Abstract
The effects of direct and indirect defenses differ among plant species, and the variation in the mode of plant defenses might reflect physiological and/or ecological constraints of each mode of defense related to the growth and reproduction of individual plant species. To evaluate the advantages and disadvantages of indirect ant-mediated defense via extrafloral nectaries (EFNs), we compared the herbivory pressure, leaf chemicals, vegetative growth, and reproduction between two species of vetches, Vicia sativa var. angustifolia (Reichard) Wahlenb (Leguminosae) with EFNs and V. hirsuta (L.) SF Gray without EFNs (or with very small EFNs). Indirect ant defense of V. sativa was not consistently reliable because of the low constancy of ant attraction. In addition, V. sativa was more vulnerable to attack by herbivores than V. hirsuta. The estimated total amount of sugars secreted by EFNs of V. sativa corresponded to 0.5% of total leaf biomass, and 0.07% of total plant biomass, indicating a low investment to the production of extrafloral nectar. Vicia sativa plants grew more rapidly than V. hirsuta plants during the reproductive stage. Therefore, we consider that V. sativa adopts the ant defense via EFNs in spite of its low reliability because the indirect ant defense supported by EFNs requires only low investment, allowing the plants to attain rapid growth in the early spring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have evolved various modes of anti-herbivory defense (Howe and Westley 1988). They are largely classified into two categories: “direct defense” and “indirect defense”. It is known that plants can defend themselves against herbivores in a direct physical manner involving structures such as spines, hairs, wax, and thick cell walls (Fernandes 1994). Many secondary metabolic substances, such as alkaloids, terpenoids, and phenolics, act as chemical toxins to reduce herbivore attacks (Feeny 1970; Larson and Berry 1984; Adler 2000).
It is also known that animals at higher trophic levels (e.g., predators and parasitoids) can limit the abundance and impact of herbivores on plants, and the plants “indirectly” defend themselves by attracting these animals (Price et al. 1980; Dicke 1999). For example, extrafloral nectaries (EFNs) function as an indirect defense by ants (Beattie 1985; Koptur 1992). EFNs secrete extrafloral nectar that attracts ants onto the plant (Koptur 1992; Katayama and Suzuki 2004). The ants exclude herbivores (Koptur et al. 1998; Katayama and Suzuki 2005) and remove pathogenic fungi (de la Fuente and Marquis 1999) from the plants, and increase plant fitness (Stephenson 1982; de la Fuente and Marquis 1999).
The effects of direct and indirect defenses differ among plant species: some plants employ direct defense, while others employ indirect defense (Itioka et al. 2000; Linsenmair et al. 2001; Agrawal et al. 2002). For example, the genus Macaranga (Euphorbiaceae) in tropical southeast Asia is known for a wide range of mutualistic associations with ants, ranging from facultative to obligate ones (Fiala et al. 1989; Itioka et al. 2000). Macaranga plants with an obligate association with ants contain less phenolics (tannin) than those with a facultative one (Eck et al. 2001). In ant-plant associations, many studies have shown a negative correlation between direct defensive traits and indirect ant-mediated defensive traits (Dyer et al. 2001; Eck et al. 2001; but see: Rudgers et al. 2004).
Indirect defense by ants attracted by EFNs may be superior in some respects to direct defenses and other indirect ones. For example, ants may be effective defenders against a wide range of insect herbivores (Keeler 1977; Beattie 1985). Specialist herbivores frequently break through chemical defenses of plants (Howe and Westley 1988). However, ants attack regardless of the susceptibilities of herbivores to the chemical defenses (Keeler 1977; Beattie 1985). In addition, the costs of extrafloral nectar production may be cheap for plants (O’Dowd 1979). The main components of the extrafloral nectar are sugars produced by photosynthesis (Koptur 1992; Heil and McKey 2003). O’Dowd (1979) reported that extrafloral nectar production in Ochroma pyramidale (Cav.) Urban utilized only 1% of the energy invested in leaf production. Furthermore, the EFNs are anatomically and morphologically simple, requiring little differentiation or additional structure for their functions (Beattie 1985).
Although many studies have focused on the advantages of indirect ant defense, those studies have tended to overlook the disadvantages constraining each mode of defense in relation to the growth and reproduction of plants. The main disadvantage of indirect defense by ants seems to be related to the uncertainty of the defensive effects. For example, the abundance of ants depends on the habitat (Bentley 1976; Horvitz and Schemske 1990), and some ant species cannot exclude herbivores from plants (Katayama and Suzuki 2005). When EFNs fail to attract ants, or attract less effective ants, plants may suffer high herbivory.
