Abstract
In addition to direct defenses, some plant species provide extrafloral nectar (EF-nectar) and/or food bodies (lipid-rich particles) to attract ants for their own indirect defenses. To ascertain why such plants use indirect defenses, we investigated the respective costs of direct and indirect defenses of Mallotus japonicus seedlings grown with and without ants present. Mallotus japonicus plants growing with ants present (ant-present) secreted larger volumes of EF-nectar, containing greater amounts of sugars, as an indirect defense trait. These plants also showed chemical defensive traits, such as the number of pellucid dots and the amount of accumulated phenolics, to a lesser degree than plants without ants (ant-absent) did. Moreover, the ant-present plants grew faster. The estimated amounts of EF-nectar sugars and food bodies were small compared to the amount of phenolics. Plant biomass was correlated negatively with pellucid dot density and phenolic concentration. Plant height was correlated negatively with phenolic concentration. Moreover, leaf biomass was correlated negatively with trichome density. Taken together, these results suggest a tradeoff between the expression of direct defense traits and plant growth. Mallotus japonicus achieves more rapid growth with ants present. We propose that this occurs because these ants provide low-cost indirect defenses allowing plants to re-allocate their energy from direct defenses to growth instead. This mutual benefit apparently facilitates ant–plant defensive mutualism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mutualism, interspecific interaction that enhances the respective fitnesses of the involved organisms, is often based on an exchange of rewards and services (Doebeli and Knowlton 1998; Leigh 2010). Defensive mutualism is one such mutualistic relation (Bronstein and Barbosa 2002; Leigh 2010). Under defensive mutualism, an organism provides nutrition or shelter to its partner. In return, it receives defense against its natural enemies (Koptur 1992; Stadler and Dixon 2008). For example, in addition to direct defenses, plants might make use of indirect defenses that attract bodyguards, such as ants, by providing alternative food via secreted extrafloral nectar (EF-nectar) and via specialized food bodies (FBs). The ants then remove herbivores from the plants (Koptur 1992). To ascertain why those organisms use indirect defenses, assessing the respective costs and benefits of direct and indirect defenses is useful. Most earlier studies have particularly examined the costs and benefits of particular indirect defenses (O’Dowd 1979; Barton 1986; Heil et al. 1997; Agrawal 2011).
Generally speaking, the expression of direct defense measures such as trichomes, leaf toughness, and chemical substances is costlier (e.g. Vickery and Vickery 1981; Redman et al. 2001; Strauss et al. 2002; Feng et al. 2009) than expression of indirect defenses (O’Dowd 1979; Heil et al. 1997; Rutter and Rausher 2004; Katayama and Suzuki 2011). Therefore, it is possible that plants which have efficient indirect defenses stop investing plant resources in direct defenses and instead redirect resources to growth and reproduction (Redman et al. 2001; Strauss et al. 2002; Feng et al. 2009). In doing so, plants can achieve higher rates of growth and reproduction (Katayama and Suzuki 2011).
The relative costs and benefits of various defense traits must be affected by biotic conditions such as the species composition of herbivores and mutualistic partners. For example, EFN-bearing plants invest more in physical and chemical defenses in areas with lower ant density because of the low effectiveness of indirect defenses (Koptur 1985; Rios et al. 2008). In contrast, in regions with higher densities of mutualistic ants, plants invest more in indirect defenses (Rudgers and Strauss 2004; Rios et al. 2008).
Biotic conditions are often heterogeneous in space and time. Plants change their intensities of anti-herbivore defenses depending on biotic factors such as the degrees of herbivory and ant activity (Risch and Rickson 1981; Heil et al. 1997, 2000, 2009; Dyer et al. 2001; Agrawal 2011; Bixenmann et al. 2011; Heil 2013). Most myrmecophytes reduce their production of FBs and EF-nectar when they are not well defended by their attendant ants (Heil et al. 2000, 2009; Heil 2013). In the attendance of mutualistic ants, some EFN-bearing plants increase their production of EF-nectar (Heil et al. 2000, 2009; Bixenmann et al. 2011) or FBs (Risch and Rickson 1981; Heil et al. 1997) and decrease their production of chemical defense substances (Dyer et al. 2001). Under such conditions, plants tend to prepare for indirect defense by ants rather than direct defense, probably because the former is more effective than the latter.
