Abstract
A survey of 93 wetlands in six catchments across the Maloti-Drakensberg is used to assess the distribution of plant functional types across altitudinal and wetness gradients. Altitudes range from 1,000 to 3,200 m a.s.l. Within each catchment, the wetlands were selected to cover the complete range in altitude and wetland types. In each of the selected wetlands, vegetation was sampled in 3 by 3 m quadrats covering the entire range of wetness represented in the wetland, from temporarily wet to permanently inundated soils. Plant species were allocated to one of 11 different functional types (examples are C3 grasses, C4 sedges, rosette plants, and shrubs), and the proportion of the vegetation in each sample occupied by each functional type was calculated from the species’ abundances. Canonical Correspondence Analysis shows that “wetness” clearly has the highest impact on the distribution of functional types, followed by altitude. The most important plant functional types in wetlands are grasses and sedges, however, at higher altitudes, forbs (especially rosette plants) and bulbous plants become a more prominent feature in the wetlands. The total amount of graminoids gradually decreases with altitude. The general trend is that sedges tend to increase with increasing wetness and C3 plants (grasses and sedges) increase with increasing altitude, but these effects are not independent. The distributions of C4 sedges and C4 grasses along an altitudinal gradient are quite different, and C4 grasses grow abundantly at much higher altitudes than C4 sedges. C4 sedges are very scarce at the altitudes represented in the Maloti-Drakensberg area, whereas C3 grasses occur in the permanently wet parts of the wetlands, especially at higher altitudes (normally mostly occupied by sedges). Shrubs are rare in wetlands and tend to be an indication of disturbance. This study complements previous studies on the distribution of grasses and sedges at the lower altitudes within KwaZulu-Natal, which found that at altitudes below 1,000 m a.s.l. C4 sedges were much more prominent, while forbs and rosette plants were largely absent. This confirms that C4 as an adaptation to hotter and warmer climates is sometimes a less favorable metabolism in wet high altitude areas. At high altitudes, rosette plants and bulbous plants become more competitive in wetlands, probably because grasses and sedges present at these altitudes generally grow smaller than they do in low altitude wetlands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The integrated conservation of large-scale ecosystems requires an understanding of the determinants of the structure and functioning of such systems (Ostfeld et al. 1997). Since the presence of many interacting species makes analyzing ecosystems potentially extremely complex, there is a need to group together species that perform a similar function in an ecosystem. Different classification schemes and terminologies exist for grouping plants into functional groups and there is no single commonly accepted classification useful for all studies (Smith et al. 1997). Plant functional types (PFTs) and guilds have been defined as “a group of species that use the same type of resources in the same way” (Smith and Huston 1989). Plant functional types have been defined on the basis of, inter alia, morphology, nutrient foraging strategies, location of perennating tissues, physiology, and life span (Boutin and Keddy 1993; Díaz and Marcelo 1997; Lavorel et al. 1997; Smith et al. 1997; Box and Fujiwara 2005). Since form and function have an evolutionary origin, many functional groups are closely linked to their phylogeny, and hence can be delineated taxonomically (Silvertown et al. 1997; Ingrouille and Eddie 2006).
Classifying plants into functional groups is often used as a means of explaining vegetation patterns resulting from the environmental factors that structure a community. An ecological gradient or a combination of ecological gradients can be described in the form of changes in overall species composition but many broad patterns elude us if we fail to look at the changes in the contribution of broad functional groups (Lavorel et al. 1997; Smith et al. 1997; Weiher and Keddy 1999). Functional groups have been applied particularly often in the context of correlating vegetation to climatic gradients (Díaz and Marcelo 1997; Smith et al. 1997; Box and Fujiwara 2005). It has been suggested that the term functional group should be used only in the specific situation that the response of species to a disturbance is taken into account (Smith et al. 1997; Lavorel et al. 1997). In this way, the term can be differentiated from several other terms that refer to different aspects of plant life cycles, environmental adaptations, and growth forms, such as “structural guilds”, “ecological species”, “character syndromes”, “functional guilds”, and many others, but at the same time, it is acknowledged that “functional type” is the most broadly used term, and that form and function are often linked in ecology (Smith et al. 1997; Box and Fujiwara 2005).