In this study, to evaluate the advantages and disadvantages of indirect defense by ants attracted by EFNs, we compared the herbivory pressure, leaf chemicals, vegetative growth, and reproduction between two species of vetches, Vicia sativa var. angustifolia (Reichard) Wahlenb (with EFNs) (Leguminosae) and V. hirsuta (L.) SF Gray (without the EFNs or with EFNs too small to attract ants) found in temperate regions. In addition, we estimated the investment to the indirect defense by ants by measuring total extrafloral nectar production of V. sativa. Our results supported the hypothesis that although indirect ant defense of V. sativa is not consistently reliable because of the low constancy of ant attraction, the ant defense would be a better strategy for rapidly growing plants, because the investment to EFN production would be small and cheap, and plants would be able to invest more resources for vegetative growth and/or reproduction.
Materials and methods
Vicia plants
The two species of the vetches, V. sativa and V. hirsuta, are annual legumes, and are frequently growing together in open land such as roadsides, grasslands, and footpaths (Brown et al. 1987; Ohta et al. 2006). In western Japan, the overwintering plants grow rapidly in early spring, and flower in March to June. Vicia sativa produces pods containing five to eleven seeds, and V. hirsuta produces pods containing two seeds, successively from May to June. Although the two species have similar life history traits, there are some differences in their traits related to attracting ants. Vicia sativa bears many stipules with EFNs. On the other hand, V. hirsuta does not secrete extrafloral nectar, or its volume is too little to attract ants in Japan (Katayama personal obs.).
Insect communities
To clarify the herbivory pressure on the plants of V. sativa and V. hirsuta, the community composition of herbivorous insects on these plants was examined during the flowering season (from early April to early May) in 2002–2004. We investigated insect communities in 27 sites of V. sativa, and 10 sites of V. hirsuta in Saga Prefecture (33°16′N, 130°18′E) in western Japan. The sites were located at least 1 km apart from each other. Although within the sites, both plants of V. sativa and V. hirsuta frequently grew together, we chose the assembly of only single species and regarded it as a population of each species. We set five quadrats (25 × 25 cm2) in each population and collected the aboveground part of the plants including animals harbored on the plants in each quadrat. Then, we brought the plant samples back to a laboratory, and counted the number of each type of herbivorous insect in each quadrat.
Leaf chemicals
From eight populations of V. sativa and eight populations of V. hirsuta in Saga Prefecture, we collected a sufficient amount of leaves (over 100 g in dry weight per population) of each species from 17 April to 21 May 2004. The collected leaves were dried naturally in a laboratory at 25°C and then the dry leaf samples were powdered using a mill for leaf chemical analysis and bioassay.
Nitrogen is an essential limiting element for survival and/or growth of many herbivorous insects (Mattson and Scriber 1987). In general, the tissue of herbivorous insects has a lower C/N ratio (higher nitrogen concentration) compared to plants (Fagan et al. 2002). This indicates that herbivores demand more nitrogen when they consume plants, and plant parts containing high nitrogen concentration are preferentially utilized by the herbivores (Fagan et al. 2002). In this study, the carbon and nitrogen contents of the leaves were measured using an elemental analyzer (CHN Corder MT-3, Yanaco, Kyoto, Japan), with 20-mg powder samples, and the foliar C/N ratio was compared between the two Vicia species, as an indicator of the plant nutritional quality for herbivores.
Since foliar phenolics are defensive substances against many herbivorous arthropods (Feeny 1970; Dudt and Shure 1994), we measured foliar phenolics of V. sativa and V. hirsuta. Total phenolics in a 20-mg leaf powder sample were extracted with 50% methanol (10 ml) for 1 h in a 40°C ultrasonic bath, and the concentration (mg/g) was measured using the Folin–Ciocalteu method (Julkunen-Tiitto 1985).
Bioassay for susceptibility of herbivores
To clarify the susceptibility of herbivores to both vetch species, we carried out a bioassay for the susceptibility of herbivores, using polyphagous caterpillars of the common armyworm, Pseudaletia separata Walker (Lepidoptera: Noctuidae), which can be regarded as a generalist herbivore with a broad host range.
We obtained a sufficient amount of leaf powder from eight populations of each vetch species. We made the artificial diet by mixing 10 g of leaf powder of each vetch population, 10 g of base diet and 70 ml of water. The base diet provided by NOSAN Corporation contains essential nutrients (soybean powder, cellulose, vitamins, and minerals), preservatives, and antibiotics. The fresh body weight of ten 4th (0 day) instar larvae of the common armyworm, P. separata, was measured and thereafter, they were individually put into 10 Petri dishes with 2 g of the artificial diet including the leaf powder of V. sativa or V. hirsuta. After 24 h, the fresh body weight of larvae was measured, and the leftover diet was dried at 60°C for 48 h, and the dry weight was measured. We also measured the water content of 2 g of the artificial diet including the leaf powder of each vetch species. Then, we estimated the amount of consumption (dry mass) by a larva. We replicated this procedure on 10 larvae for each vetch population, and the average amount of consumption by the 10 larvae was used for statistical analysis comparing V. sativa and V. hirsuta (n = 8 in both vetch species).