This paper presents and evaluates the hypothesis that defensive mutualism with ants in EFN-bearing plants favors growth. A shift from direct to indirect defense in the presence of mutualistic ants must make more energy available to the plant if the costs of indirect defense are lower than the costs of direct defense. That energy resource is expected to be allocated to growth. To test this hypothesis, we examined Mallotus japonicus (Euphorbiaceae), a pioneer plant that grows in gaps and disturbed areas in temperate regions of eastern Asia. Mallotus japonicus has physical, chemical, and biotic defenses against herbivores (Yamawo et al. 2012a, b). Trichomes, which are produced on leaf surfaces, function as a physical defense for the plants (Yamawo et al. 2012b). Pellucid dots, which are also present on leaf surfaces, typically contain toxic metabolic substances or essential oils (Wittstock and Gershenzon 2002). The plant bears EFNs on its leaf edges and FBs on its leaf and stem surfaces as biotic defenses (Yamawo et al. 2012a). The EFNs and FBs attract ants, which subsequently remove herbivores from the plant (Yamawo et al. 2012b).
Materials and Methods
Effects of Ant Presence on Defense Trait Expression and Plant Growth
Fifty seeds of M. japonicus were collected during May 2011 from five trees (10 from each) growing on Amami Island (28°37′N, 129°49′E). On November 15, 2012, a plastic container (45 × 35 × 15 cm) was filled to 10 cm depth with wet soil. The collected seeds were sown at 1 cm depth. This container was kept in a growth chamber (Biotron; NK System, Osaka, Japan) for 24 h at 35 °C under a 12L–12D photoperiod because M. japonicus seeds germinate after experiencing high temperatures (Washitani and Takenaka 1987). Then the container was kept at 25 °C under the same photoperiod for 30 days. It was watered on alternate days.
On December 15, 2012, 30 randomly selected shoots were transplanted into plastic pots (20 × 20 × 25 cm) containing 70 % tuff loam and 30 % humus. The pots, placed in the same conditioned chambers, were cultivated for 20 days. Water was applied on alternate days. No pest insect was allowed to invade the chambers.
Thirty pots were then assigned evenly to ant-present and ant-absent (control) conditions (n = 15 each) on January 4, 2013. At the start of experiment, each shoot had cotyledons and the first leaf. To assess the possibility of grouping bias of the plants, we examined the height and defense traits on the first leaf of each plant, such as quantities of EFNs and FBs, volume of EF-nectar, and the densities of trichomes and pellucid dots, on the first day of grouping. The plant height was measured using a ruler to accuracy of 10−1 cm. All FBs on the plant were removed using a fine brush. To measure the nectar volume secreted from EFNs, all leaves of cultivated plants were washed with distilled water to remove the accumulated EF-nectar. The leaf surfaces were wiped softly with towels (Kim-towels; Jujo Kimberly, Tokyo, Japan). After 24 h, the FBs produced during one day were counted on each plant. Then the nectar secreted within 24 h was collected from all EFNs using a 0.5-µl microcapillary tube (Drummond Scientific Co., Broomall, Pennsylvania, USA). The nectar concentration (amount of sugars) for each plant was measured immediately after collection using a portable, temperature-compensated refractometer (ATAGO hand refractometer; L. Kubler, Karlsruhe, Germany). The EFNs on the first leaf were counted. The densities of trichomes and pellucid dots were examined as follows. A small center area of the leaf undersurface was selected and photographed using a digital camera (Cyber-shot T10; Sony Corp., Tokyo, Japan). Then trichomes and pellucid dots in the 0.79-cm2 photographed area were counted. The respective densities (cm−2) of these materials were calculated for each leaf.
As the experimental ant species in the ant-present condition, we used Pheidole noda Smith (Hymenoptera: Formicidae) workers, which frequently visit M. japonicus (Yamawo et al. 2012b). Fifteen colonies of P. noda were collected from Mt. Kinryu (33°33′N, 130°31′E, alt. 40–250 m), Kanzaki, Saga, during June and August of 2012. After collection, every colony was adjusted to include 1000 workers, one queen, and 50 broods. Then each colony of P. noda was confined in a glass test tube (1.5 × 15 cm long), which was used as an artificial ant nest. To arrange an ant entrance, a vinyl chloride tube (6 × 10 cm long) was connected to the opening of each tube using a cotton mass. Each artificial ant nest was placed in a plastic container (35 × 15 × 5 cm), the inner surfaces of which had been covered with talc (Wako Pure Chemical Industries Ltd., Osaka, Japan) to prevent ants’ escape from the container.
