Abstract
Rare weeds are currently under pressure due to intensifying arable management practices, and as a consequence of climate change, these practices will likely become even more intensive, together with a greater uniformity of land use. As a result, ecological stresses will increase for most species of rare weeds, in some cases leading to their further decline or even extinction. Moreover, climate change will alter the suitability of the environment for many plants, since average temperatures are predicted to increase and precipitation extremes to become more common. For most arable weed species it is unclear, whether the anticipated changes in environmental conditions are disadvantageous or beneficial. Little is known about specific biological responses of rare weeds to climate changes, and this study attempts to close some of these knowledge gaps. Here, the rare arable weed Lithospermum arvense and the endangered arable species Scandix pecten-veneris were investigated with regard to the effects of higher temperature and different crop densities on flowering time, shoot development, plant height, dry mass and seed production. Semi-field experiments were conducted with winter wheat crop for 3 years, involving 48 climate cages, in which every second was a variant of warmer temperature and contrasting crop density. We observed that S. pecten-veneris flowered earlier under warmer conditions and had fewer seeds and less biomass in the dense wheat crop compared to control conditions, while L. arvense grew taller, it produced fewer seeds in the high density crop. We suggest that such data concerning the biological responses of weeds can improve the precision of bioclimatic distribution models. Finally, we discuss strategies, such as relocation or non-intrusive management practices, for preventing further disappearances of rare arable weeds. Our results should be of considerable interest for the fields of plant ecology, biodiversity research and conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several weeds that were previously common in arable lands became rare due to mechanisation and intensification of arable management practices. The first declines occurred about 150 years ago, when mineral fertiliser and mechanised management were introduced (Holzner and Immonen 1982), and additional declines in both abundance and diversity were initiated approximately 60 years ago with the adoption of widespread herbicide use (Küster 1995; Baessler and Klotz 2006). Climate change may represent a third threat for rare weeds, as increases in the use of mineral fertilisers, greater crop densities, as well as altered sowing dates and longer growing seasons as a result of higher temperatures are likely in the future (Maillet and Lopez-Garcia 2000; Howden et al. 2007; Neve et al. 2009). Furthermore, cereal development may be accelerated during winter and summer months, and warmer temperatures will favour the planting of climate-adapted cereal cultivars (Kenny et al. 1993; Wardlaw and Wrigley 1994; Batts et al. 1998). Rare arable weed species will most likely have disadvantages from these changed management practices and also from more uniform land use (Olesen and Bindi 2002; Cimalová and Lososová 2009), and this may lead to a further decline or even the extinction of rare species.
It is known that most rare weeds show specific trait syndromes, and these traits are often related to a short plant height, low competitive abilities, large seeds, poor seed dispersal, narrow germination timeframes, easy seed cleaning, late flowering, and thus also late seed ripening (Kazanis and Arianoutsou 2004; Storkey 2006; Lososová et al. 2008). Importantly, most of these traits became obsolete or even disadvantageous in the context of modern arable farming (Andreasen et al. 1996; Sutcliffe and Kay 2000; Baessler and Klotz 2006; Petit et al. 2011). The number and the extent of the rare trait syndromes reflect the low phenotypic plasticity of rare weeds in the context of changes in arable conditions (Ohlemüller et al. 2006; Lososová et al. 2008; Pompe et al. 2009). According to Baker (1965), a general-purpose genotype (the extent to which phenotypic plasticity is exhibited by a species) is one of the most important factors for the success of weeds growing on arable lands. As the suitability of the environment will be altered as a result of future climate change, the degree of phenotypic plasticity is likely an important determinant of the future distribution of arable weeds (Bradshaw and McNeilly 1991; Pautasso et al. 2010).
At present, little is known about the specific biological responses of rare arable weeds to climate changes. Nevertheless, it is likely that direct effects of climate change, such as rising temperatures and less precipitation during summer months, will increase ecological stresses for rare weeds and create environmental conditions that are less favourable for many rare weeds (Patterson 1995; Marshall et al. 2003). In contrast, by removing species from the species pool, extreme climate events or other ecological disturbances can result in less competition by other species, which can even be an opportunity for certain rare weeds (Hobbs and Huenneke 1992; Storkey et al. 2010; Sheppard et al. 2012). Currently, most rare weeds use the C3 photosynthetic pathway. Despite the positive effects of rising levels of atmospheric CO2, higher temperatures negate the effect of elevated CO2 for most C3 plants (Batts et al. 1998; Morison and Lawlor 1999).