Extrafloral nectar production
We collected over 100 seeds per population from eight populations of V. sativa and eight populations of V. hirsuta in Saga Prefecture. Collected seeds were put in a glass pot (3 cm in diameter, 7.5 cm in depth), and were kept in an incubator at 10°C under dark conditions.
In November 2004, 20 seeds of each population were sown into polyethylene pots (7 cm in diameter and 6.5 cm in depth) filled with commercial soil (Hana to Yasai no Baiyoudo®, Tachikawa Heiwa Nouen Co., LTD.), and the pots were placed in an experimental field of the Center for Ecological Research, Kyoto University in Otsu City, Shiga Prefecture (35°01′N, 135°51′E).
From middle March to late May in 2005, we randomly selected one plant in each V. sativa and V. hirsuta population once a week (total 11 times), and removed extrafloral nectar in EFNs on all stipules on the plant using 0.5-μl microcapillary tubes (Drummond Scientific Company, Broomall, Pennsylvania, USA). After removing extrafloral nectar, the plants were located in an outdoor climate chamber (25°C, and natural light conditions) to exclude ants and other insects that would forage extrafloral nectar. After 1 day, we collected extrafloral nectar from each plant using 0.5-μl microcapillary tubes. The total volume of collected extrafloral nectar was estimated by multiplying the proportion of the length of the tube filled with extrafloral nectar relative to the total length of the tube by 0.5 μl.
Extrafloral nectar samples were dissolved in 15 μl of Milli-Q-Water. Five microliters of the sample solution were used for sugar analysis by high-pressure liquid chromatography (HPLC), using a Wakosil 5NH2-MS packed column (4.6 × 150 mm; Wako Pure Chemical, Osaka, Japan) and an 80% acetonitrile mobile phase at room temperature. Peak size for the various sugars present in the extrafloral nectar samples was calculated directly using a refractive index detector (RID; Shimadzu Corp., Kyoto, Japan). Extrafloral nectar samples were optimized using six sugar standards (xylose, fructose, glucose, sucrose, maltose, and melezitose), and the composition of each sample was determined by comparison of retention times with those from a standard sample measured on the same day.
Total sugar amount excreted by EFNs was estimated by integrating “amount of sugars per d”. Therefore, we estimated total sugar secretion of the eight populations of the vetches during the reproductive stage as follows:
where EF is total amount of sugars secreted during the reproductive stage, A i is total sugar secretion per day on the ith (i = 1–11) collection of extrafloral nectar, and t i is the sampling day of the ith collection of extrafloral nectar. In this formula, we assumed that the plants produced new nectar everyday even if the nectar was not consumed based on a preliminary experiment demonstrating that Vicia faba, which is closely related to V. sativa, newly secreted extrafloral nectar everyday (N. Katayama, unpublished data).
Biomass of vegetative and reproductive organs
Just after collecting extrafloral nectar, we divided each V. sativa and V. hirsuta plant into leaves, reproductive organs (i.e., buds, flowers, and pods) and other parts (i.e., stems and roots). They were oven-dried at 60°C for 48 h, and the dry mass of leaves, reproductive organs, and other plant parts were weighed.
Statistical procedures
Abundance of each herbivore was compared among V. sativa with ants, V. sativa without ants, and V. hirsuta (without ants) using GLMs with a log link function assumed for the Poisson distribution. The Bonferroni correction was used for multiple comparisons. Carbon, nitrogen, C/N ratio and total phenolics of leaves, bodyweight of common armyworm larvae, and the amount of consumption of the diet by the larvae were compared between V. sativa and V. hirsuta using a t-test. A repeated measures ANOVA was used to compare plant biomass, and the proportion of leaf biomass and reproductive organs relative to total plant mass between V. sativa and V. hirsuta. Tukey HSD was used as a post hoc test.
All statistical procedures were conducted using JMP ver. 7 (SAS Institute Inc.).