Wetland plants can be grouped into several functional types. Boutin and Keddy (1993) recognize three basic functional types, namely matrix species, interstitial species, and ruderals, based on a number of measured traits. Several traits, such as floating leaves and aerenchyma in the stem, are specific adaptations to wetlands (Cronk and Fennessy 2002; Keddy 2000), and many different lineages of vascular plants have acquired these traits and conquered this habitat. The most abundant families of wetland plants are the grasses (Poaceae) and sedges (Cyperaceae) together with some smaller monocot families (Kotze and O’Connor 2000; Thompson and Hamilton 1983). Many of these species are clonal, sprouting at the base, and they form the bulk of what Boutin and Keddy (1993) refer to as “matrix species”. Grasses and sedges differ in their basic morphology, with most sedges having closed leaf sheaths that are attached to the stem at the base level, giving them a basal “rosette” of narrow leaves and grasses having open leaf sheaths attached to nodes on the stem, so that the leaves are further apart. In general, sedges tend to occur in the wetter parts of the wetland whereas grasses tend to dominate toward the drier end of the spectrum.
Both grasses and sedges have evolved C4 metabolism in parallel multiple lineages (Sage 2004; Osborne and Beerling 2006; Christin et al. 2008). It is generally thought that a C4 metabolism is more adapted for hot, semi-arid to arid high-light environments (e.g., Li et al. 1999; Osborne 2008), yet many C4 plants occur in wetlands (Keeley 1998; Kotze and O’Connor 2000). A more nuanced view on the origins of the C4 metabolism is necessary as it may have evolved independently in response to different environmental conditions.
In this study, we investigate the contribution to the overall vegetation cover of various functional groups in wetlands in high altitude areas of South Africa. The Maloti-Drakensberg area in the central-eastern part of South Africa receives rainfall in the form of warm moist air from the Indian Ocean that cools down when rising up a high mountain escarpment and this has resulted in a large number of wetlands occurring at a range of altitudes (Sieben et al. in prep.). We investigated the distribution of plants with different morphologies and different metabolisms (C3 vs. C4 plants) along an altitudinal gradient (across the mountain range), along which many climate variables that directly affect plant growth vary, and along small-scale gradients in soil wetness within wetlands of the Maloti-Drakensberg range.
These distribution patterns will reveal the relevance of specific growth forms and types of metabolism as a strategy for adaptation to wetland habitats at different altitudes. This is particularly relevant in monitoring high-altitude wetland ecosystems in the face of wetland degradation and imminent climate change. It also will give additional insights into the evolutionary history of some of the strategies that are particularly successful in such habitats. The gradient analysis expands on the study by Kotze and O’Connor (2000) by extending the altitudinal range right up onto the Maloti-Drakensberg escarpment, and by including additional functional types (i.e., rosette plants, forbs, shrubs, and submerged aquatics), which tend to be more abundant at the higher altitudes (Van Zinderen Bakker and Werger 1974; Backeus and Grab 1995).
Methods
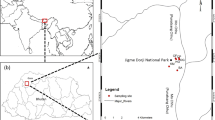
Vegetation relevés were located in 93 wetlands across all altitudinal zones in six catchments distributed at intervals along the Maloti-Drakensberg range (Fig. 1). Maps were consulted to examine where wetlands were located within these catchments and an attempt was made to visit all wetlands within a catchment. Within each wetland, several vegetation units could be recognized across the wetness gradient and for each of them at least one vegetation relevé was recorded using the Braun-Blanquet method (Westhoff and van der Maarel 1978). In most cases, wetland vegetation occurs in discrete units but, in some cases, a transitional zone between two units is present. A homogeneous relevé of 3 by 3 m was chosen to represent the vegetation unit as a whole. Several environmental variables were determined for each relevé. This has resulted in a database of 257 vegetation relevés.
All plant species were recorded with a Braun-Blanquet cover abundance value, which was later converted into a percentage value, and each species was allocated to one of 11 different functional groups, following the classification in Table 1. Sedges and grasses were subdivided into C3 and C4 types, following the literature (Gibbs Russell et al. 1991; Bruhl and Wilson 2007). Sedges and grasses have also been subdivided into tuft (short or no rhizomes) and mat (long rhizomes or stolons) forming species, but this subdivision has not been used in all analyses. Especially in sedges, it is not always clear how to allocate species to either a tuft or a mat growth form. The proportion of each plant functional group in each plot was calculated by subdividing the total cover of a certain functional group by the total cover of all plant species.