To date, only a few studies have analysed the effects of climate change on rare arable weeds (Morison and Lawlor 1999), so there is a lack of knowledge concerning their capacity to exhibit adaptive responses to changing climate conditions and the role of phenotypic plasticity. This study addresses these issues experimentally, using two rare weed species. The first species, Lithospermum arvense subsp. arvense L. (Syn. Buglossoides arvensis subsp. arvensis (L.) I. M. Johnst.) (Corn Gromwell, or Field Gromwell), is a white flowering weed of the family Boraginaceae. This species is mostly found in warm and exposed locations in Central Europe. It prefers sandy-clay soils, which are rich in nutrients, slightly basic and moderately calcareous (Moss et al. 2004). The second species, Scandix pecten-veneris L. (Venus’ Comb, or Shepherd’s Needle), is a short-statured weed of the family Apiaceae. This species prefers calcareous soils with low to moderate nitrogen levels and is typically found in warm exposed locations with moderate water availability (Moss et al. 2004; Chantre et al. 2009).
These two weed species have a different history of dispersal in Central Europe. While L. arvense originated in summer-warm continental regions in the far east, and seeds were found in Europe dating back to the Neolithic Era (approximately 5,000 years ago), S. pecten-veneris most likely originated in continental Eurosibiric or sub-Mediterranean regions and migrated to Central Europe along with cereal cultivation about 600–700 years ago (Tuexen 1950; Willerding 1986; Küster 1995). The two weeds were common in European fields until about 150 years ago (Schneider et al. 1994), when sophisticated seed-cleaning mechanisms were developed, mineral fertilisers were introduced and management methods became increasingly mechanised, all of which have been proposed as reasons for their first decline (Holzner and Immonen 1982; Küster 1995). Both species started to experience a second decline about 60 years ago upon the introduction and widespread use of certain herbicides (Sala et al. 2000; Chapin et al. 2000; Baessler and Klotz 2006).
In present day Central Europe, both species are the relicts of extensively managed fields, and they are often restricted to field boundaries (Chantre et al. 2009). Furthermore, while L. arvense is currently common only in parts of Poland and mountainous European regions, S. pecten-veneris is near extinction in most parts of Central Europe (Schneider et al. 1994). In recent decades in the UK, the use of herbicides, together with earlier harvesting of winter crops, had a strong negative effect on S. pecten-veneris (Rich and Woodruff 1995; Robinson and Sutherland 2002). In contrast, over the last few decades the abundance of L. arvense in Great Britain increased slightly (Potts et al. 2010), and it became a troublesome neophyte in winter crops in the semiarid regions of Southern Argentina and in the United States (Chantre et al. 2009). As is typical for species from the family Boraginaceae, L. arvense has silicate hairs that may cause a reduced uptake of water-bound herbicides into the plant. However, it should be noted that despite this characteristic, the most European populations of L. arvense are still susceptible to herbicides (Neururer and Herwisch 1976).
In this study, we aim at analysing the morphological responses of the two rare weeds to warmer conditions and high crop density in a semi-field experiment. We elucidate the influence of phenotypic plasticity and the role of certain rare weed trait syndromes with regard to warmer conditions and changes in the management practices.
Materials and methods
The seeds of L. arvense and S. pecten-veneris were collected near Göttingen, Germany in 2000. Wild plants originated from different field populations in that area. Both species were kept in ex situ cultivation in the experimental area of the University of Rostock, where the semi-field experiments were also conducted. Seeds were stored until the experiments were started. The experiment was initiated with a preliminary study in the season of 2009/2010. Five different densities of a winter wheat crop (Triticum aestivum cultivar Dekan) were used for a preliminary study involving 250–450 plants/m2, increasing in each treatment in increments of 50 plants/m2. Crop densities of 200 and 400 plants/m2 were used in the following two seasons (2010/2011 and 2011/2012) (Fig. 1).
For the semi-field experiments, 48 established barrels were used that were embedded in the ground since 1998, and so contained consolidated soil. They were cropped annually with cereals, oilseed rape or a grass crop. Each barrel had a diameter of 0.94 m and a depth of 1.50 m. The distance to the adjacent barrels was 2.06 m (Fig. 1). The barrels were arranged in 6 rows with 8 barrels per row. Plot size was 20 m by 26 m (Fig. 1). Winter wheat was sown in varying densities in the last week of September in the barrels (sowing period, Table 1). In the preliminary experiment, weed seeds were sown simultaneously in germination trays, and young plants were then planted in the barrels during the second week of March. In the following two seasons, a different approach was taken: manual broadcast sowing of weeds was carried out directly in the wheat crop in the sowing period. Each barrel was protected with a glass cover to prevent birds and mice from interfering with germination and growth during the first 4 weeks. No other actions were performed during the winter.
