ONE 10(9):e0138783. https://doi.org/10.1371/journal.pone.0138783
Debinski DM, Holt RD (2000) A survey overview of habitat fragmentation experiments. Conserv Biol 14:342–355
Didham RK, Lawton JH, Hammond PM, Eggleton P (1998) Trophic structure stability and extinction dynamics of beetles (Coleoptera) in tropical forest fragments. Philos Trans R Soc B: Biol Sci 353:437–451
Dirzo R, Miranda A (1991) Altered patterns of herbivory and diversity in the forest understory: a case study of the possible consequences of contemporary defaunation. In: Price PW, Lewinsohn TW, Fernandes GW, Benson WW (eds) Plant-animal interaction: evolutionary ecology in tropical and temperate regions. Wiley, New York, pp 273–287
Dupin MGV, Espírito-Santo MM, Leite ME, Silva JO, Rocha AM, Barbosa RS, Anaya FC (2018) Land use policies and deforestation in Brazilian tropical dry forests between 2000 and 2015. Environ Res Lett 13(3):035008
Eckert CG, Kalisz S, Geber MA et al (2009) Plant mating systems in a changing world. Trends Ecol Evol 25:35–43
Ellis RH, Roberts EH (1981) The quantification of ageing and survival in orthodox seeds. Seed Sci Technol 9:373–409
Emer C, Galetti M, Pizo MA, Guimarães PR Jr, Moraes S, Piratelli A, Jordano P (2018) Seed-dispersal interactions in fragmented landscapes – a metanetwork approach. Ecol Lett 21:484–493
Emer C, Galetti M, Pizo MA, Jordano P, Verdú M (2019) Defaunation precipitates the extinction of evolutionarily distinct interactions in the Anthropocene. Sci Adv. https://doi.org/10.1126/sciadv.aav6699
Espírito-Santo MM, Sevilha AC, Anaya F, Barbosa R, Fernandes GW, Sánchez-Azofeifa GA, Scariot A, Noronha SE, Sampaio CA (2009) Sustainability of tropical dry forests: two case studies in southeastern and central Brazil. Forest Ecol Manag 258:922–930
Frankie GW, Haber WA, Vinson SB, Bawa KS, Ronchi PS, Zamora N (2004) Flowering phenology and pollination systems diversity in the seasonal dry forest. In: Frankie GW, Mata A, Vinson SB (eds) Biodiversity conservation in Costa Rica: learning the lessons in a seasonal dry forest. University of California Press, Berkeley, pp 17–29
Frishkoff LO, Hadly EA, Daily GC (2015) Thermal niche predicts tolerance to habitat conversion in tropical amphibians and reptiles. Glob Chang Biol 21:3901–3916
Fuchs EJ, Lobo JA, Quesada M (2003) Effects of forest fragmentation and flowering phenology on the reproductive success and mating patterns of the tropical dry forest tree Pachira quinata. Conserv Biol 17:149–157
Galetti M, Guevara R, Côrtes MC et al (2013) Functional extinction of birds drives rapid evolutionary changes in seed size. Sci 340:1086–1090
Ghazoul J (2005) Pollen and seed dispersal among dispersed plants. Biol Rev 80:413–443
Ghazoul J, Liston KA, Boyle TJB (1998) Disturbance-induced density-dependent seed set in Shorea siamensis (Dipterocarpaceae), a tropical forest tree. J Ecol 86:462–473
Haber WA, Frankie GW (1989) A tropical hawkmoth community: Costa Rican dry forest Sphingidae. Biotropica 21:155–172
Haddad NM et al (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 2:1–9
Hamrick JL (2004) Response of forest trees to global environmental changes. Forest Ecol Managt 197:323–335
Hamrick JL, Apsit VJ (2004) Breeding structure of neotropical dry forest tree species in fragmented landscapes. In: Frankie GW, Mata A, Vinson SB (eds) Biodiversity conservation in Costa Rica: learning the lessons in a seasonal dry forest. University of California Press, Berkeley, pp 30–37