Wetlands have been grouped into different hydrogeomorphic types (Kotze et al. 2006; Ewart-Smith et al. 2006). In the Maloti-Drakensberg area, the following wetland types have been recognized: bedrock pools, pans (depressions), valley bottom wetlands, valley-head seepages, slope seepages, footslope seepages, and floodplains. A wetness index was allocated on the basis of the occurrence of hydromorphic characters of the soil. Mottling, gleying, and peat formation are all phenomena that occur when the soil is at least temporarily wet, and on the basis of these characters, a soil can be characterized as non-wetland, temporary, seasonal, or permanently wet, or a transition between those categories (Kotze et al. 1996). Clay content of the soil was estimated on a scale ranging from 1 to 8 using the field method of Ball (1986) and the organic matter contents was assigned to one of three classes on the basis of the blackness and feel of the soil: mineral non-humic, mineral humic, and organic (the last class consists of peaty soils, which have a fibrous to clayey feel in the hand, and the water expressed from some soil squeezed in hand is not murky, as in a clay soil).
For all localities, climate variables were included in the data analysis, so that the influence of climate on the vegetation composition could be assessed more directly than by using altitude as a surrogate for many of these (Körner 2006). The climate data are interpolated values at a resolution of 1’ by 1’ of a degree, derived from a network of recording stations using regression-type approaches (Schulze 2006; for method of interpolation, see Dent et al. 1987). A list and explanation of the climate and environmental variables that have been used in the analysis is given in Table 2. An important question is whether the grid cell size used to resolve climate data is sufficiently small to be able to correlate vegetation patterns with climate at the small sampling scale used in the study. If this is not the case, altitude itself may be the best surrogate to explain vegetation patterns in the wetlands of the Maloti-Drakensberg range. Some interaction variables, which are the product of two different variables, are considered and forward selection is used to find out which ones of these can be retained.
The wetlands in the Maloti-Drakensberg are subject to various types of disturbances, such as grazing and fire. Although no recently burnt sites were sampled, the wetlands in the present study may vary with respect to the grazing pressure that they are subject to.
The pattern of joint variation between altitude and all climate variables was initially investigated using a standardized principal component analysis (PCA; Hill 1973; Jongman et al. 1987) to reveal multicollinearity and redundancy in the data. Thereafter, the influence of environmental and climate variables on the functional composition of the wetlands was examined by means of canonical correspondence analysis (CCA; ter Braak 1986; ter Braak and Prentice 1988) using the program CANOCO 4.5 (ter Braak and Šmilauer 2002). A sub-set of variables that were most strongly related to the main pattern of variation in functional composition was identified using forward selection based on a Monte Carlo permutation test (n = 499) to select significant variables (P-value for conditional effect ≤0.05), with the initial order of variables for selection based on a ranking of the marginal contribution of each variable in the CCA.
Regression tree analysis (RTA) was used to identify variables that had the greatest influence on the relative abundance (%) of each functional group in the wetlands surveyed. Regression trees are binary decision trees for numerical response variables, showing at each dichotomy the explanatory variable which best divides the data into relatively homogeneous groups (Breiman et al. 1984; De’ath and Fabricius 2000). RTA is a non-parametric approach that does not seek to identify a single dominant structure—viz. a general model—in the data (as in CCA), but rather aims to reveal multiple structures in heterogeneous data by identifying the hierarchy of context dependent (i.e., interactive) effects of explanatory variables. Importantly, RTA, unlike generalized (additive or linear) modelling, is not subject to the statistical problems caused by multicollinearity arising from correlated explanatory variables, which was the case in the environmental and climate data set used in the present study. Regression trees were developed for each functional group, using the default settings in CART v. 6.0 (Salford Systems 2006), with all measured environmental and climate variables (Table 2) supplied as potential predictors of the relative abundance of that group.
Results
A vegetation classification for the wetlands in the Maloti-Drakensberg area can be found in Sieben et al. (in prep.). A PCA analysis was conducted to observe the collinearity between a large number of the climatic variables. For many climatic variables, the values for July (driest season) are correlated to the values in January (wettest season). The wettest areas in the Maloti-Drakensberg are located in the Mlambonja and Umkomazi transects, both located in KwaZulu-Natal. In this area, the change in altitude is the highest and warm air from the Indian Ocean results in a high incidence of orographic rainfall here. Both to the north (Klerkspruit river) and to the south, there is actually much less rainfall than in the central parts of the Maloti-Drakensberg. The incidence of frost at the lower altitudes is most common in the southern parts of the Maloti-Drakensberg and the Klerkspruit catchment. Altitude can be a good surrogate here for climatic indices, especially, since most climatic variables are only available at a very coarse scale. It is worth to consider, however, in what way climate is correlated to altitude, as there are more complicated patterns (for example rain shadows), especially, when considering a large stretch of a mountain range (Körner 2006).