On January 5, 2013, ant-present plants were prepared as follows. First, to prevent the ant path from reaching the ground, Tanglefoot (The Tanglefoot Co., Grand Rapids, Mich.) was applied to the lower stem of each plant at approximately 1 cm above the ground surface. Next, one end of a 20-cm cotton string was tied to the upper stem of the plant, approximately 1 cm above the Tanglefoot. The other end was tied to the vinyl chloride tube of an artificial ant nest, allowing the ants to visit the plant. These ants were provided no bait during the experiment. For each control plant, Tanglefoot was applied similarly. Then a 3 cm string was tied on the upper stem with no connection. Both ant-present and ant-absent plants were kept for 30 days at 25 °C under a 12L–12D photoperiod. They were watered on alternate days. During cultivation, the ants on each ant-present plant were counted five times at one-week intervals from the second day of setting, between 9:00 and 12:00.
After cultivation, on February 4, 2013, the string tied to each plant was loosened. All ants were removed from the plant. Then the leaves were counted. To ascertain the expression of defense traits on the plant, the fully expanded fifth leaf of each plant, each with more than six leaves, was selected because defense traits were best expressed on the fifth leaf (Yamawo et al. 2012b). All FBs on the plant were removed using a fine brush. All leaves were washed with distilled water and were wiped softly with Kim-towels. The productivity of EF-nectar and FBs in M. japonicus was examined for these ant-free plants. After 24 h, the FBs produced during one day on the fifth leaf were collected. The EF-nectar secreted within 24 h was collected from EFNs after counting. The total amount of sugars in EF-nectar was then measured. The collected FBs were counted and dried at 80 °C for 3 days. The dried FBs were weighed as a mass to a precision of 10−3 mg using an electronic balance (MC5; Sartorius, Goettingen, Germany).
Thereafter, all plants were collected and sandwiched for 14 days in moisture-absorbing paper. They were then divided into leaves and other parts. These two parts of each plant were weighed separately to the nearest 10−4 g using an electronic balance (BP211D; Sartorius, Goettingen, Germany). The stem length of each plant was measured using a ruler to an accuracy of 10−1 cm. It was recorded as the plant height. The densities of trichomes and pellucid dots on the fifth leaf were examined as follows. Two small areas near both sides of the midrib on the leaf under surface were selected. The areas were located approximately on the one-fifth line of the leaf length. The trichomes and pellucid dots in each of the 23.7 mm2 selected areas were counted under a microscope (40×). Then the densities (cm−2) of these traits were calculated.
Finally, the foliar phenolic contents of the plant were measured because foliar phenolics function as a defense against many herbivorous arthropods (Feeny 1970; Dudt and Shure 1994). The dried fifth leaf of each plant was powdered using a mill. Total phenolics in a 20-mg leaf powder sample were extracted using 50 % methanol (10 ml) for 1 h in a 40 °C ultrasonic bath. Then the phenolic concentration (mg/g) was measured using Folin–Ciocalteu method (Julkunen-Tiitto 1985).
Statistical Analyses
The plant heights, amounts of FBs, volumes of EF-nectar, pellucid dot densities, concentrations of nectar and phenols, and dry weights of leaves and whole plants were compared between the groups using a generalized linear model (GLM) with a Gaussian distribution and an identity link. The numbers of leaves, quantities of EFNs, and quantities of FBs were compared using a GLM with a Poisson distribution after log-link transformation. The ant condition, either presence or absence, was used as a fixed factor. The false discovery rate control was applied for multiple tests following GLM. The relations between growth characteristics and several leaf characteristics (i.e., trichome density, pellucid dot density, phenolic concentration, amount of sugars, and amount of FBs) were examined using a multivariate GLM with a Gaussian distribution and identity link after confirming correlation among all leaf characteristics (Table S1). Numbers of ants at five counts were analyzed using GLM with a Gaussian distribution. Thereafter, multiple comparisons were performed using Tukey–HSD tests. Statistical analyses were conducted using software (R ver. 2.15.1; R Development Core Team 2012).
Results
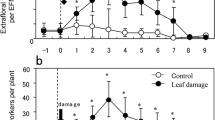
No grouping bias was detected between ant-present and ant-absent (control) plants (n = 15 each) for any leaf characteristic examined at the start of the experiment: number of EFNs, χ 2 = 0.34, P = 0.73; number of FBs, χ 2 = 0.51, P = 0.73; volume of EF-nectar, F = 0.67, P = 0.73; amount of sugars, F = 0.32, P = 0.73; trichome density, F = 0.56, P = 0.73; and pellucid dot density, F = 2.22, P = 0.73. Plant heights were not different between the groups (control: 3.1 ± 0.2 cm (mean ± SD); ant-present: 3.1 ± 0.4 cm, F = 0.12, P = 0.73).