Results
Insect communities on plants
Ants
Although in total we found 12 ant species that visited V. sativa plants in 27 sampling populations, ants did not always visit V. sativa plants. In 35.6% (48/135) of quadrats, there were no ants. We seldom observed ants on V. hirsuta plants (6.0%, 3/50), and the number of ants per quadrat on V. hirsuta was significantly smaller than that on V. sativa plants (V. sativa (n = 135): 6.83 ± 10.85 individuals per quadrat (mean ± SD), V. hirsuta (n = 50): 0.10 ± 0.46, GLM, χ 1,183 = 531.9, P < 0.001).
Herbivores
The number of overall herbivores on V. sativa plants was 12-fold greater than that on V. hirsuta plants (V. sativa (n = 135): 91.07 ± 207.12 (mean ± SD), V. hirsuta (n = 50): 7.58 ± 10.18, GLM, χ1,183 > 1,000, P < 0.001). The number of overall herbivores on V. sativa plants without ants was significantly greater than that on V. sativa plants with ants or that on V. hirsuta plants (Table 1).
On V. sativa plants, we frequently observed larvae of the alfalfa weevil, Hypera postica Gyllenhal (Coleoptera: Curculionidae), which fed on the leaves, flower buds, and flowers of V. sativa. The number of the weevil larvae on V. sativa plants decreased in the presence of ants (Table 1). The number of the weevil larvae on V. sativa plants was 42-fold greater than that on V. hirsuta (V. sativa (n = 135): 31.60 ± 47.95 (mean ± SD), V. hirsuta (n = 50): 0.76 ± 3.00, GLM, χ1,183 > 1,000, P < 0.001).
Although lepidopteran larvae, orthopteran nymphs, and hemipteran adults (stinkbugs) were occasionally observed on V. sativa and V. hirsuta plants, the numbers were very small on both the vetch species (Table 1).
One ant-tended aphid species, Aphis craccivora Koch, and two non-ant-tended aphid species, Acyrthosiphon pisum Harris and Megoura crassicauda Mordvilko (Hemiptera: Aphididae), parasitized on V. sativa plants, and two aphid species, Ap. craccivora and Ac. pisum parasitized on V. hirsuta plants. The numbers of ant-tended aphids and non-ant-tended aphids on V. sativa plants were 126-fold and 6.3-fold greater than those on V. hirsuta, respectively (ant-tended aphids, V. sativa (n = 135): 17.62 ± 80.36 (mean ± SD), V. hirsuta (n = 50): 0.14 ± 0.86, GLM, χ 1,183 > 1,000, P < 0.001, non-ant-tended aphids, V. sativa (n = 135): 41.73 ± 186.88 (mean ± SD), V. hirsuta (n = 50): 6.58 ± 9.91, GLM, χ 1,183 > 1000, P < 0.001). On V. sativa plants with ants, the number of ant-tended aphids was greater but the number of non-ant-tended aphids was smaller than those on V. sativa plants without ants (Table 1).
Leaf chemicals
The carbon content of the leaves of V. sativa was lower than that of the leaves of V. hirsuta (V. sativa (n = 8): 434.6 ± 6.5 mg/g (mean ± SD), V. hirsuta (n = 8): 446.7 ± 4.0 mg/g, t-test, t 14 = 4.658, P < 0.001), and the nitrogen content was higher than that of the leaves of V. hirsuta (V. sativa (n = 8): 52.2 ± 2.8 mg/g (mean ± SD), V. hirsuta (n = 8): 43.6 ± 3.1 mg/g, t-test, t 14 = −6.038 P < 0.001). Therefore, the C/N ratio of the leaves of V. sativa was lower than that of the leaves of V. hirsuta (t-test, t 14 = 7.181, P < 0.001, Fig. 1).
Total phenolics in the leaves did not differ between V. sativa and V. hirsuta (V. sativa (n = 8): 17.3 ± 2.0 mg/g (mean ± SD), V. hirsuta (n = 8): 17.8 ± 1.2 mg/g, t-test, t 14 = 0.547, P = 0.593).
Bioassay for susceptibility of herbivores
The water content (%) of 2.00 g of the artificial diet including the leaf powder of V. sativa and V. hirsuta was 72.9 ± 0.7% (mean ± SD) and 73.4 ± 1.0%, respectively (t-test, t 14 = 0.977, P = 0.345).
The initial fresh body weight of caterpillars before feeding did not differ between treatments (V. sativa (n = 80): 0.218 ± 0.027 g (mean ± SD); V. hirsuta (n = 80): 0.224 ± 0.026 g, t-test, t 158 = 1.378, P = 0.170), but the fresh body weight of larvae fed on the artificial diet including the leaf powder of V. sativa was heavier than that of larvae fed on the artificial diet including the leaf powder of V. hirsuta (V. sativa (n = 80): 0.387 ± 0.056 g (mean ± SD), V. hirsuta (n = 80): 0.349 ± 0.047 g, t-test, t 158 = −4.651, P < 0.001). The change in body weight of larvae before and after feeding on the artificial diet including the leaf powder of V. sativa was greater than that of larvae fed on the artificial diet including the leaf powder of V. hirsuta (V. sativa (n = 80): 0.168 ± 0.038 g (mean ± SD), V. hirsuta (n = 80): 0.125 ± 0.031 g, t-test, t 158 = −7.971, P < 0.001).