During the second week of March (early growth period, Table 1), seedlings were removed to ensure the same number of seedlings in each barrel. Every other barrel was surrounded with a metal frame (1.70 m in height) that was completely covered with transparent foil other than the top, which was made of transparent gauze with a mesh breadth of 0.5 mm, to allow necessary air exchange and to ensure a uniform temperature inside. These structures are referred here as cages with warmer conditions (barrels represented by a solid thick line in Fig. 1). In the season of 2011/2012 the remaining barrels were also equipped with metal frames that were covered with a white mesh (mesh breadth of 0.5 cm). The mesh was used to create comparable light conditions in both variants, since the transparent foil used with the warm cages absorbed a fraction of the radiation originating from the sun. The mesh was also useful to prevent birds from interfering. These are referred here as cages with control conditions (barrels with a dashed line in Fig. 1).
During the second week of May, young plants were counted; their height was measured, and the BBCH stage was recorded according to Hess et al. (1997) (flowering period, Table 1). Both wheat and weeds were harvested during the first week of August (harvesting period, Table 1). The dry mass (without seeds or roots) was determined, and the seeds were counted for each plant. Interfering weeds were removed by hand during the season. Snails were controlled by molluscicides as required. Each barrel was fertilised with 75 g Compo Hakaphos® Blue (equal to 112.5 kg N and 75 kg P ha−1) before sowing in the last week of March.
Data loggers were needed to assess the difference in the conditions of the cages with control conditions from the cages with warmer conditions. At an interval of 30 min, weather conditions (temperature and air moisture) were recorded with data loggers at a point 5 cm above the ground, one for each climate variant. The recorded weather data were used to calculate the growing degree days using the daily minimum and maximum air temperatures for the time in which the measurements were performed at the flowering and harvesting period. The following base temperatures were used: 3.8 °C for L. arvense and 8.0 °C for S. pecten-veneris (Chantre et al. 2009).
Statistical analysis was carried out using the R software package (Ihaka and Gentleman 1996), together with the additional packages MASS, lme4, languageR, gplots, coin and agricolae. To compare the different biological parameters, generalised linear models (GLM) were applied, using climate and wheat density as fixed factors. For the main experiments in 2010/2011 and 2011/2012, the year was chosen as an additional fixed factor. BBCH stages were compared using the Wilcoxon–Mann–Whitney test (function wilcox_test in R). Data were transformed before statistical analysis, if the conditions of normality were not met, to improve homogeneity of variances (see Tables 2, 3). The distribution and homogeneity of variances were visually checked with the help of histograms and diagnostic plots, performed by the glm and glm.nb functions in R, respectively (Zuur et al. 2009). The residual versus fitted plots and the normal qq-plots were examined for each tested parameter according to Faraway (2006).
Results
Measured conditions in the cages
A comparison of the cages with warmer conditions and those with control conditions revealed that the difference in the temperatures between the two varied with weather conditions. The average temperature was 0.9 °C higher in the cages with warmer conditions, ranging from a difference of 3.2 °C in sunlight during the day and a mean of 0.5 °C at night. Wind decreased the temperature inside the cages. At the flowering time, there was a difference of 16 growing degree days between the cages with control conditions and the cages with warmer conditions. At the harvesting time, the difference was 59 growing degree days between the two climate variants. As a result of the higher base temperature, S. pecten-veneris had approx. 2/3 fewer growing degree days available when compared to L. arvense. Relative humidity was about 79 % in the cages with control conditions and 1.5 % higher in the cages with warmer climate in the first and second season, and 1.1 % lower in the third season.
Results of the preliminary experiment
Lithospermum arvense plants in the flowering period grown under control conditions were the tallest in the wheat crop grown at a density of 300 plants/m2. However, the tallest plants were found in the crop with a density of 350 plants/m2 grown under warmer conditions (Fig. 2). In the harvesting period, L. arvense grown under control conditions were the tallest in the crop with the lowest density; this relationship was reversed in the cages with warmer conditions (Fig. 2). Similar results were found for the seed production per plant and plant dry mass, although the significant differences were apparent only for the plant height (Table 2). In the flowering period, most S. pecten-veneris plants were taller under warmer conditions (Fig. 3; Table 2), and this relationship was reversed at the time of harvesting. S. pecten-veneris plants were smaller and produced significantly fewer seeds under warmer conditions than under control conditions (Fig. 3; Table 2); upon reaching the harvesting period, no plants survived in the highest wheat density under warmer conditions (Fig. 3).