During 30-day cultivation, no ants were observed on control plants. The plants under the ant-present condition were commonly visited by ant workers. The mean ± SD numbers of ant workers observed at the five counts were 0.4 ± 0.5, 1.3 ± 0.8, 2.7 ± 2.1, 4.3 ± 2.5, and 12.4 ± 11.9 (n = 15 each), which underscored the increasing tendency. The ants of the last count were significantly more numerous than those of other counts (Tukey–HSD, P < 0.001).
After 30-day cultivation, the plant height and biomass of ant-present plants were significantly higher and greater, respectively, than those of control plants (P < 0.01 for both; Table 1). The numbers of leaves were not different between the groups. The trichome density did not differ significantly between control and ant-present plants. However, other direct traits such as the pellucid dot density and phenolic concentration in the leaves of control plants were significantly higher than those of ant-present plants (Table 1). By contrast, the volumes of EF-nectar and amounts of sugars in ant-present plants were significantly larger than those in control plants (P < 0.05). The numbers, or amounts, of FBs on ant-present plants were approximately three times greater than those on control plants, although not significantly greater (Table 1).
The trichome density was correlated significantly and negatively with leaf biomass (P < 0.05; Table 2). The phenolic concentration was significantly and negatively correlated with plant height, leaf biomass, and whole biomass (P < 0.05 for all). The pellucid dot density was correlated negatively with the leaf biomass and with the whole-plant biomass (P < 0.05 for both). Amounts of sugars were positively correlated with the leaf biomass (P < 0.05), but no significant correlation was found between the FB amount and growth characteristics (Table 2).
Discussion
Ant presence in the EFN-bearing plant M. japonicus promotes increased expression of indirect defense traits and decreased expression of direct ones. These indirect defenses are probably less costly than direct defenses, leaving resources that the plants can redirect to growth. These results support our hypothesis that defensive mutualism with ants in EFN-bearing plants favors growth: a readily apparent benefit of indirect defense by ants.
Growth characteristics of M. japonicus were correlated negatively with the expression of direct defense traits such as the densities of both trichomes and pellucid dots and leaf phenolic concentration (Table 2). Such a relation is often called a tradeoff, suggesting that the expression of direct defense traits is costly. Many earlier works have demonstrated that the expression of trichomes and chemical substances impedes plant growth and reproduction (e.g. Redman et al. 2001; Strauss et al. 2002), although the expression of indirect defense traits such as EFNs and EF-nectar has little effect (Rutter and Rausher 2004). Rutter and Rausher (2004) reported that EF-nectar production in Chamaecrista fasciculata did not reduce plant growth or seed production. In addition, ant attendance for C. fasciculata showed no influence on plant growth (Barton 1986). These results suggest that the costs for displaying indirect defense traits are lower than those for displaying direct defense traits.
In Thea (=Camellia) sinensis, the cost of secondary-compound production accounted for 30 % of leaf dry-weight (Vickery and Vickery 1981). In myrmecophytic Macaranga tanarius (Euphorbiaceae), the phenolic content was about 20 % of the leaf biomass (Lim et al. 2009). In contrast, the cost of EFN-production was as low as approximately 1 % of the total energy invested in leaves in Ochroma pyramidale (O’Dowd 1979). In M. triloba, the production cost of FBs was about 5 % of the above-ground biomass production in unbranched saplings (Heil et al. 1997). In M. japonicus, the production costs of indirect defense traits proved to be lower than those of direct defense traits (Table 1). Our results are therefore consistent with the results of several earlier studies.
Differences in costs between those of indirect defenses and those of direct, or physical/chemical, defenses must reflect the differences in materials used to produce the respective defense traits. Actually, trichomes consist fundamentally of cellulose (Betancur et al. 2010). Pellucid dots typically contain toxic metabolic substances or essential oils (Wittstock and Gershenzon 2002; Schilmiller et al. 2008; Sirikantaramas et al. 2008). Although the pellucid dot contents in M. japonicus have not been identified, the pellucid dots of an allied species, M. tanarius, secrete the prenylated flavanone nymphaeol-C (Guhling et al. 2005). Food bodies (FBs) of facultative Macaranga species contain higher amounts of carbohydrates in the form of common soluble sugars compared to lipids and proteins (Heil et al. 1998). The FBs of M. japonicus probably include these substances. EF-nectar contains primary sugars (Heil et al. 2001; Katayama and Suzuki 2011). Synthesis costs of cellulose and the prenylated flavanone nymphaeol-C are regarded as higher than those of carbohydrates or primary sugars (Scott 2008).