Henle K, Davies KF, Kleyer M, Margules C, Settele J (2004) Predictors of species sensitivity to fragmentation. Biodivers Conserv 13:207–251
Herrerías-Diego Y, Quesada M, Stoner KE, Lobo JA (2006) Effects of forest fragmentation on phenological patterns and reproductive success of the tropical dry forest tree Ceiba aesculifolia. Conserv Biol 20:1111–1120
Herrerías-Diego Y, Quesada M, Stoner KE, Lobo JA, Hernández-Flores Y, Montoya GS (2008) Effect of forest fragmentation on fruit and seed predation of the tropical dry Forest tree Ceiba aesculifolia. Biol Conserv 141:241–248
Hooper ER, Ashton MS (2020) Fragmentation reduces community-wide taxonomic and functional diversity of dispersed tree seeds in the Central Amazon. Ecol Appl 30:e02093
Janzen DH (1969) Seed eaters versus seed size, number, toxicity and dispersal. Evol 23:1–27
Janzen DH (1977) The interaction of seed predators and seed chemistry. In: Labeyrie V (ed) Comportement des insects et milieu trophique. Colloques Internationaux du C.N.R.S, Paris, pp 415–428
Janzen DH (1982) Variation in average seed size and fruit seediness in a fruit crop of a Guanacaste tree (Leguminosae: Enterolobium cyclocarpum). Am J Bot 69:1169–1178
Janzen DH (1986) The future of tropical ecology. Annu Rev Ecol Syst 17:305–324
Janzen DH (1988) Management of habitat fragments in a tropical dry forest: growth. Ann Mo Bot Gard 75:105–116
Johnson CD (1985) Potential useful tropical Legumes and their relationships with Bruchid beetles. In: Misra KC (ed) Ecology resource management in tropics: resented papers, silver jubilee Symposium of International Society for Tropical Ecology. Bhargava Book Depot, Varanasi, pp 206–223
Kolb A (2008) Habitat fragmentation reduces plant fitness by disturbing pollination and modifying response to herbivory. Biol Conserv 141:2540–2549
Kollman J, Buschor M (2003) Edges effects on seed predation by rodents in deciduous forests of northern Switzerland. Plant Ecol 164:249–261
Labouriau LG (1983) A germinação das sementes. Secretaria Geral da Organização dos Estados Americanos, Washington
Leishman MR, Wright IJ, Moles AT, Westoby M (2000) The evolutionary ecology of seed size. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities. CAB International, Wallingford, pp 31–57
Link D, Costa EC (1995) Danos causados por insetos em sementes de timbaúva, Enterolobium contortisiliquum (Vell.) Morong. Cienc Florest 5:113–122
Lohbeck M, Lebrija-Trejos E, Martínez-Ramos M, Meave JA, Poorter L, Bongers F (2015) Functional trait strategies of trees in dry and wet tropical forests are similar but differ in their consequences for succession. PLoS ONE 10(4):e0123741. https://doi.org/10.1371/journal.pone.0123741
Manning AD, Fischer J, Lindenmayer DB (2006) Scattered trees are keystone structures – Implications for conservation. Biol Conserv 132:311–321
Marjakangas EL, Abrego N, Grøtan V et al (2019) Fragmented tropical forests lose mutualistic plant–animal interactions. Divers Distrib 26:154–168
Mass JM (1995) Conservation of tropical dry forest to pasture and agriculture. In: Bullock SH, Mooney HA, Medina E (eds) Seasonally dry tropical forests. Cambridge University Press, New York, pp 399–422
Mendes CP, Ribeiro MC, Galetti M (2016) Patch size, shape and edge distance influence seed predation on a palm species in the Atlantic forest. Ecography 39:465–475
Menges ES (1991) Seed germination percentage increases with population size in a fragmented prairie species. Conserv Biol 5:158–164
Miles L, Newton AC, DeFries RS, Ravilious C, May I, Blyth S, Kapos V, Gordon JE (2006) A global overview of the conservation status of tropical dry forests. J Biogeogr 33:491–505