Preliminary experiment: Plant height measured in the flowering period, and plant height, seeds per plant and dry mass measured in the harvesting period for Lithospermum arvense. Circles represent medians under control conditions, whereas triangles represent medians under warmer conditions. Lines are drawn to differentiate the five contrasting wheat densities in the climate cages under control conditions (dotted line) from the cages with warmer conditions (solid line). n = 240 for both species
Preliminary experiment: Plant height measured in the flowering period, and plant height, seeds per plant and dry mass measured in the harvesting period for Scandix pecten-veneris. Circles represent medians under control conditions, whereas triangles represent medians under warmer conditions. Lines are drawn to differentiate the five contrasting wheat densities in the climate cages under control conditions (dotted line) from the cages with warmer conditions (solid line). n = 240 for both species
Measurements at flowering period
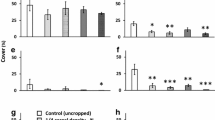
Under control conditions, L. arvense grew taller in the wheat crop with low density than with high density, while under warmer conditions plant heights were significantly greater in the dense wheat crop than in the low density crop (Fig. 4; Table 3). Under control conditions, L. arvense produced significantly more shoots in the crop with low density compared to the dense wheat crop (Fig. 4). Finally, the onset time and duration of flowering (the BBCH stages measured) did not differ significantly between the treatments (Table 3). S. pecten-veneris plants were smaller under warmer conditions than under control conditions, and plants were smaller in the dense wheat crop than in the low density crop (Fig. 5; Table 3). S. pecten-veneris produced significantly fewer shoots under warmer conditions than under control conditions (Table 3) and had fewer shoots in the high density wheat crop than the low density crop (Fig. 5). Generally, a lower phenotypic plasticity, and thus less variation in plant height and plant stature, was observed for S. pecten-veneris compared to L. arvense. Plants of S. pecten-veneris flowered earlier and are also shorter when grown under warmer conditions (Table 3). Thus, BBCH stages were higher under warmer conditions (Table 3). The species also showed an earlier onset of flowering in the dense wheat crop (Table 3).
Characteristics measured in the flowering period (plant height and shoots per plant) and in the harvesting period (seeds per plant and dry mass) for L. arvense in two different wheat densities (200 and 400) in climate cages under control conditions (n) and warm climate cages (w). n = 1,409 for both species
Properties measured in the flowering period (plant height and shoots per plant) and in the harvesting period (seeds per plant and dry mass) for S. pecten-veneris in two different wheat densities (200 and 400) in climate cages under control conditions (n) and warm climate cages (w). n = 1,409 for both species
Measurements at harvesting period
On average, L. arvense plants produced 132 seeds/plant in the dense wheat crop and 152 seeds/plant in the low density crop. Occasionally, single plants produced up to 4,000 seeds (Fig. 4). Such outliers were only observed in the low density wheat crop. L. arvense plants had significantly less dry mass when grown in the dense wheat crop than in the low density crop (Fig. 4). However, the two climate conditions had no significant effect on seed production and dry mass of L. arvense in wheat crops of both densities (Table 3). On average, S. pecten-veneris produced 44 seeds/plant under control conditions and 32 seeds/plant under warmer conditions (Fig. 5). Seed production was likewise significantly reduced in the dense wheat crop compared to the low density crop (Table 3), and similarly, a significant reduction was observed for S. pecten-veneris dry mass (Table 3).
Discussion
In this experiment, both species responded differently to the experimentally warmer climate changes and also to crop density.
The warmer conditions had a stronger negative impact on the generative reproduction of S. pecten-veneris than on L. arvense. Concerning warmer conditions, S. pecten-veneris exhibited low phenotypic plasticity mostly regarding life history traits, which likely affect negatively the persistence in the long term of the weed (Storkey et al. 2010). One example is dispersal capacity, which is strongly associated with morphological traits such as seed production, seed size and the number of shoots (Gaudet and Keddy 1988). S. pecten-veneris grows very large seeds, which is an unusual trait for weeds growing on arable lands. The large size is an adaptation that can be advantageous if nutrients are limiting (Storkey et al. 2010); however, the ability to retain nutrients provides no advantages under current agricultural management methods, where the nutrients are typically not a limiting factor (Reich et al. 1992; Aerts 1999). On the other hand, L. arvense germinates in a narrow timeframe in autumn and spring (Svensson and Wigren 1986a). If future crop sowing occurs outside of this window, fewer L. arvense plants may emerge (Chantre et al. 2009).