The amount of consumption (dry weight) of the diet by a larva fed on the artificial diet including the leaf powder of V. sativa was significantly greater than that by a larva fed on the artificial diet including the leaf powder of V. hirsuta (t-test, t 14 = −3.554, P = 0.003, Fig. 2).
Extrafloral nectar production
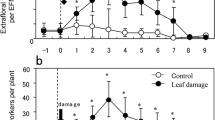
Although V. hirsuta did not secrete extrafloral nectar, V. sativa secreted extrafloral nectar from mid-April to late May (Fig. 3). Extrafloral nectar of V. sativa contained fructose, glucose, and sucrose, and V. sativa most actively secreted sugars on May 3. The estimated total amount of sugars secreted by V. sativa from April 18 to May 31 was 3.50 ± 1.19 mg (n = 8, mean ± SD).
Biomass of vegetative and reproductive organs
Vicia sativa and V. hirsuta grew from March to May, and they produced pods starting in mid-May. All plants were withered in early June.
The original size (initial size) did not differ between V. sativa and V. hirsute. However, V. sativa rapidly grew, and the biomass of V. sativa was greater than that of V. hirsuta (repeated measures ANOVA, plant species: F 1,14 = 272.40, P < 0.001, time: F 10,6 = 37.75, P < 0.001), and there is a significant interaction between plant species and time (F 10,6 = 40.83, P < 0.001; Fig. 4a). The biomass of V. sativa was greater than that of V. hirsuta from May 11 to May 31.
Seasonal changes in a the biomass of a whole plant, and the proportion of biomass of b leaves and c reproductive organs. Solid and open circles represent Vicia sativa (n = 8) and Vicia hirsuta (n = 8), respectively. Bars show SD. Asterisks indicate significant difference between Vicia angustifolia and Vicia hirsuta (Tukey HSD, *P < 0.05)
The proportion of leaf biomass relative to total plant mass differed significantly between V. sativa and V. hirsuta (repeated measures ANOVA, plant species: F 1,14 = 9.69, P = 0.007, time: F 10,6 = 47.80, P < 0.001), and there was a significant interaction between plant species and time (F 10,6 = 13.98, P = 0.002; Fig. 4b). The proportion of leaf biomass of V. hirsuta was greater than that of V. sativa on May 3.
The proportion of biomass of the reproductive organs relative to total plant mass differed significantly between V. sativa and V. hirsuta (repeated measures ANOVA, plant species: F 1,14 = 62.26, P < 0.001, time: F 10,6 = 274.39, P < 0.001), and there was a significant interaction between plant species and time (F 10,6 = 18.39, P = 0.003; Fig. 4c). The proportion of biomass of the reproductive organs of V. sativa was greater than that of V. hirsuta from May 18 to May 31.
Discussion
Herbivory pressure on the two vetch species
The numbers of weevil larvae and non-ant-tended aphids were markedly smaller in the presence of ants than in the absence of ants on V. sativa plants (Table 1). This is because ants attracted by EFNs of V. sativa attack herbivorous insects, and exclude them from the plants (Koptur 1979; Suzuki et al. 2004; Katayama and Suzuki 2005). It is well known that ants exclude herbivorous insects from plants (Bentley 1977). This is due to the omnivory and territory-defending behaviors of ants (Hölldobler and Wilson 1990). Ants attack and prey on various insects on the plants. Many kinds of organisms, including insects such as Lepidoptera and Hemiptera (Way 1963; Pierce et al. 1987; Gibernau and Dejean 2001) and plants (Bentley 1977; Koptur 1992; Linsenmair et al. 2001) employ ants as efficient bodyguards. The results of our field census (Table 1) showed that EFNs of V. sativa functioned as an indirect ant defense.
However, our finding that 35.6% of sites lacked ants showed that the effect of indirect ant defense by EFNs was not constant. Ant attraction by EFNs is affected by biotic factors, such as the presence of other carbohydrate resources for ants (Buckley 1983; Horvitz and Schemske 1990; Katayama and Suzuki 2003) and the absence of ants near the plant. Competition for ants frequently occurs among plants and/or ant-tended insects (Becerra and Venable 1989; Apple and Feener 2001). Therefore, plants occasionally fail to attract ants by their EFNs.