Patterns in the occurrence of functional groups
Canonical correspondence analysis
The results of the CCA (Fig. 2) show the arrangement of the functional groups in multi-dimensional space projected onto two ordination axes. A large part of the climatic variables have extremely high inflation factors and after stepwise selection only mean annual precipitation (MAP), altitude and number of frost days have been retained. Among the interaction variables (products between two different variables), only the product between wetness and number of frost days has been retained. The sum of all canonical eigenvalues is 0.925, while Total Inertia is 5.982; therefore, 15.5% of all variation is explained by the variables provided. Altitude and wetness, which are the two most important variables (altitude is mostly correlated to the first axis, whereas wetness is mostly correlated to the second axis), together make up 58.4% of the explained variation.
Plot of centroids for plant functional types (black dots) and environmental variables (arrows) along the first two axes of a canonical correspondence analysis (CCA) of plant functional type proportional data from 257 sample plots of the wetlands of Maloti-Drakensberg, South Africa. Grasses and sedges have been split up into tuft-formers and mat-formers. See Table 2 for the explanation of environmental variables and Table 1 for details of functional types
It is conspicuous that several functional groups only become prominent at higher altitudes. The total cover of all graminoids (sedges, grasses, and other graminoids) slightly decreases with altitude whereas the total cover of forbs, especially rosette plants, increases with altitude. Also, the proportion of bulbous plants increases with altitude.
The functional types differ in their distribution along the wetness and altitudinal gradients (Fig. 3). When data from all habitats and altitudes are merged, the most prominent functional types are C4 grasses (26.4%), C3 sedges (22.1%), C3 grasses (15.6%), and forbs (9.4%). When wetland relevés are arranged along a gradient from temporary to permanent inundation, an increasing trend is observed for aquatics, bulbous plants, C3 grasses, C3 sedges, and graminoids, whereas a decreasing trend is observed for C4 grasses, forbs, and shrubs. C4 sedges are most common in seasonally wet habitats. With increasing altitude, several functional types increase (bulbs, C3 grasses, C3 sedges, graminoids, and rosette plants), while others decline (C4 sedges and C4 grasses).
Distribution of the functional types across wetness and altitude gradients in the study area. See Table 1 for details of functional types
In most cases, there is not a very clear distinction between tuft and mat forming graminoids, although in most cases, mat formers occur at lower altitudes and wetter conditions than tuft formers. The only case where there is a really clear distinction is among the C3 grasses. The group of “other graminoids” consist of only four species, and among these, Typha capensis is really distinct from the other three.
Distribution of C3 and C4 plants
The distribution of C3 and C4 metabolism is different among sedges and grasses. Figure 4a–e shows the environmental variables that explain the occurrence of the most relevant and distinct groupings (C3 grasses, C3 sedges, C4 grasses, C4 sedges, and rosette plants) in the form of regression trees. From these figures, it becomes clear to which environmental variable the difference in dominance of each functional type is most strongly linked.
Regression trees for the effect of climate and soil variables on the relative proportion (%) of the main functional types occurring in 257 sample plots of the wetlands of the Maloti-Drakensberg, South Africa: (a) C4 grasses, (b) C4 sedges, (c) C3 grasses, (d) C3 sedges, and (e) rosette plants. At each dichotomy the level of the variable that best splits the preceding node is presented (left branch for ≤level, right branch for >level) along with the mean (±SE) percentage of the functional type in each of the resultant non-terminal (broken rectangles) or terminal nodes (rectangles). See Table 2 for explanation of environmental variables used in the regression trees
C3 grasses have a mean cover of more than 25% in situations that have less than 179 Heat Units in July (the coldest month), whereas in “warmer” situations, their contribution to the total vegetation is much less (Fig. 4c). This implies a certain amount of time that surpasses a threshold temperature of 10°C (this is implied by the definition of Heat Units), and the variable that expresses this is strongly correlated to altitude.
For C3 sedges, wetness is the most important variable that determines their occurrence (Fig. 4d). In seasonal to permanent wetlands, the average contribution of C3 sedges is about 30%, whereas in temporary wetlands, they generally do not exceed 15% in cover.
In the case of C4 grasses, their occurrence in wetlands is more complicated. They achieve high cover values (40–60% on average) in cases where wetlands are temporary and the Heat Units exceed 179 in the coldest month, but they are also common in seasonal wetlands, where the Heat Units in the coldest month exceed 259 (Fig. 4a). In all other situations, their cover values are much less, so here, there is a clear complementary effect between altitude (which has an impact on the amount of heat units in the coldest month) and wetness.
C4 sedges occur mostly with high cover values in those areas that have a large number of Heat Units in the warmest month and that have dark or peaty soils (Fig. 4b). These cover values are still highly variable (between 15 and 45%), and have been found only in three relevés. Indeed, this functional type rarely reaches high cover values in the Maloti-Drakensberg and the only species that really can become dominant is Cyperus fastigiatus.