The disadvantage of large seeds, fewer shoots and lower seed production under warmer conditions of S. pecten-veneris is more limiting and less likely to be changed by adaptation or compensated by variation than the narrow germination period of L. arvense. Thus, the mentioned rare weed syndromes for S. pecten-veneris have likely more negative impact on the generative reproduction rather than those of L. arvense (Fried et al. 2010). Under warmer conditions, L. arvense will, therefore, probably be able, to some degree, to migrate to a favourable climate gradient as the seeds are also distributed more easily due to their small size (Fahrig and Merriam 2002). However, L. arvense abundance may decrease in Central Europe if climate change occurs too rapidly and future agricultural management becomes more intensive. The lower generative reproduction of S. pecten-veneris under warmer conditions will likely increase the risk of extinction for S. pecten-veneris (Wilson 2006).
In this experiment, the seed output of the two species was lower in the dense crop at the time of harvesting. Both species responded negatively to increasing wheat crop densities, probably as a result of the increased shading caused by the high crop density (Korsmo 1930; Svensson and Wigren 1986b). However, our results showed that some L. arvense plants grew very tall and produced an unusually high number of seeds under warmer conditions in the dense wheat crop (Fig. 4). If these differences were caused by genetic diversity, this could lead to biotypes of L. arvense that are better adapted to the future climate conditions. Thus, this species may even show an increase in abundance in high density crops under warmer conditions. Similar selection patterns may explain the high competitive abilities of the invasive populations in Argentina and the United States, although the climatic selection of L. arvense has not been studied to date (Chantre et al. 2009). We note that such suggestions about the significance of selective effects are still speculative, and further research is needed to assess the importance of regional biotypes of L. arvense.
Warmer and dryer conditions can also have an accelerating effect on the flowering time of plants (Ballaré and Casal 2000). Due to climate change, the flowering period of winter wheat will most likely be shorter and harvesting may occur earlier (Kenny et al. 1993). Conversely, accelerated flowering may be advantageous for some weeds grown on arable lands as they are expected to produce more ripe seeds in a shorter time before harvesting (Baker 1965; Kenny et al. 1993; Ballaré and Casal 2000). S. pecten-veneris flowered earlier and for a shorter time under warmer conditions, which resulted in significantly fewer seeds at the harvesting time. For S. pecten-veneris, this strategy ensures fertile and fully ripened seeds before harvesting, but is only advantageous on nutrient-poor soils and not under current management practices (Morison and Lawlor 1999; Storkey et al. 2010). Thus, due to low phenotypic plasticity, this weed species is also less buffered against the changes in other agronomic practices, such as sowing time or harvesting time, which affect the flowering period of the weed. This has previously been suggested as a reason for the general decline of rare weed species (Wilson and Wright 1990; Robinson and Sutherland 2002).
Clustering weed communities from arable lands by phytosociological characteristics have a long tradition in Central Europe (Tuexen 1950; Hüppe and Hofmeister 1990). L. arvense and S. pecten-veneris belong to the phytosociological class Secalinetea Br.-Bl. 1936 (Tuexen 1950; Küster 1995). They are typically found in communities such as Caucalidion platycarpi R. Tx. ex von Rochow 1951 (Tuexen 1950; Hüppe and Hofmeister 1990). Both species are competitive only under relatively nutrient-poor, calcareous soils and in crop conditions with low densities (Fried et al. 2010). Modern agricultural management practices encourage species of the phytosociological class Chenopodietea that are most competitive in nutrient-rich arable ecosystems (Otte 1990). Weed communities will be altered due to the climate change because ‘old’ species with obsolete traits are being replaced by ‘new’ species with opportunistic traits (Storkey et al. 2010). Species niches will change, and weed communities will have a different composition (Silvertown 2004; Broennimann et al. 2006; Cimalová and Lososová 2009). Thus, rare weed species may have to adapt to competition from different species in altered plant communities, in which they may not be able to effectively compete. While S. pecten-veneris is restricted to the aforementioned communities, the phenotypic plasticity of L. arvense allows the species to also appear in half-ruderal grassland (Convolvulo-Agropyrion repentis Görst 1966). This may allow L. arvense to sustain populations in alternative plant associations.
Finally, some of our results regarding S. pecten-veneris suggest a contradiction with recent bioclimatic modelling of the future species’ distribution in the UK (Berry et al. 2007). This is most likely due to the spatial approach of the most current models, which only include species distribution data and bioclimatic or agronomic parameters (Morin and Thuiller 2009). Moreover, the comparative study of Morin and Thuiller (2009) indicates the need to connect spatial modelling with biological and ecological data, and future research should incorporate these factors to improve model precision (Summers et al. 2012; Kubisch et al. 2013). Since conservation measures often rely on accurate prediction of bioclimatic distribution models, additional biological and ecological data are vital for conservation strategies to succeed under future conditions (Loss et al. 2011; Bernazzani et al. 2012). Our results provide a first step towards such integration under certain climate change scenarios.