Our results demonstrate that ant-present M. japonicus plants express more indirect defense traits and fewer direct defense traits than ant-absent plants do. The phenolic concentration in ant-present plants is approximately 15 mg/g lower than that of ant-absent plants, representing a substantial difference. The expressions of indirect defense traits such as EF-nectar and FBs are higher in ant-present plants, but the costs of indirect defenses are evidently low compared with the costs of direct defense (Table 1). Therefore, ant-present plants invest less in defenses than ant-absent plants do.
Mallotus japonicus plants in ant-present conditions are regarded as allocating the saved energy to growth. Ant-attendant M. japonicus shoots grew higher. Their biomass became greater than that of the ant-absent shoots. In other words, M. japonicus can achieve rapid growth by entrusting their defense mainly to indirect defense by ants. Millán-Cañongo et al. (2014) reported that plant growth and EF-nectar secretion depend on sugar flux in the phloem. Ant attendance is apparently beneficial to M. japonicus. In several EFN-bearing plant species, ant attendance promotes the induction of EF-nectar (Heil et al. 2000, 2009; Bixenmann et al. 2011) or the reduction of the chemical defense level (Dyer et al. 2001). Such a phenomenon by which resources are conserved in ant-attendant conditions through the principal use of indirect defense might be common in EFN-bearing plants. Recently, several reports have described that some plants can receive nutrient benefits from ants (e.g. Wagner and Nicklen 2010; Chanam et al. 2014). However, our experimental design eliminated that possibility because the ants were given no bait and were isolated completely from outside influences during the experiment.
How do plants sense the presence of ants? Reportedly, in M. tanarius (Heil et al. 2000) and in Acacia spp. (Heil et al. 2009; Heil 2013), the plants can alter EF-nectar volume in response to nectar removal. Mallotus japonicus plants might detect the presence of ants through the removal of EF-nectar. In addition, plants can recognize the visitation of ants directly through tactile and/or chemical stimuli (Braam 2005; Hilker and Meiners 2010). Ants have adhesive pads on their legs and leave chemical footprints on a substrate (Hölldoble and Palmer 1989; Federle et al. 2002). It is probable that ant-derived chemicals inform a plant about the presence of ants. Further studies must be conducted to reveal plants’ ant-recognition mechanisms.
In obligate ant–plant mutualism, plants bear a few specific ant species. EF-nectar secretion is induced only by mutualistic ant species (Heil et al. 2009; Heil 2013). In facultative ant–plant mutualism, not a few ant species are attracted by EF-nectar, exhibiting a loose relation. Young M. japonicus plants in the field are visited by many ant species (Yamawo et al. 2012b, 2014), among which the workers of P. noda are most frequently observed as being effective at excluding herbivores (A.Y. unpublished). Some ant species seem to be less effective. The difference of ant species on the plants might affect the switching of defense tactics of M. japonicus. However, this effect is not probable because our results were obtained under herbivore-free conditions and because the ant–M. japonicus system is loose. If herbivores are present, then differences of ant species, expecially in terms of whether they are effective for herbivore exclusion or not, might affect the results. This point remains to be investigated in future studies.
The modification of defense tactics in M. japonicus must be promoted by plant hormones such as jasmonic acid (JA). The induction of EF-nectar production often depends on JA (Heil et al. 2001, 2004), which is increased by herbivory (Heil et al. 2001), tactile stimuli (Chehab et al. 2012), and chemical stimuli (review in Heil and Karban 2009). Therefore, ant tactile stimuli might increase the amount of JA in M. japonicus. Although a large amount of JA generally inhibits plant growth (review in Wasternack 2007; Radhika et al. 2010; Izaguirre et al. 2013), several studies have demonstrated that a small amount of JA facilitates growth (Martín-Closas et al. 2003; Toro et al. 2003). For instance, Toro et al. (2003) reported positive effects of jasmonates on cabbage growth (Brassica oleracea L. var Capitata L.). It is probable that tactile and/or chemical stimuli by ants facilitate the growth of M. japonicus plants through the action of JA.