Vicia sativa with EFNs tended to be more vulnerable to attack by herbivores than V. hirsuta. The numbers of weevil larvae and aphids on V. sativa plants were significantly larger than those on V. hirsuta plants. In addition, our bioassay experiment demonstrated the smaller mass gains of the caterpillars and lower amount of diet consumption by the larvae when fed on an artificial diet including the leaf powder of V. hirsuta than when fed on one containing that of V. sativa (Fig. 2). This might indicate that the nutrient quality of V. hirsuta for herbivores is lower than that of V. sativa, and/or the strength of chemical defense by V. hirsuta is greater than that by V. sativa. Although the concentration of total phenolics did not differ between leaves of V. sativa and V. hirsuta, leaves of V. hirsuta may contain other defensive substances because V. hirsuta in the field was hardly attacked by herbivorous insects. For example, V. hirsuta contains a specific hemiterpene glucoside that works on a chemical barrier against a bean aphid, Megoura crassicauda (Ohta et al. 2006). Vicia plants have several neurotoxic amino acids (Bell and Tirimanna 1965). However, the chemical substances that create the difference in herbivore community between V. sativa and V. hirsuta have not been well explored.
Although we did not measure the plant biomass in the field census, we suspected that the biomass of V. sativa was greater than that of V. hirsuta, particularly in the later reproductive stage. Then, V. sativa may potentially attract more herbivores than V. hirsute.
Our study did not consider the habitat effects on plant growth and defensive traits.
Investment for the production of extrafloral nectar
We estimated that the total amount of sugars secreted by V. sativa was 3.50 mg (Fig. 3). The average leaf mass and total plant biomass of V. sativa on May 25 were 0.702 g and 4.780 g, respectively (Fig. 4). Therefore, the estimated total amount of sugar secretion during the reproductive stage corresponded to 0.5% of total leaf biomass, and to 0.07% of total plant biomass on May 25. This result may mean that investment for the production of extrafloral nectar is small, compared with that for other defensive substances. Quantitative defensive substances, such as polyphenols, in leaves of Thea sinnensis correspond to 30% of leaf biomass (Vickery and Vickery 1981). Plants with an obligate mutualism with ants invest great resources for ant-mediated defense (Heil et al. 1997; Hatada et al. 2002). The energy cost for extrafloral nectar production seems to be smaller than that for other defensive substances (O’Dowd 1979).
Why does V. sativa adopt the ant-mediated defense by EFNs?
Extrafloral nectaries of Vicia plants have a prominent role to exclude herbivores on the plants (Koptur 1979; Katayama and Suzuki 2004). Our study strongly supported the results of the previous studies (see Table 1). However, our study also suggested that the indirect ant defense of V. sativa was not consistently reliable because the plants occasionally failed to attract ants. Then, we consider the reason why V. sativa adopts the ant defense by EFNs in spite of the low reliability.
Vicia sativa grew more rapidly and reproduced earlier than V. hirsuta without EFNs. Rapidly growing plants are compelled to reduce the investments in defenses because of the trade-off between defense and growth and/or reproduction (Redman et al. 2001). Indirect ant defense via EFNs seems to require relatively low investment (O’Dowd 1979). Our study also indicated that the investment to the secretion of the extrafloral nectar was small. Furthermore, rapidly growing plants bear leaves with high nitrogen to attain a higher photosynthetic rate (Evans 1989). However, a great variety of herbivores tend to attack leaves with higher nitrogen content because the leaves are more palatable (Mattson and Scriber 1987). Indirect ant defense via EFNs seems to be effective against a wide range of herbivores (Keeler 1977). Then, we consider that V. sativa might adopt the inexpensive indirect ant-mediated defense via EFNs. On the other hand, V. hirsuta may conduct strong direct chemical defense. Vicia hirsuta, which is smaller than V. sativa, would suffer serious damage if it would be attacked by the equivalent abundant herbivores with the case of V. sativa. Thus, V. hirsuta may adopt reliable direct defenses instead of facultative indirect ant defenses.
Many plant species with EFNs were pioneer plants (Bentley 1976, 1977). Heil and McKey (2003) discussed that the fact that ant defenses were highly developed in pioneer plants was consistent with the resource availability hypothesis (Coley et al. 1985) that the rapidly growing plants favor low investments in anti-herbivore defenses. In addition, the ant defense strategy is favorable for plants in disturbed habitats. Koptur (1979) demonstrated that extrafloral nectar of V. sativa was preferred by exotic ant species. In that report, she discussed that a tight coevolutionary process was not needed to maintain the facultative mutualism with ants, and that the vetches might be successful weeds because of their ability to attract ants in non-native locations. Vicia sativa do not selectively attract specific ant species (Katayama and Suzuki 2005). In this process, it sometimes fails to attract ants, or attracts non-mutualistic ants. However, it adopts the ant-mediated defense by EFNs because of the above advantages of extrafloral nectaries.