Conclusions
We found that changes in the crop density had a stronger negative effect than warmer temperature alone. Thus, the influence of climate-mediated changes on the intensification of management practices can outweigh the sole effect of direct climate changes on rare weeds. We suggest that conservation biologists should promote extensive and non-intrusive management for the two rare weed species under warmer climate conditions (Brooke 2008). Effective conservation also requires the maintenance of the communities in which both species typically occur. This will mainly mean the promotion of extensive agriculture on calcareous or nutrient-poor soils (Swetnam et al. 1999). However, it is also possible that different species-specific responses to warmer climate conditions will result in entirely novel communities (Williams et al. 2007). Conservation efforts could attempt to anticipate changes by translocating rare weed species in communities that would occur under warmer conditions (Harris et al. 2006). This concept is also known as managed relocation (Richardson et al. 2009) or assisted colonisation (Loss et al. 2011). Knowledge of biological responses of rare weeds and their responses within communities is vital if relocation measures are to succeed. Further studies of the responses of rare weeds to community changes are needed to successfully incorporate the findings into adaptation strategies to future climate scenarios (Brooke 2008).
References
Aerts R (1999) Interspecific competition in natural plant communities: mechanisms, trade-offs and plant-soil feedbacks. J Exp Bot 50(330):29–37
Andreasen C, Stryhn H, Streibig JC (1996) Decline of the flora in Danish arable fields. J Appl Ecol 33:619–626
Baessler C, Klotz S (2006) Effects of changes in agricultural land-use on landscape structure and arable weed vegetation over the last 50 years. Agric Ecosyst Environ 115:43–50
Baker HG (1965) Characteristics and modes of origin of weeds. The genetics of colonizing species. In: Proceedings of the first international union of biological sciences, pp 147–168
Ballaré CL, Casal JJ (2000) Light signals perceived by crop and weed plants. Field Crop Res 67:149–160
Batts GR, Ellis RH, Morison JIL et al (1998) Yield and partitioning in crops of contrasting cultivars of winter wheat in response to CO2 and temperature in field studies using temperature gradient tunnels. J Agric Sci 130:17–27
Bernazzani P, Bradley BA, Opperman JJ (2012) Integrating climate change into habitat conservation plans under the U.S. Endangered Species Act. Environ Manag 49:1103–1114
Berry PM, O’Hanley JR, Thomson CL, et al. (2007) MONARCH 3 (Modelling Natural Resource Responses To Climate Change)—Technical Report. UKCIP, University of Oxford. http://www.eci.ox.ac.uk/research/biodiversity/downloads/Monarch3_FullTechnical.pdf. Accessed 14 Jan 2014
Bradshaw AD, McNeilly T (1991) Evolutionary response to global climatic change. Ann Bot 67(1):5–14
Broennimann O, Thuiller W, Hughes G et al (2006) Do geographic distribution, niche property and life form explain plants’ vulnerability to global change? Glob Chang Biol 12:1079–1093
Brooke C (2008) Conservation and adaptation to climate change. Conserv Biol 22(6):1471–1476
Chantre GR, Batlla D, Sabbatini MR, Orioli G (2009) Germination parameterization and development of an after-ripening thermal-time model for primary dormancy release of Lithospermum arvense seeds. Ann Bot 103:1291–1301
Chapin FS III, Zavaleta ES, Eviner VT et al (2000) Consequences of changing biodiversity. Nature 405:234–242
Cimalová S, Lososová Z (2009) Arable weed vegetation of the northeastern part of the Czech Republic: effects of environmental factors on species composition. Plant Ecol 203:45–57
Fahrig L, Merriam G (2002) Conservation of fragmented populations. Conserv Biol 8(1):50–59
Faraway JJ (2006) Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. Chapman & Hall, London
Fried G, Petit S, Reboud X (2010) A specialist-generalist classification of the arable flora and its response to changes in agricultural practices. BioMed Cent Ecol 10(20):1–11
Gaudet CL, Keddy PA (1988) A comparative approach to predicting competitive ability from plant traits. Nature 334:242–244