Moles AT, Westoby M (2004) Seedling survival and seed size: a synthesis of the literature. J Ecol 92:372–383
Morandini MN, Viana ML (2009) Depredación pre-dispersiva de semillas en tres poblaciones del árbol Enterolobium contortisiliquum (Fabaceae). Rev Biol Trop 57:781–788
Moreira PA, Brandão MM, Araújo NH, Oliveira DA, Fernandes GW (2015) Genetic diversity and structure of the tree Enterolobium contortisiliquum (Fabaceae) associated with remnants of a seasonally dry tropical forest. Flora 210:40–46
Morrison BML, Mendenhall CD (2020) Hummingbird–plant interactions are more specialized in forest compared to coffee plantations. Divers. https://doi.org/10.3390/d12040126
Murphy PG, Lugo AE (1986) Ecology of tropical dry forests. Annu Rev Ecol Evol Syst 17:67–88
Nason JD, Hamrick JL (1997) Reproductive and genetic consequences of forest fragmentation: two case studies of neotropical canopy trees. J Hered 88:264–276
Neal JM, Hardner CM, Gross CL (2010) Population demography and fecundity do not decline with habitat fragmentation in the rainforest tree Macadamia integrifolia (Proteaceae). Biol Conserv 143:2591–2600
Newbold T, Bentley LF, Hill SLL, Edgar MJ, Horton M, Su G, Şekercioğlu ÇH, Collen B, Purvis A (2019) Global effects of land use on biodiversity differ among functional groups. Funct Ecol. https://doi.org/10.1111/1365-2435.13500
Olinda RG, Medeiros RMT, Dantas AFM, Lemos RAA, Riet-Correa F (2015) Intoxicação por Enterolobium contortisiliquum em bovinos na região Nordeste do Brasil. Pesq Vet Bras 35:44–48
Oliveira-Filho AT (2006) Catálogo das árvores nativas de Minas Gerais: mapeamento e inventário da flora nativa e dos reflorestamentos de Minas Gerais. Editora UFLA, Lavras
Pineda-García F, Paz H, Meinzer FC (2013) Drought resistance in early and late secondary successional species from a tropical dry forest: the interplay between xylem resistance to embolism, sapwood water storage and leaf shedding. Plant Cell Environ 36:405–418
Portillo-Quintero CA, Sánchez-Azofeifa GA (2010) Extent and conservation of tropical dry forests in the Americas. Biol Conserv 143:144–155
Quesada M, Stoner KE, Lobo JA, Herrerías-Diego Y, Palacios-Guevara C, Munguía-Rosas MA, Salazar KAO, Rosas-Guerrero V (2004) Effects of forest fragmentation on pollinator activity and consequences for plant reproductive success and mating patterns in bat-pollinated Bombacaceous trees. Biotropica 36:131–138
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ Accessed 13 February 2019
Rocha OJ, Aguilar G (2001a) Variation in the breeding behavior of the dry forest tree, Enterolobium cyclocarpum (Guanacaste) in Costa Rica. Am J Bot 88:1600–1606
Rocha OJ, Aguilar G (2001b) Reproductive biology of the dry forest tree Enterolobium cyclocarpum (Guanacaste) in Costa Rica: a comparison between trees left in pastures and trees in continuous forest. Am J Bot 88:1607–1614
Rodrigues L (2000) Formação econômica do norte de Minas e o período recente. In: Oliveira MFM, Rodrigues L, Machado JMA, Botelho TR (eds) Formação social e econômica do norte de Minas Gerais. Editora Unimontes, Montes Claros, pp 105–170
Rosas F, Quesada M, Lobo JA, Sork VL (2011) Effects of habitat fragmentation on pollen flow and genetic diversity of the endangered tropical tree Swietenia humilis (Meliaceae). Biol Conserv 144:3082–3088
Sala OE et al (2000) Global biodiversity scenarios for the year 2100. Sci 287:1770–1774