References
Adler LS (2000) Alkaloid uptake increases fitness in a hemiparasitic plant via reduced herbivory and increased pollination. Am Nat 156:92–99
Agrawal AA, Janssen A, Bruin J, Posthumus MA, Sabelis MW (2002) An ecological cost of plant defence: attractiveness of bitter cucumber plants to natural enemies of herbivores. Ecol Lett 5:377–385
Apple JL, Feener DH Jr (2001) Ant visitation of extrafloral nectaries of Passiflora: the effects of nectary attributes and ant behavior on patterns in facultative ant-plant mutualisms. Oecologia 127:409–416
Beattie AJ (1985) The evolutionary ecology of ant-plant mutualisms. Cambridge University Press, New York
Becerra JXI, Venable DL (1989) Extrafloral nectaries: a defense against ant-Homoptera mutualisms? Oikos 55:276–280
Bell EA, Tirimanna ASL (1965) Associations of amino acids and related compounds in the seeds of forty-seven species of Vicia: their taxonomic and nutritional significance. Biochem J 97:104–111
Bentley BL (1976) Plants bearing extrafloral nectaries and the associated ant community: interhabitat differences in the reduction of herbivore damage. Ecology 57:815–820
Bentley BL (1977) Extrafloral nectaries and protection by pugnacious bodyguards. Ann Rev Ecol Syst 8:407–427
Brown VK, Gange AC, Evans IM, Storr AL (1987) The effect of insect herbivory on the growth and reproduction of two annual Vicia species at different stages in plant succession. J Ecol 75:1173–1189
Buckley R (1983) Interaction between ants and membracid bugs decreases growth and seed set of host plant bearing extrafloral nectarines. Oecologia 58:132–136
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 22:895–899
de la Fuente MAS, Marquis RJ (1999) The role of ant-tended extrafloral nectaries in the protection and benefit of a neotropical rainforest tree. Oecologia 118:192–202
Dicke M (1999) Specificity of herbivore-induced plant defences. In: Chadwick DJ, Goode JA (eds) Insect-plant interactions and induced plant defence. Wiley, Chichester, pp 43–59
Dudt JF, Shure DJ (1994) The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology 75:86–98
Dyer LA, Dodson CD, Beihoffer J, Letourneau DK (2001) Trade-offs in antiherbivore defenses in Piper cenocladum: ant mutualists versus plant secondary metabolites. J Chem Ecol 27:581–592
Eck G, Fiala B, Linsenmair KE, Hashim RB, Proksch P (2001) Trade-off between chemical and biotic antiherbivore defense in the South East Asian plant genus Macaranga. J Chem Ecol 27:1979–1996
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Fagan WF, Siemann E, Mitter C, Denno RF, Huberty AF, Woods HA, Elser JJ (2002) Nitrogen in insects: implications for trophic complexity and species diversification. Am Nat 160:784–802
Feeny PP (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
Fernandes GW (1994) Plant mechanical defenses against insect herbivory. Rev Bras Entomol 38:421–433
Fiala B, Maschwitz U, Pong TY, Helbig AJ (1989) Studies of a South East Asian ant-plant association: protection of Macaranga trees by Crematogaster borneensis. Oecologia 79:463–470
Gibernau M, Dejean A (2001) Ant protection of a Heteropteran trophobiont against a parasitoid wasp. Oecologia 126:53–57
Hatada A, Itioka T, Yamaoka R, Itino T (2002) Carbon and nitrogen contents of food bodies in three myrmecophytic species of Macaranga: implications for antiherbivore defense mechanisms. J Plant Res 115:179–184
Heil H, McKey D (2003) Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu Rev Ecol Evol Syst 34:425–553
Heil M, Fiala B, Linsenmair KE, Zotz G, Menke P, Maschwitz U (1997) Food body production in Macaranga triloba (Euphorbiaceae): a plant investment in anti-herbivore defence via symbiotic ant partners. J Ecol 85:847–861
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Horvitz CC, Schemske DW (1990) Spatiotemporal variation in insect mutualists of a neotropical herb. Ecology 71:1085–1097
Howe HF, Westley LC (1988) Ecological relationships of plants and animals. Oxford University Press, New York
Itioka T, Nomura M, Inui Y, Itino T, Inoue T (2000) Difference in intensity of ant defense among three species of Macaranga myrmecophyte in a Southeast Asian dipterocarp forest. Biotropica 32:318–326