In a few other cases, strong relations have been found between environmental factors, and the dominance of certain functional groups: rosette plants clearly become more dominant at the higher altitudes (above 2,450 m a.s.l.) and even then they only attain cover values of about 10% (Fig. 4e).
Discussion
The current distribution of various functional types may be determined by adaptation to current ecological conditions or it may represent remnants or refugia of previous vegetation types. In some cases, it can be argued that dominance of a certain functional type is not created by adaptation of that type to a certain habitat, but by “competitive release” within that habitat, since the groups that are really well-adapted to a certain habitat have less vitality or a lower cover under certain circumstances. In other words, the “typical” wetland plants that are to be expected cannot cope well with the cold, therefore they are not as vigorous as usual, which leaves a lot of niches open in the wetland habitat at high altitudes. This may explain the occurrence of more forbs and especially rosette plants in high-altitude wetlands. Grasses and sedges in these high altitudes are generally very low in stature and this leaves a lot of niches open for forbs and rosette plants that are common in the surrounding dry alpine grasslands to evolve into wetland species. In some cases, species that are common at low altitudes, but only occur with low cover in wetlands, can become quite dominant at high altitudes, as it is easier to compete with other wetland plants here. An example is Ranunculus meyeri, which is common across all altitudes, but only achieves high cover values at the higher altitudes.
Kotze and O’Connor (2000), dealing with the lower altitudinal regions in the eastern regions of South Africa, adjacent to the areas dealt with in the present study, showed that with increasing altitude, the proportional contribution to the overall number of graminoid species per wetland decreased for C4 grasses and C4 sedges and increased for C3 grasses and C3 sedges. At all altitudes, the abundance of C4 grasses declined and C4 sedges increased from the temporary to the permanent zone. C3 grasses showed a less straightforward trend. At altitudes lower than 1,800 m a.s.l., there was no significant difference in the mean relative cover of C3 grasses between any of the wetness classes, but at the lowest altitudes, the mean relative cover of the permanently wet zone was significantly higher than the temporary zone, primarily as a result of Phragmites australis and Leersia hexandra, which are abundant in the permanently wet areas. At higher altitudes, C3 sedges were significantly more abundant in the permanently wet zone than in the temporarily wet zone, but at lower altitudes C3 sedges were generally of low abundance, and the declining abundance across the wetness gradient is less evident.
Grasses and sedges may be seen as the matrix species that create the niches for many of the other species in wetlands. At the lower altitudes, they are certainly the most dominant functional types in wetlands (Kotze and O’Connor 2000) and only at higher altitudes have other functional types a chance to achieve high cover. In the Maloti-Drakensberg area, C3 grasses and sedges generally occur at higher (and colder) habitats and also in habitats with an increased wetness as compared to C4 taxa. Between the two groups of C4 plants, C4 grasses seem to be able to grow at much higher altitudes than C4 sedges. In fact, C4 sedges seem to be everywhere rare above 1,300 m a.s.l., whereas C4 grasses can still be quite dominant at 2,400 m a.s.l.; although, they tend to occur there toward the drier edges of the wetland. Even in cases found in literature, where C4 plants have adapted to the aquatic environment, it seems to have an impact on associated traits, such as the Kranz anatomy (Keeley 1998).
The difference in growth form in graminoids, tufts versus mats, is not very significant in most groups, but the tendency is for mat-formers to grow in wetter habitats and lower altitudes than the tuft-formers. The only group where this distinction is very important is the C3 grasses, but here mat-formers are only represented by two species: Leersia hexandra and Phragmites australis. These are the same two species that Kotze and O’Connor (2000) reported as common across all the lower altitudes, but it seems to be an unusual functional type in wetlands.
We can summarize our findings and those of Kotze and O’Connor (2000) for the main functional groups as follows:
-
C3 grasses occur in most wetlands. At the higher altitudes they occur across the wetness gradient, whereas at altitudes lower than 1,800 m a.s.l., there are two common mat-forming species occurring only in the wettest parts of the wetlands.
-
C3 sedges are common in the wettest parts of wetlands at altitudes above 1,000 m a.s.l., but are replaced mostly by C4 sedges at the lowest altitudes.
-
C4 grasses are common in the temporarily wet zones of the wetland across all altitudes, but tend to decrease at altitudes higher than 2,400 m a.s.l.
-
C4 sedges occur mostly in the permanently wet zones of wetlands at altitudes below 1,300 m a.s.l.
-
Forbs and bulbous plants only become dominant at the highest altitudes.