Mutualism has evolved, facilitated by the competitive processes of natural selection (Darwin 1862; Palmer et al. 2008; Degnan et al. 2009). Actually, EFNs and FBs are more common in pioneer plant species, but their expression of these indirect defense traits is reportedly influenced by light availability (Bentley 1977; O’Dowd 1982; Schupp and Feener 1991; Koptur 1992). Pioneer plants such as M. japonicus, which are under severe light competition and herbivory pressures, are expected to benefit most from rapid growth, which can be achieved by investing less in defense traits because the large investment in direct defense traits reduces plant growth. Therefore, plants must balance rapid growth against effective defense in a tradeoff relation. The adoption of indirect defenses by ants contributes not only to the reduction of herbivory but also to the increase of resource allocation to growth. This dual benefit can be regarded as facilitating ant–plant defensive mutualism.
References
Agrawal, A. A. (2011). Current trends in the evolutionary ecology of plant defence. Functional Ecology, 25(2), 420–432.
Barton, A. M. (1986). Spatial variation in the effect of ants on extrafloral nectary plant. Ecology, 67(2), 495–504.
Bentley, B. L. (1977). Extrafloral nectaries and protection by pugnacious bodyguards. Annual Reviews Ecology, Evolution and Systematics, 8, 407–427.
Betancur, L., Singh, B., Rapp, R. A., Wendel, J. F., Marks, M. D., Roberts, A. W., & Haigler, C. H. (2010). Phylogenetically distinct cellulose synthase genes support secondary wall thickening in Arabidopsis shoot trichomes and cotton fiber. Journal of Integrative Plant Biology, 52(2), 205–220.
Bixenmann, R. J., Coley, P. D., & Kursar, T. A. (2011). Is extrafloral nectar production induced by herbivores or ants in a tropical facultative ant–plant mutualism? Oecologia, 165(2), 417–425.
Braam, J. (2005). In touch: plant responses to mechanical stimuli. New Phytologist, 165(2), 373–389.
Bronstein, J. L., & Barbosa, P. (2002). Multitrophic/multispecies mutualistic interactions: the role of non-mutualists in shaping and mediating mutualisms. In T. Tscharntke & B. A. Hawkins (Eds.), Multitrophic level interactions (pp. 44–66). New York: Cambridge University Press.
Chanam, J., Sheshshayee, M. S., Kasinathan, S., Jagdeesh, A., Joshi, K. A., & Borges, R. M. (2014). Nutritional benefits from domatia inhabitants in an ant–plant interaction: interlopers do pay the rent. Functional Ecology, 28(5), 1107–1116.
Chehab, E. W., Yao, C., Henderson, Z., Kim, S., & Braam, J. (2012). Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Current Biology, 22(8), 701–706.
Darwin, C. (1862). On the various contrivances by which British and foreign orchids are fertilised by insects, and on the good effects of intercrossing. London: John Murray.
Degnan, P. H., Yu, Y., Sisneros, N., Wing, R. A., & Moran, N. A. (2009). Hamiltonella defensa, genome evolution of protective bacterial endosymbiont from pathogenic ancestors. Proceedings of the National Academy of Sciences of the United States of America, 106(22), 9063–9068.
Development, R. (2012). R: a language and environment for statistical computing. Vienna: Austria.
Doebeli, M., & Knowlton, N. (1998). The evolution of interspecific mutualisms. Proceedings of the National Academy of Sciences of the United States of America, 95(15), 8676–8680.
Dudt, J. F., & Shure, D. J. (1994). The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology, 75(1), 86–98.
Dyer, L. A., Dodson, C. D., Beihoffer, J., & Letourneau, D. K. (2001). Trade-offs in antiherbivore defenses in Piper cenocladum: ant mutualists versus plant secondary metabolites. Journal of Chemical Ecology, 27(3), 581–592.
Federle, W., Riehle, M., Curtis, A. S., & Full, R. J. (2002). An integrative study of insect adhesion: mechanics and wet adhesion of pretarsal pads in ants. Integrative and Comparative Biology, 42(6), 1100–1106.
Feeny, P. (1970). Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology, 51(4), 565–581.
Feng, Y. L., Lei, Y. B., Wang, R. F., Callaway, R. M., Valiente-Banuet, A., Inderjit, Li, Y. P., et al. (2009). Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 1853–1856.
Guhling, O., Kinzler, C., Dreyer, M., Bringmann, G., & Jetter, R. (2005). Surface composition of myrmecophilic plants: cuticular wax and glandular trichomes on leaves of Macaranga tanarius. Journal of Chemical Ecology, 31(10), 2323–2341.
Heil, M. (2013). Let the best one stay: screening of ant defenders by Acacia host plants functions independently of partner choice or host sanctions. Journal of Ecology, 101(3), 684–688.