Julkunen-Tiitto R (1985) Phenolics constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agr Food Chem 33:213–217
Katayama N, Suzuki N (2003) Changes in the utilization of extrafloral nectaries of Vicia faba (Leguminosae) and honeydew of aphids by ants with increasing aphid density. Ann Entomol Soc Am 96:579–584
Katayama N, Suzuki N (2004) Role of extrafloral nectaries of Vicia faba for attraction of ants and herbivores exclusion by ants. Entomol Sci 7:119–124
Katayama N, Suzuki N (2005) The importance of the encounter rate between ants and herbivores and of ant aggressiveness against herbivores in herbivore exclusion by ants on Vicia angustifolia L. (Leguminosae) with extrafloral nectaries. Appl Entomol Zool 40:69–76
Keeler KH (1977) The extrafloral nectaries of Ipomoea carnea (Convolvulaceae). Am J Bot 64:1182–1188
Koptur S (1979) Facultative mutualism between weedy vetches bearing extrafloral nectaries and weedy ants in California. Am J Bot 66:1016–1020
Koptur S (1992) Extrafloral nectary-mediated interactions between insects and plants. In Bernays E (ed) Insect-plant interaction, vol IV. CRC press, Boca Raton, pp 81–129
Koptur S, Rico-Gray V, Palacios-Rios M (1998) Ant protection of the nectaried ferrn Polypodium plebeium in central Mexico. Am J Bot 85:736–739
Larson KC, Berry RE (1984) Influence of peppermint phenolics and monoterpenes on two spotted spider mite (Acari: Tetranychidae). Environ Entomol 13:282–285
Linsenmair KE, Heil M, Kaiser WM, Fiala B, Koch T, Boland W (2001) Adaptations to biotic and abiotic stress: Macaranga-ant plants optimize investment in biotic defence. J Exp Bot 52:2057–2065
Mattson WJ, Scriber JM (1987) Nutritional ecology of insect folivores of woody plants: nitrogen, water, fiber, and mineral considerations. In: Slansky F, Rodrigues JG (eds) Nutritional ecology of insects Mites spiders, and related invertebrates. Wiley, New York, pp 105–146
O’Dowd DJ (1979) Foliar nectar production and ant activity on a neotropical tree, Ochroma pyramidale. Oecologia 43:233–248
Ohta N, Mori N, Kuwahara Y, Nishida R (2006) A hemiterpene glucoside as a probing deterrent of the bean aphid, Megoura crassicauda, from a non-host vetch, Vicia hirsuta. Phytochemistry 67:584–588
Pierce NE, Kitching RL, Buckley RC, Taylor MFJ, Benbow KF (1987) The costs and benefits of cooperation between the Australian lycaenid butterfly, Jalmenus evagoras, and its attendant ants. Behav Ecol Sociobiol 21:237–248
Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Redman AM, Cipollini DF Jr, Schultz JC (2001) Fitness costs of jasmonic acid-induced defense in tomato, Lycopersicon esculentum. Oecologia 126:380–385
Rudgers JA, Strauss SY, Wendel JF (2004) Trade-offs among anti-herbivore resistance traits: insights from Gossypieae (Malvaceae). Am J Bot 91:871–880
Stephenson AG (1982) The role of the extrafloral nectaries of Catalpa speciosa in limiting herbivory and increasing fruit production. Ecology 63:663–669
Suzuki N, Ogura K, Katayama N (2004) The efficiency of herbivore exclusion by ants on the vetch Vicia angustifolia L. (Leguminosae), mediated by ant attraction to aphids. Ecol Res 19:275–282
Vickery ML, Vickery B (1981) Secondary plant metabolism. Macmillan, London
Way MJ (1963) Mutualism between ants and honeydew-producing Homoptera. Annu Rev Entomol 8:307–344
Acknowledgments
We thank H. Kagata, T. Kawagoe, and A. Yamawo for advice on this research, and Y. Hayakawa and M. Hironaka for providing the materials for these experiments. We thank R. Yamaoka, M. K. Hojo, and T. Nishida for advice on the chemical analysis. We also thank E. Nakajima for correcting the English of the text. This study was partly supported by the Global COE program A06 of Kyoto University, and by a JSPS Research Fellowship for Young Scientists to N. Katayama.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katayama, N., Suzuki, N. Anti-herbivory defense of two Vicia species with and without extrafloral nectaries. Plant Ecol 212, 743–752 (2011). https://doi.org/10.1007/s11258-010-9862-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-010-9862-2