-
Shrubs are unusual in wetlands and tend to occur mostly at the driest edge of the wetlands.
The C4 pathway, which is hypothesized to have evolved in response to decreases in atmospheric CO2 over geological time scales (e.g., Collatz et al. 1998), is a metabolic adaptation which greatly assists plant survival under water stressed conditions (Teeri and Stowe 1976; Ellis et al. 1980; Pearcy and Ehleringer 1984; Sage 2004). Among the Poales (including grasses and sedges), C4 has evolved independently at least 23 times (Christin et al. 2008) and reversals from C4 to C3 forms are known to occur in some species (e.g., Alloteropsis; Ibrahim et al. 2009). C4 graminoids predominate over C3 graminoids in habitats characterized by high levels of irradiance, high daytime temperatures, and low levels of water availability. With increasing ambient temperatures and decreasing humidity, C4 species increase in abundance relative to C3 species. The main environmental variable limiting C4 plants appears to be low temperature during the growing season (Teeri and Stowe 1976; Ellis et al. 1980; Teeri et al. 1980). There is a higher photosynthetic efficiency of C4 taxa in situations with high temperatures compared to C3 taxa (e.g., Sage 2004). Our data shows that at low altitudes and drier habitats, there is a dominance of C4 grasses and sedges, while C3 grasses and sedges are more prominent at higher altitudes and wetter habitats. These patterns can be seen from the present study and similar observations on grass distribution have been reported in Kenya (Tieszen et al. 1979).
It is quite commonly known that generally sedges are better adapted to wet areas than grasses (Kotze and O’Connor 2000; Thompson and Hamilton 1983). At most altitudinal ranges in this study except the lowest, C3 sedges are most prominent in permanently wet habitats, but it is noted that the percentage of C4 sedges in the study area is low compared to the percentage of C4 grasses in the study area (Vogel and Ellis 1978; Stock et al. 2004). Global sedge distribution shows that C3 taxa are more common in temperate areas while C4 taxa are found in the tropics (Govaerts et al. 2007). Three out of four tribes of the Cyperaceae that have evolved the C4 mechanism are recorded in our study area (Abildgaardiae, Eleocharidae, and Cypereae; Christin et al. 2008) and within these tribes, the C4 taxa comprise about 71% of the species.
The decreased efficiency of C4 photosynthesis at low temperatures (Kubien and Sage 2004) may explain the reduction of C4 taxa at higher altitudes. Wetlands are generally located at valley bottoms where the cold air sinks and where frost is very common in winter. Therefore, plants from the wetter parts of the wetland are likely to generally have more stress to endure during the cold season than plants from the drier parts of the wetland (especially, since the cold season in the Maloti-Drakensberg is also the dry season). The unusual distribution of C4 taxa in habitats which routinely experience freezing temperatures, such as the high Andes, has been attributed to the evolution of freezing tolerance (Marquez et al. 2006) and such taxa are hypothesized to be occupying habitats that were previously suitable for C4 plants (Boom et al. 2001).
An ecological study on the distribution of C3 and C4 taxa on an ecological gradient helps us to understand the implications of this trait in evolutionary history. The lack of fully resolved phylogenies and the need for a reconstruction of ancestral photosynthetic mechanisms for various lineages further limits the formulation of hypotheses on whether multiple origins of the C4 metabolism in the grass and sedge families or reversals from C4 to C3 have occurred. An increasing number of phylogenetic studies (e.g., Christin et al. 2008; Muasya et al. 2009) and screening for photosynthetic mechanisms (e.g., Gibbs Russell et al. 1991; Bruhl and Wilson 2007) will allow the interpretation of C4 evolution within a phylogenetic context.