Heil, M., Fiala, B., Baumann, B., & Linsenmair, K. E. (2000). Temporal, spatial and biotic variations in extrafloral nectar secretion by Macaranga tanarius. Functional Ecology, 14(6), 749–757.
Heil, M., Fiala, B., Kaiser, W., & Linsenmair, K. E. (1998). Chemical contents of Macaranga food bodies adaptations to their role in ant attraction and nutrition. Functional Ecology, 12(1), 117–122.
Heil, M., Fiala, B., Linsenmair, K. E., Zotz, G., Menke, P., & Maschwitz, U. (1997). Food body production in Macaranga triloba (Euphorbiaceae): a plant investment in anti-herbivore defence via symbiotic ant partners. Journal of Ecology, 85(6), 847–861.
Heil, M., González-Teuber, M., Clement, L. W., Kautz, S., Verhaagh, M., & Bueno, J. C. S. (2009). Divergent investment strategies of Acacia myrmecophytes and the coexistence of mutualists and exploiters. Proceedings of the National Academy of Sciences of the United States of America, 106(43), 18091–18096.
Heil, M., Greiner, S., Meimberg, H., Krüger, R., Noyer, J. L., Heubl, G., et al. (2004). Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature, 430(6996), 205–208.
Heil, M., & Karban, R. (2009). Explaining evolution of plant communication by airborne signals. Trends in Ecology & Evolution, 25(3), 134–144.
Heil, M., Koch, T., Hilpert, A., Fiala, B., Boland, W., & Linsenmair, K. E. (2001). Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect defensive response elicited by jasmonic acid. Proceedings of the National Academy of Sciences of the United States of America, 98(3), 1083–1088.
Hilker, M., & Meiners, T. (2010). How do plants “notice” attack by herbivorous arthropods? Biological Reviews, 85, 267–280.
Hölldoble, B., & Palmer, J. M. (1989). Footprint glands in Amblyopone australis (Formicidae, Ponerinae). Psyche, 96(1–2), 111–122.
Izaguirre, M. M., Mazza, C. A., Astigueta, M. S., Ciarla, A. M., & Ballaré, C. L. (2013). No time for candy: passionfruit (Passiflora edulis) plants down-regulate damage-induced extra floral nectar production in response to light signals of competition. Oecologia, 173(1), 213–221.
Julkunen-Tiitto, R. (1985). Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. Journal of Agricultural and Food Chemistry, 33(2), 213–217.
Katayama, N., & Suzuki, N. (2011). Anti-herbivory defense of two Vicia species with and without extrafloral nectaries. Plant Ecology, 212(5), 743–752.
Koptur, S. (1985). Alternative defenses against herbivores in Inga (Fabaceae: Mimosoideae) over an elevational gradient. Ecology, 66(5), 1639–1650.
Koptur, S. (1992). Extrafloral nectary-mediated interactions between insects and plants. In E. A. Bernays (Ed.), Insect–plant interactions (Vol. 4, pp. 81–129). Boca Raton: CRC Press.
Leigh, E. G, Jr. (2010). The evolution of mutualism. Journal of Evolutionary Biology, 23(12), 2507–2528.
Lim, T. Y., Lim, Y. Y., & Yule, C. M. (2009). Evaluation of antioxidant, antibacterial and anti-tyrosinase activities of four Macaranga species. Food Chemistry, 114(2), 594–599.
Martín-Closas, L., Toro, F. J., Calvó, G., & Pelacho, A. M. (2003). Effect of methyl jasmonate on the first developmental stages of globe artichoke. Acta Horticulturae, 660, 185–190.
Millán-Cañongo, C., Orona-Tamayo, D., & Heil, M. (2014). Phloem sugar flux and jasmonic acid-responsive cell wall invertase control extrafloral nectar secretion in Ricinus communis. Journal of Chemical Ecology, 40(7), 760–769.
O’Dowd, D. J. (1979). Foliar nectar production and ant activity on a neotropical tree, Ochroma pyramidale. Oecologia, 43(2), 233–248.
O’Dowd, D. J. (1982). Pearl bodies as ant food: an ecological role for some leaf emergences of tropical plants. Biotropica, 14(1), 40–49.
Palmer, T. M., Stanton, M. L., Young, P. T., Goheen, J. R., Pringle, R. M., & Karban, R. (2008). Breakdown of an ant-plant mutualism follows the loss of large herbivores from an African savanna. Science, 319(5860), 192–195.
Radhika, V., Kost, C., Mithöfer, A., & Boland, W. (2010). Regulation of extrafloral nectar secretion by jasmonates in lima bean is light dependent. Proceedings of the National Academy of Sciences of the United States of America, 107(40), 17228–17233.