Sánchez-Azofeifa GA, Kalacska M, Quesada M, Calvo-Alvarado JC, Nassar JM, Rodríguez JP (2005) Need for integrated research for a sustainable future in tropical dry forests. Conserv Biol 19:1–2
Sánchez-Azofeifa GA, Quesada M, Cuevas-Reyes P, Castillo A, Sánchez-Montoya G (2009) Land cover and conservation in the area of influence of the Chamela-Cuixmala Biosphere Reserve, Mexico. Forest Ecol Manag 258:907–912
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Schupp EW (1988) Factors affecting post-dispersal seed survival in a tropical forest. Oecol 76:525–530
Senior RA, Hill JK, del Pliego PG, Goode LK, Edwards DP (2017) A pantropical analysis of the impacts of forest degradation and conversion on local temperature. Ecol Evol 7:7897–7908
Skogen KA, Jogesh T, Hilpman ET, Todd SL, Rhodes MK, Still SM, Fant JB (2016) Land-use change has no detectable effect on reproduction of a disturbance-adapted, hawkmoth-pollinated plant species. Am J Bot 103:1950–1963
Souza-Silva H, Machado LF, Silva JO, Espírito-Santo MM (2015) Consequences of habitat disturbance on seed fate of a Brazilian tropical dry forest tree Cavanillesia arborea (Malvaceae). Austral Ecol 40:726–732
Vázquez-Yanes C, Orozco-Segovia A (1993) Patterns of seed longevity and germination in the tropical rain forest. Annu Rev Ecol Syst 24:69–87
Westoby M, Leishman MR, Lord JM (1996) Comparative ecology of seed size and seed dispersal. Philos Trans R Soc B 351:1309–1318
White GM, Boshier DH, Powell W (2002) Increased pollen flow counteracts fragmentation in a tropical dry forest: an example from Swietenia humilis Zuccarini. Proc Natl Acad Sci USA 99:2038–2042
Wilcock C, Neiland R (2002) Pollination failure in plants: why it happens and when it matters. Trends Plant Sci 7:270–277
Winfree R, Bartomeus I, Cariveau DP (2011) Native pollinators in anthropogenic habitats. Annu Rev Ecol Evol Syst 42:1–22
Acknowledgements
The authors thank Universidade Estadual de Montes Claros for logistical support during our laboratory work, FC Nascimento, TBS Prates, SAS Sousa, and FA Oliveira for their kind help with laboratory work. They thank CS Ribeiro-Costa from Universidade Federal do Paraná for insect identification. They are very grateful to the Instituto Estadual de Florestas (IEF) for logistical support during field work. They thank the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) (APQ-04127-10, APQ-00372-11). FSN are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for his scholarship.
Funding
This study was funded by Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) (APQ-04127–10, APQ-00372–11).
Author information
Authors and Affiliations
Contributions
PAM contributed to data curation, methodology, and writing—original draft, and review and editing. FSN was involved in methodology, formal analysis, and writing—original draft, and review and editing. JAL contributed to writing––review and editing and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This study was approved by the State Forest Institute (Instituto Estadual de Florestas – IEF/Protocol # 017/10).
Consent for participate
All co-authors have read the submitted version and have agreed with the submission.
Additional information
Communicated by E.T.F Witkowski.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Abreu Moreira, P., de Siqueira Neves, F. & Lobo, J.A. Consequences of tropical dry forest conversion on diaspore fate of Enterolobium contortisiliquum (Fabaceae). Plant Ecol 222, 525–535 (2021). https://doi.org/10.1007/s11258-021-01124-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-021-01124-6