References
Backeus I, Grab S (1995) Mires in Lesotho. Gunneria 70:243–250
Ball DF (1986) Site and soils. In: Moore PD, Chapman SB (eds) Methods in plant ecology, 2nd edn. Blackwell, Osney Mead, Oxford
Boom A, Mora G, Cleef AM, Hooghiemstra H (2001) High altitude C4 grasslands in the Northern Andes: relicts from glacial conditions? Rev Palaeobot Palynol 115:147–160
Boutin C, Keddy PA (1993) A functional classification of wetland plants. J Veg Sci 4:591–600
Box EO, Fujiwara K (2005) Vegetation types and their broad-scale distribution. In: Van der Maarel E (ed) Vegetation ecology. Blackwell, Malden, pp 106–128
Breiman L, Friedman JH, Olshen RA, Stone CG (1984) Classification and regression trees. Wadsworth, Belmont
Bruhl JJ, Wilson KL (2007) Towards a comprehensive survey of C-3 and C-4 photosynthetic pathways in Cyperaceae. Aliso 23:99–148
Christin PA, Salamin N, Muasya AM, Roalson ER, Russier F, Besnard G (2008) Evolutionary switch and genetic convergence on rbcL following the evolution of C4 photosynthesis. Mol Biol Evol 25:2361–2368
Collatz GJ, Berry JA, Clark JS (1998) Effects of climate and atmospheric CO2 partial pressure on the global distribution of C4 grasses: past, present and future. Oecologia 114:441–454
Cronk JK, Fennessy MS (2002) Wetland plants: biology and ecology. CRC Press, Boca Raton
De’ath G, Fabricius KE (2000) Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81:3178–3192
Dent MC, Lynch SD, Schulze RE (1987) Mapping mean annual precipitation and other rainfall statistics over Southern Africa. Agricultural Catchments Research Unit Report No. 27. Water Research Commission Report No. 109/1/89
Díaz S, Marcelo C (1997) Plant functional types and ecosystem function in relation to global change. J Veg Sci 8:463–474
Ellis RP, Vogel JC, Fuls A (1980) Photosynthetic and the geographical distribution of grasses in South West Africa/Namibia. S Afr J Sci 76:307–314
Ewart-Smith J, Ollis D, Day J, Malan H (2006) National Wetland Inventory: development of a Wetland Classification System for South Africa. Report prepared for the Water Research Commission and South African National Biodiversity Institute
Gibbs Russell GE, Watson L, Koekemoer M, Smook L, Barker NP, Anderson HM, Dallwitz MJ (1991) Grasses of Southern Africa. Memoirs of the Botanical Survey of South Africa No. 58. National Botanical Gardens, Botanical Research Institute, South Africa
Govaerts R, Simpson D, Goetghebeur P, Wilson K, Egorova T, Bruhl JJ (2007) World checklist of Cyperaceae. Royal Botanic Gardens, Kew
Hill MO (1973) Reciprocal averaging: an eigenvector method of ordination. J Ecol 61:237–249
Ibrahim DG, Burke T, Ripley BS, Osborne CP (2009) A molecular phylogeny of the genus Alloteropsis (Panicoideae, Poaceae) suggests an evolutionary reversion from C4 to C3 photosynthesis. Ann Bot 103(1):127–136
Ingrouille MJ, Eddie B (2006) Plants: diversity and evolution. Cambridge University Press, Cambridge, p 440
Jongman RHG, Ter Braak CJF, Van Tongeren OFR (1987) Data analysis in community and landscape ecology. Pudoc, Wageningen
Keddy P (2000) Wetland ecology: principles and conservation. Cambridge studies in ecology. Cambridge University Press, Cambridge, p 614
Keeley J (1998) C4 Photosynthetic modifications in the evolutionary transition from land to water in aquatic grasses. Oecologia 116:85–97
Körner C (2006) The role of ‘altitude’ in ecological studies. Trends Ecol Evol 22(11):569–574
Kotze DC, O’Connor TG (2000) Vegetation variation within and among palustrine wetlands along an altitudinal gradient in Kwazulu-Natal, South Africa. Plant Ecol 146:77–96
Kotze DC, Hughes JC, Klug JR, Breen CM (1996) Improved criteria for classifying hydric soils in South Africa. S Afr J Plant Soil 13:67–73
Kotze DC, Sieben EJJ, Morris CD (2006) A classification and health assessment of the wetlands of the Maloti-Drakensberg planning area. Report produced for the Maloti-Drakensberg Transfrontier Park
Kubien DS, Sage RF (2004) Dynamic photoinhibition and carbon gain in a C4 and a C3 grass native to high latitudes. Plant Cell Environ 27:1424–1435
Lavorel S, McIntyre S, Landsberg Forbes TDA (1997) Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends Ecol Evol 12:474–477
Li M-R, Wedin DA, Tieszen LL (1999) C3 and C4 photosynthesis in Cyperus (Cyperaceae) in temperate eastern North America. Can J Bot 77:209–218
Marquez E, Rada F, Farinas M (2006) Freezing tolerance in grasses along an altitudinal gradient in the Venezuelan Andes. Oecologia 150:393–397