Gunton RM, Petit S, Gaba S (2011) Functional traits relating arable weed communities to crop characteristics. J Veg Sci 22:541–550
Harris JA, Hobbs RJ, Higgs E, Aronson J (2006) Ecological restoration and global climate change. Restor Ecol 14(2):170–176
Hess M, Barralis G, Bleiholder H et al (1997) Use of the extended BBCH scale—general for the descriptions of the growth stages of mono- and dicotyledonous weed species. Weed Res 37:433–441
Hobbs RJ, Huenneke LF (1992) Disturbance, diversity, and invasion: implications for conservation. Conserv Biol 6(3):324–337
Holzner W, Immonen R (1982) The agrestal weed flora and vegetation of the world. In: Holzner W, Numata M (eds) Biology and ecology of weeds. The Hague, Boston, pp 203–226
Howden SM, Soussana J-F, Tubiello FN et al (2007) Adapting agriculture to climate change. PNAS 104(50):19691–19696
Hüppe J, Hofmeister H (1990) Syntaxonomische Fassung und Übersicht über die Ackerunkrautgesellschaften der Bundesrepublik Deutschland. Berichte der Reinhold-Tüxen-Gesellschaft 2:61–81
Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5(3):299–314
Kazanis D, Arianoutsou M (2004) Long-term post-fire vegetation dynamics in Pinus halepensis forests of Central Greece: a functional group approach. Plant Ecol 171:101–121
Kenny GJ, Harrison PA, Oleson JE, Parry ML (1993) The effects of climate change on land suitability of grain maize, winter wheat and cauliflower in Europe. Eur J Agron 2(4):325–338
Korsmo E (1930) Unkräuter im Ackerbau der Neuzeit—Biologische und Praktische Untersuchungen. Springer, Berlin
Kubisch A, Degen T, Hovestadt T, Poethke HJ (2013) Predicting range shifts under global change: the balance between local adaptation and dispersal. Ecography 36:873–882
Küster H (1995) Herkunft und Ausbreitungsgeschichte einiger Secalietea-Arten. Mitteilungen der Floristisch-Soziologischen Arbeitsgemeinschaft Göttingen 5:89–98
Loarie SR, Duffy PB, Hamilton H et al (2009) The velocity of climate change. Nature 462:1052–1057
Lososová Z, Chytry M, Kühn I (2008) Plant attributes determining the regional abundance of weeds on central European arable land. J Biogeogr 35:177–187
Loss SR, Terwilliger LA, Peterson AC (2011) Assisted colonization: integrating conservation strategies in the face of climate change. Biol Conserv 144:92–100
Maillet J, Lopez-Garcia C (2000) What criteria are relevant for predicting the invasive capacity of a new agricultural weed? The case of invasive American species in France. Weed Res 40:11–26
Marshall EJP, Brown VK, Boatman ND et al (2003) The role of weeds in supporting biological diversity within crop fields. Weed Res 43:77–89
Morin X, Thuiller W (2009) Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 90(5):1301–1313
Morison JIL, Lawlor DW (1999) Interactions between increasing CO2 concentration and temperature on plant growth. Plant Cell Environ 22:659–682
Moss SR, Storkey J, Cussans JW et al (2004) The Broadbalk long-term experiment at Rothamsted: what has it told us about weeds? Weed Sci 52:864–873
Neururer H, Herwisch W (1976) Die wichtigsten Unkräuter im Getreide-, Hackfrucht-, Obst-, Wein- und Gartenbau. Bundesanstalt für Pflanzenschutz. pp 91
Neve P, Vila-Aiub M, Roux F (2009) Evolutionary-thinking in agricultural weed management. New Phytol 184:783–793
Ohlemüller R, Gritti ES, Sykes MT, Thomas CD (2006) Towards European climate risk surfaces: the extent and distribution of analogous and non-analogous climates 1931–2100. Glob Ecol Biogeogr 15:395–405
Olesen JE, Bindi M (2002) Consequences of climate change for European agricultural productivity, land use and policy. Eur J Agron 16:239–262
Otte A (1990) Die Entwicklung von Ackerwildkraut-Gesellschaften auf Böden mit guter Ertragsfähigkeit nach dem Aussetzen von Unkrautregulierungsmaßnahmen. Phytocoenologia 19:43–92
Patterson DT (1995) Weeds in a changing climate. Weed Sci 43:685–701
Pautasso M, Dehnen-Schmutz K, Holdenrieder O et al (2010) Plant health and global change—some implications for landscape management. Biol Rev 85(4):729–755
Petit S, Boursault A, Le Guilloux M et al (2011) Weeds in agricultural landscapes—a review. Agron Sustain Dev 31:309–317
Pompe S, Hanspach J, Badeck F et al (2008) Climate and land use change impacts on plant distributions in Germany. Biol Lett 4:564–567