Redman, A. M., Cipollini, D. F, Jr, & Schultz, J. C. (2001). Fitness costs of jasmonic acid-induced defense in tomato, Lycopersicon esculentum. Oecologia, 126(3), 380–385.
Rios, R. S., Marquis, R. J., & Flunker, J. C. (2008). Population variation in plant traits associated with ant attraction and herbivory in Chamaecrista fasciculata (Fabaceae). Oecologia, 156(3), 577–588.
Risch, S. J., & Rickson, F. (1981). Mutualism in which ants must be present before plants produce food bodies. Nature, 291(14), 149–150.
Rudgers, J. A., & Strauss, S. Y. (2004). A selection mosaic in the facultative mutualism between ants and wild cotton. Proceedings of the Royal Society B, 271(1556), 2481–2488.
Rutter, M. T., & Rausher, M. D. (2004). Natural selection on extrafloral nectar production in Chamaecrista fasciculata: the costs and benefits of a mutualism trait. Evolution, 58(12), 2657–2668.
Schilmiller, A. L., Last, R. L., & Pichersky, E. (2008). Harnessing plant trichome biochemistry for the production of useful compounds. Plant Journal, 54(4), 702–711.
Schupp, E. W., & Feener, D. H. (1991). Phylogeny, lifeform, and habitat dependence of ant-defended plants in a Panamanian forest. In C. R. Huxley & D. F. Cutler (Eds.), Ant–plant interactions (pp. 175–197). Oxford: Oxford Univ. Press.
Scott, P. (2008). Physiology and behaviour of plants. Chichester: Wiley.
Sirikantaramas, S., Yamazaki, M., & Saito, K. (2008). Mechanisms of resistance to self-produced toxic secondary metabolites in plants. Phytochemistry Reviews, 7(3), 467–477.
Stadler, B., & Dixon, T. (2008). Mutualism: ants and their insect partners. Cambridge: Cambridge University Press.
Strauss, S. Y., Rudgers, J. A., Lau, J. A., & Irwin, R. E. (2002). Direct and ecological costs of resistance to herbivory. Trends in Ecology & Evolution, 17(6), 278–285.
Toro, F. J., Martín-Closas, L., & Pelacho, A. M. (2003). Jasmonates promote cabbage (Brassica oleracea L. var Capitata L.) root and shoot development. Developments in Plant and Soil Sciences, 101, 77–83.
Vickery, M. L., & Vickery, B. (1981). Secondary plant metabolism. London: Macmillan.
Wagner, D., & Nicklen, E. F. (2010). Ant nest location, soil nutrients and nutrient uptake by ant-associated plants: does extrafloral nectar attract ant nests and thereby enhance plant nutrition? Journal of Ecology, 98(3), 614–624.
Washitani, I., & Takenaka, A. (1987). Gap-detecting mechanism in the seed germination of Mallotus japonicus (Thunb.) Muell. Arg., a common pioneer tree of secondary succession in temperate Japan. Ecological Research, 2(3), 191–201.
Wasternack, C. (2007). Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany, 100(4), 681–697.
Wittstock, U., & Gershenzon, J. (2002). Constitutive plant toxins and their role in defense against herbivores and pathogens. Current Opinion in Plant Biology, 5(4), 300–307.
Yamawo, A., Katayama, N., Suzuki, N., & Hada, Y. (2012a). Plasticity in the expression of direct and indirect defence traits of young plants of Mallotus japonicus in relation to soil nutritional conditions. Plant Ecology, 213(1), 127–132.
Yamawo, A., Suzuki, N., Tagawa, J., & Hada, Y. (2012b). Leaf ageing promotes the shift in defence tactics in Mallotus japonicus from direct to indirect defence. Journal of Ecology, 100(3), 802–809.
Yamawo, A., Tagawa, J., Hada, Y., & Suzuki, N. (2014). Different combinations of multiple defence traits in an extrafloral nectary-bearing plant growing under various habitat conditions. Journal of Ecology, 102(1), 238–247.
Acknowledgments
This work was supported in part by Research Fellowships from the Japan Society for the Promotion of Science for Young Scientists (234305) and (251712).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamawo, A., Tokuda, M., Katayama, N. et al. Ant-Attendance in Extrafloral Nectar-Bearing Plants Promotes Growth and Decreases the Expression of Traits Related to Direct Defenses. Evol Biol 42, 191–198 (2015). https://doi.org/10.1007/s11692-015-9310-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-015-9310-2