Muasya AM, Simpson DA, Verboom GA, Goetghebeur P, Naczi RFC, Chase MW, Smets E (2009) Phylogeny of Cyperaceae based on DNA sequence data: current progress and future prospects. Bot Rev 75:2–21
Osborne C (2008) Atmosphere, ecology and evolution: what drove the Miocene expansion of C4 grasslands? J Ecol 96:35–45
Osborne C, Beerling D (2006) Nature’s green revolution: the remarkable evolutionary rise of C4 plants. Phil Trans R Soc B 361:173–194
Ostfeld RS, Pickett STA, Shachak M, Likens GE (1997) Operationalizing ecology under a new paradigm: an African perspective. In: Pickett STA, Ostfeld RS, Shachak M, Likens GE (eds) Enhancing the ecological basis of conservation: heterogeneity, ecosystem function and biodiversity. Chapman and Hall, New York
Pearcy RW, Ehleringer J (1984) Comparative ecophysiology of C3 and C4 plants. Plant Cell Environ 7:1–13
Sage R (2004) The evolution of C4 photosynthesis. New Phytol 161:341–370
Salford Systems (2006) CART 6.0. Salford Systems, San Diego, CA
Schulze RE (ed) (2006) South African Atlas of climatology and agrohydrology. Water Research Commission, Pretoria. WRC Report 1489/1/05
Sieben EJJ, Kotze DC, Morris CD (in prep.) The vegetation of the wetlands in the Maloti-Drakensberg, South Africa. Bothalia
Silvertown J, Franco M, Harper JM (1997) Plant life histories: ecology, phylogeny and evolution. Cambridge University Press, Cambridge, p 313
Smith TM, Huston M (1989) A theory of spatial and temporal dynamics of plant communities. Vegetatio 69:47–55
Smith TM, Shugart HH, Woodward FI (eds) (1997) Plant functional types: their relevance to ecosystem properties and global change. International Geosphere-Biosphere Programme Book Series 1. Cambridge University Press
Stock W, Chuba D, Verboom G (2004) Distribution of South African C3 and C4 species of Cyperaceae in relation to climate and phylogeny. Austral Ecol 29:313–319
Teeri JA, Stowe LG (1976) Climatic patterns and the distribution of C4 grasses in North America. Oecologia 23:1–12
Teeri JA, Stowe LG, Livingstone DA (1980) The distribution of C4 species of the Cyperaceae in North America in relation to climate. Oecologia 47:307–310
ter Braak CJF (1986) Canonical correspondence analysis a new eigenvector technique for multivariate direct gradient analysis. Ecology 67(5):1167–1179
ter Braak CJF, Prentice IC (1988) A theory of gradient analysis. Adv Ecol Res 18:272–317
ter Braak CJF, Šmilauer P (2002) Canoco 4.5, Canoco reference manual and Canodraw for Windows User’s guide. Software for Canonical Community Ordination (version 4.5) Microcomputer Power. Ithaca, New York, p 500
Thompson K, Hamilton AC (1983) Peatlands and swamps of the African continent. In: Gore AJP (ed) Ecosystems of the world, 4B. Mires: swamp, bog, fen and moor. Elsevier, Amsterdam
Tieszen LL, Senyimba MM, Imbamba SK, Troughton JH (1979) The distribution of C3 and C4 grasses and carbon isotope discrimination along an altitudinal and moisture gradient in Kenya. Oecologia 37:337–350
Van Zinderen Bakker EM, Werger MJA (1974) Environment, vegetation and phytogeography of the high-altitude bogs of Lesotho. Vegetatio 29:37–49
Vogel HJ, Ellis TP (1978) The geographical distribution of Kranz grasses in South Africa. S Afr J Sci 74:209–215
Weiher E, Keddy PA (1999) Ecological assembly rules perspectives advances, retreats. Cambridge University Press, Cambridge
Westhoff V, van der Maarel E (1978) The Braun-Blanquet approach. In: Whittaker RH (ed) Classification of plant communities. Dr. W. Junk, The Hague, pp 287–399
Acknowledgments
This study was carried out as part of an assessment of the wetlands in the Maloti-Drakensberg Transfrontier Park Planning area. We would like to thank Richard Lechmere-Oertel for coordinating the project and for making wetlands a priority in conservation planning within the area, and Boyd Escott for providing us with the climatic data. Many thanks to Prof. Kathy Gordon-Gray who helped greatly with the identification of grass and sedge species. And, at last thanks to the many people who have joined us in the field to collect the data: Doug McCullogh, Samantha Adey, Craig Cowden, Dean Ricketts, Eric Qonya, Mike Cunningham, and many others.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sieben, E.J.J., Morris, C.D., Kotze, D.C. et al. Changes in plant form and function across altitudinal and wetness gradients in the wetlands of the Maloti-Drakensberg, South Africa. Plant Ecol 207, 107–119 (2010). https://doi.org/10.1007/s11258-009-9657-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-009-9657-5