Pompe S, Berger S, Walther G-R et al (2009) Mögliche Konsequenzen des Klimawandels für Pflanzenareale in Deutschland. Natur und Landschaft 84(1):2–7
Potts GR, Ewald JA, Aebischer NJ (2010) Long-term changes in the flora of the cereal ecosystem on the Sussex Downs, England, focusing on the years 1968–2005. J Appl Ecol 47:215–226
Reich PB, Walters MB, Ellsworth DS (1992) Leaf life-span in relation to leaf, plant and stand characteristics among diverse eco-systems. Ecol Monogr 62(3):365–392
Rich TCG, Woodruff ER (1995) Changes in the vascular plant floras of England and Scotland between 1930–1960 and 1987–1988: the BSBI monitoring scheme. Biol Conserv 75:217–229
Richardson DM, Hellmann JJ, McLachlan JS et al (2009) Multidimensional evaluation of managed relocation. PNAS 106(24):9721–9724
Robinson RA, Sutherland WJ (2002) Post-war changes in arable farming and biodiversity in Great Britain. J Appl Ecol 39:157–176
Sala OE, Chapin FS III, Armesto JJ et al (2000) Global biodiversity scenarios for the Year 2100. Science 287:1770–1774
Schneider C, Sukopp U, Sukopp H (1994) Biologisch-ökologische Grundlagen des Schutzes gefährdeter Segetalpflanzen. Schriftenreihe für Vegetationskunde 26:1–340
Sheppard CS, Alexander JM, Billeter R (2012) The invasion of plant communities following extreme weather events under ambient and elevated temperature. Plant Ecol 213:1289–1301
Silvertown J (2004) Plant coexistence and the niche. TREE 19(11):605–611
Storkey J (2006) A functional group approach to the management of UK arable weeds to support biological diversity. Weed Res 46:513–522
Storkey J, Moss SR, Cussans JW (2010) Using assembly theory to explain changes in a weed flora in response to agricultural intensification. Weed Sci 58:39–46
Summers DM, Bryan BA, Crossman ND, Meyer W (2012) Species vulnerability to climate change: impacts on spatial conservation priorities and species representation. Glob Chang Biol 18:2335–2348
Sutcliffe OL, Kay QON (2000) Changes in the arable flora of central southern England since the 1960s. Biol Conserv 93:1–8
Svensson R, Wigren M (1986a) Sminkrotens historia och biologi i Sverige (History and biology of Lithospermum arvense in Sweden). Svensk Botanisk Tidskrift 80:107–131
Svensson R, Wigren M (1986b) A survey of the history, biology and preservation of some retreating synanthropic plants. Acta Universitatis Upsaliensis 25(4):1–74
Swetnam TW, Allen CD, Betancourt JL (1999) Applied historical ecology: using the past to manage for the future. Ecol Appl 9(4):1189–1206
Tuexen R (1950) Grundriss einer Systematik der nitrophilen Unkrautgesellschaften in der Eurosibirischen Region Europas. Mitteilungen der floristisch-soziologischen Arbeitsgemeinschaft 2:94–176
Wardlaw IF, Wrigley CW (1994) Heat tolerance in temperate cereals: an overview. Aust J Plant Physiol 21:695–703
Willerding U (1986) Zur Geschichte der Unkräuter Mitteleuropas. Karl Wachholtz, Neumünster
Williams JW, Jackson ST, Kutzbach JE (2007) Projected distributions of novel and disappearing climates by 2100 AD. PNAS 104(14):5738–5742
Wilson P (2006) UK Biodiversity Action Plan for Scandix pecten-veneris. Plantlife International, The Wild Plant Conservation Charity. http://www.plantlife.org.uk/uploads/documents/Scandix_pecten-veneris_dossier.pdf. Accessed 14 Jan 2014
Wilson BJ, Wright KJ (1990) Predicting the growth and competitive effects of annual weeds in wheat. Weed Res 30:201–211
Zuur AF, Ieno EN, Walker NJ et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
This study was supported by the Ministry for Science and Culture of Lower Saxony within the network KLIFF—climate impact and adaptation research in Lower Saxony. We thank Ingolf Gliege, Martina Goltermann, Diego Piedra Garcia and Katharina Beer for assisting in the experiments. We also thank PlantScribe for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. P. Messina.
Rights and permissions
About this article
Cite this article
Peters, K., Gerowitt, B. Response of the two rare arable weed species Lithospermum arvense and Scandix pecten-veneris to climate change conditions. Plant Ecol 215, 1013–1023 (2014). https://doi.org/10.1007/s11258-014-0358-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-014-0358-3